Abstract
Inherited deficiency of the housekeeping enzyme triosephosphate isomerase (TPI) is the most severe clinical disorder of glycolysis. Homozygotes manifest congenital hemolytic anemia and progressive neuromuscular impairment, which in most cases pursues an inexorable course with fatal outcome in early childhood. No effective therapy is available. Hitherto specific enzyme replacement has not been attempted in disorders of glycolysis. Primary skeletal muscle myoblasts and Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines generated from homozygous TPI-deficient patients were cultured in the presence of exogenous enzyme or cocultured with human K562 erythroleukemia cells as an exogenous source of TPI. Uptake of active enzyme by TPI-deficient cells resulted in reversal of intracellular substrate accumulation, with a reduction in dihydroxyacetone phosphate (DHAP) concentration to levels seen in TPI-competent cells. Evidence of successful metabolic correction of TPI deficiency in vitro establishes the feasibility of enzyme replacement therapy, and has important implications for the potential role of allogeneic bone marrow transplantation and gene therapy as a means of sustained delivery of functional enzyme in vivo.
TRIOSEPHOSPHATE ISOMERASE (TPI, EC5.3.1.1) catalyzes the interconversion of dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate in the Embden-Meyerhof pathway, with the reaction favoring formation of DHAP by a ratio of 20:1.1 TPI deficiency is an autosomal recessive multisystem disorder characterized by congenital hemolytic anemia, progressive neuromuscular dysfunction, susceptibility to bacterial infection, and cardiomyopathy. The majority of affected children fail to survive beyond 5 years of age.1-3 Homozygotes exhibit markedly reduced enzyme activity in all tissues studied, accompanied by metabolic block in glycolysis with intracellular accumulation of DHAP, particularly in red blood cells, which lack the capacity to metabolize DHAP in the glycerophosphate shuttle via α-glycerophosphate dehydrogenase.1,2 Genetic studies of multiple unrelated families have shown that a single mutation, G to C transversion at codon 104, accounts for 80% of mutant alleles, reflecting a founder effect.3 The substitution of aspartate for glutamate at residue 104, which lies at the subunit interface of the TPI dimer, results in loss of activity due to instability of the mutant enzyme. Several other causative mutations (8 missense, 2 nonsense, and 1 frameshift) distributed throughout the TPI gene have been defined.4-7 Progress towards understanding the molecular basis of TPI deficiency has overcome the limitations of biochemical assessment for in utero diagnosis.8 9 However, there is no effective therapy for the generalized manifestations of the disease.
Metabolic correction of several inborn errors, including severe combined immunodeficiency due to adenosine deaminase deficiency, X-linked adenoleukodystrophy, and the lysosomal storage disorders Gaucher disease, mucopolysaccharidosis (types I, II, and VII), and metachromatic leukodystrophy, has been achieved by enzyme replacement therapy and bone marrow transplantation.10-15 To date, neither approach has been attempted in TPI deficiency.
Heterozygotes have approximately 50% normal TPI activity, but manifest no evidence of metabolic block or clinical effects, implying this level of enzyme activity is sufficient to maintain normal metabolic function.5-8 Restoration of enzyme activity to comparative levels is therefore expected to produce substantial clinical benefit in homozygous TPI deficiency. The capacity of enzyme-replete cells to secrete functional enzyme that can be captured by deficient cells in cross-correction studies provides a basis for investigating the feasibility of intracellular enzyme delivery. Primary skeletal muscle myoblasts and Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines derived from patients with homozygous TPI deficiency manifest the biochemical characteristics of TPI deficiency1,2,16 17 and thus provide a model system for the disease.
In a preliminary study, transfer of functional enzyme in vitro from cells with normal TPI activity to lymphoblastoid cells derived from a TPI-deficient patient was demonstrated.16 In the present study, the feasibility of somatic correction was studied in primary skeletal muscle myoblasts and lymphoblastoid cells derived from TPI-deficient patients with different mutant genotypes. Restoration of intracellular enzyme activity with reduction in DHAP to normal levels was achieved in deficient primary skeletal muscle myoblasts and lymphoblastoid cells cultured in the presence of exogenous human plasma or purified rabbit muscle TPI, and after coculture with human K562 erythroleukemia cells as a renewable source of functional enzyme. Reversal of the metabolic defect in deficient cells, in particular primary skeletal muscle myoblasts, confirms the feasibility of enzyme replacement therapy for TPI deficiency and has implications for the potential role of allogeneic bone marrow transplantation and gene therapy as strategies for sustained enzyme delivery in vivo.
MATERIALS AND METHODS
Patients and samples.
The study was approved by the institutional ethics committee. Muscle biopsy tissue and blood samples of affected children were obtained with informed parental consent. Details of the 3 TPI-deficient patients studied are listed in Table 1. Patient A is homozygous for the codon 104 mutation (Glu104Asp). Patients B and C are compound heterozygous for codon 104 and 41 (Cys41Tyr) mutations and codon 104 and 170 (Ile170Val) mutations, respectively. All patients presented with congenital hemolytic anemia and exhibit marked reduction in erythrocyte enzyme activity and DHAP accumulation.
Characteristics of Three Unrelated TPI-Deficient Patients
| Subject . | Age (yr) . | Codon Mutation . | RBC TPI Activity (IU/g Hb) . | RBC DHAP Level (nmol/g Hb) . | Symptoms From Birth to Date . | ||
|---|---|---|---|---|---|---|---|
| Jaundice . | Hemolytic Anemia . | Neuromuscular Dysfunction . | |||||
| A | 4 | 104/104 | 138 | 1,750 | Yes | Yes | Yes |
| B | 8 | 104/41 | 330 | 600 | Yes | Yes | Yes |
| C | 13 | 104/170 | 64 | 3,772 | Yes | Yes | No |
| Normal | — | — | 810-1,423 | 26-48 | — | — | — |
| Subject . | Age (yr) . | Codon Mutation . | RBC TPI Activity (IU/g Hb) . | RBC DHAP Level (nmol/g Hb) . | Symptoms From Birth to Date . | ||
|---|---|---|---|---|---|---|---|
| Jaundice . | Hemolytic Anemia . | Neuromuscular Dysfunction . | |||||
| A | 4 | 104/104 | 138 | 1,750 | Yes | Yes | Yes |
| B | 8 | 104/41 | 330 | 600 | Yes | Yes | Yes |
| C | 13 | 104/170 | 64 | 3,772 | Yes | Yes | No |
| Normal | — | — | 810-1,423 | 26-48 | — | — | — |
Abbreviations: RBC, red blood cell; Hb, hemoglobin.
Cell lines.
EBV-transformed lymphoblastoid cell lines derived from patients A, B, and C, a heterozygote (D; mother of patient A), and a normal subject were maintained in culture at 0.5 to 1 × 106 cells/mL in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 2 mmol/L glutamine, 50 μg/mL streptomycin, and 1 mg/mL penicillin (GIBCO Life Technologies, Glasgow, UK). Human K562 erythroleukemia cells were maintained in the same medium. Human muscle cell line HS94MU (European Collection of Animal Cell Cultures, Wiltshire, UK) was maintained in culture at 1 × 105 cells/mL in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% FCS, 2 mmol/L glutamine, 50 μg/mL streptomycin, and 1 mg/mL penicillin. Human astrocytoma cell line MOG-G-UVW (European Collection of Animal Cell Cultures [ECACC]) was maintained in culture at 2 × 104 cells/mL in Ham’s F10 nutrient medium mixed with DMEM (1:1) supplemented with 10% FCS, 2 mmol/L glutamine, 50 μg/mL streptomycin, and 1 mg/mL penicillin. All cell lines were cultured at 37°C, 5% CO2, 95% air and used for experiments after the third passage.
Primary skeletal muscle myoblasts.
Quadriceps muscle tissue from patient A was rapidly transferred after open biopsy to the tissue culture laboratory where primary skeletal muscle myoblasts were isolated. Muscle tissue was minced finely in phosphate-buffered saline (PBS) containing 25 μg/mL fungizone (amphotercin B; Flow Laboratories, Lichfield, Staffordshire, UK) and 1% streptomycin/penicillin to obtain fragments of approximately 1 to 2 mm3 in size. These were washed 3 times in growth medium (GM: Ham’s F-10 nutrient medium supplemented with 20% FCS, 2% glutamine, and 1% streptomycin/penicillin), after which they were resuspended in 0.5 mL GM. Approximately 5 to 10 explants were transferred with GM to a tissue culture flask coated with sterile 0.01% gelatin and incubated at 37°C, 5% CO2, 95% air. As soon as cells migrated from explants, the cultures were trypsinized (0.25% trypsin-EDTA) at 37°C and cells and explants were replated separately. Explanted muscle cells were maintained in culture at 1 × 105 cells/mL in GM and cultured in a humidified environment (37°C, 5% CO2, 95% air). Preplating of the primary cultures was performed once to separate fibroblasts and myoblasts. Confluent myoblasts were subcultured and used for all experiments after the third passage. The myogenicity of explanted primary skeletal muscle cells was determined by the capacity for myotube formation in vitro (fusion index) and expression of the muscle-specific protein, desmin. The fusion index was 82.9%, and 91.2% of cells were desmin-positive.
Culture of TPI-deficient cells in the presence of plasma or purified enzyme.
Fresh-frozen plasma (FFP) was initially used as a source of normal human TPI. TPI activity was determined in FFP (range, 16 to 79.8 U/mL; n = 4) obtained from the hospital blood bank. Serum-free medium (GIBCO Life Technologies, Europe) was mixed with FFP to obtain a TPI concentration of 40 U/mL. Lymphoblastoid cells (1 × 105 cells) from patient A were cultured for 21 hours at 37°C, 5% CO2 , 95% air in the FFP-treated medium. Control lymphoblastoid cells were cultured under similar conditions in untreated serum-free medium. To exclude the possibility that any increase of intracellular TPI activity in lymphoblastoid cells cultured in FFP is dependent on protein synthesis, parallel experiments were performed in which cycloheximide (10 μg/mL) was added to the culture medium18 and incubated for 21 hours at 37°C, 5% CO2, 95% air.
Lysates were prepared by freezing cells in liquid nitrogen and thawing on ice. After culture, lymphoblastoid cells were washed 3 times in ice-cold PBS and lysed by 3 cycles of freezing and thawing. Lysates were centrifuged (3,000 rpm, 10 minutes, 4°C) and the supernatant collected on ice. An aliquot of the supernatant was assayed for TPI activity and total cellular protein. For determination of DHAP levels, aliquots of the supernatant were deproteinized immediately by mixing with ice-cold 5% perchloric acid, incubated on ice for 20 minutes, and centrifuged (3,000 rpm, 10 minutes, 4°C). The supernatant was neutralized with 3 mol/L K2CO3solution containing 1 mol/L Tris. After centrifugation, the clear supernatant was stored at −70°C and assayed within 24 hours.
Primary skeletal muscle myoblasts (1 × 105 cells) from patient A, lymphoblastoid cells (1 × 105 cells) from patients A, B, C, a heterozygote, D, a nondeficient subject, and cell lines HS94MU (1 × 104 cells) and MOG-G-UVW (1 × 104 cells) were incubated for 24 hours in 1 mL serum-free medium containing 50 U of purified rabbit muscle TPI (Boehringer Mannheim, Mannheim, Germany) sterilized by filtration (Millipore; Sartorius Ltd, Epsom, Surrey, UK). Control cells were cultured under identical conditions in serum-free medium alone.
At the end of the culture period, primary skeletal muscle myoblasts, human muscle, and astrocytoma cell lines were washed 3 times in ice-cold PBS, trypsinized (0.25% trypsin-EDTA) at 37°C for 10 minutes, frozen in liquid nitrogen, and homogenized on ice for 2 minutes. Homogenates were centrifuged and an aliquot of supernatant was assayed for TPI activity and total cellular protein. Aliquots of supernatant for DHAP determination were immediately deproteinized as described earlier.
Coculture of TPI-deficient cells and K562 erythroleukemia cells.
Uptake of exogenous TPI by deficient primary skeletal muscle myoblasts from patient A and lymphoblastoid cells from patients A, B, and C was examined in a coculture model using human K562 erythroleukemia cells as a source of functional enzyme.16 K562 cells were plated into 6-well cell-companion culture dishes (5 × 105cells/well) containing serum-free medium. To prevent direct cell-to-cell contact, primary skeletal muscle cells (1 × 105 cells/well) and lymphoblastoid cells (5 × 105 cells/well) were plated separately into inserts containing a semipermeable membrane of 1 μm pore size (Becton-Dickinson Labware, Oxford, UK). The cocultures were incubated at 37°C, 5% CO2, 95% air for 24 hours. Control muscle and lymphoblastoid cells cultured in serum-free medium in the absence of K562 cells were maintained under similar conditions.
DHAP levels in protein-free extracts.
To exclude the possibility that membrane adherent or encapsulated enzyme contributed to the reduction in DHAP concentration observed in cell lysates, lymphoblastoid cells (1 × 105 cells) from patient A (Table 1) were cultured in serum-free medium (control) or in the presence of exogenous rabbit muscle TPI (50 U/mL) and sampled at 0, 3, 6, 9, 12, and 24 hours. Cultured cells were immediately deproteinized by the addition of ice-cold 5% perchloric acid. The deproteinized cell suspension was incubated on ice for 20 minutes, centrifuged (3,000 rpm, 10 minutes, 4°C), and the supernatant neutralized as described earlier and assayed for DHAP.
In parallel experiments, lymphoblastoid cells (1 × 105 cells) from patient A (Table 1) cultured in serum-free medium (control) or in the presence of rabbit muscle TPI (50 U/mL) were sampled at various intervals. Cultured cells were either deproteinized with 5% perchloric acid as described or lysed by immediately freezing cells in liquid nitrogen and thawing on ice. Aliquots of lysates were deproteinized with 5% perchloric acid and neutralized. DHAP assays were performed as describe earlier.
Assay of TPI activity and DHAP level.
TPI activity in FFP, culture medium, cell lysates, and homogenates was determined according to the method recommended by the International Committee for Standardisation in Haematology (ICSH) for quantification of enzyme activity in erythrocytes.19,20Briefly, culture fluid or cell lysates were added to a cuvette in the presence of DL-glyceraldehyde-3-phosphate as substrate in an α-glycerophosphate dehydrogenase/NADH-coupled assay. Decrease in optical density was measured at 340 nm, 30°C, 10 minutes. DHAP was estimated by fluorimetric analysis as previously described.8,16 Protein concentration in homogenates and lysates was determined by the method of Bradford, with bovine serum albumin as standard.21
Statistical analysis.
All results are given as the mean ± SD of 6 separate experiments in triplicate, unless otherwise indicated. Student’s t-test was used to establish whether between-group differences were statistically significant.
RESULTS
Culture of TPI-deficient cells with plasma.
Initial experiments assessed the feasibility of enzyme replacement in vitro with FFP. Lymphoblastoid cells from patient A (Table 1) cultured in the presence of FFP as an exogenous source of enzyme showed a 5-fold reduction in intracellular DHAP level (84 ± 35 μmol/L/μg protein) with a concomitant rise in TPI activity from 37 ± 15 to 361 ± 33 U/μg protein (Fig 1A and B). The corresponding DHAP level and TPI activity in lymphoblastoid cells cultured in the absence of FFP were 388 ± 60 μmol/L/μg protein and 30 ± 15 U/μg protein, respectively. At the end of the culture period, TPI activity in the culture medium had decreased by approximately 2-fold, whereas no TPI activity was detectable in the control medium (Fig 1C). Lymphoblastoid cells (1 × 106 cells) cultured in the presence of FFP for 21 hours, washed in PBS, and reintroduced into FFP-medium or serum-free medium (control) for a further 24 hours, showed that the intracellular DHAP level was 178 ± 48 μmol/L/μg protein in cells maintained in FFP and 1,023 ± 380 μmol/L/μg protein in controls. The corresponding TPI activity was 188 ± 10 U/μg protein and 21 ± 4 U/μg protein in treated and control cells, respectively.
Reversal of metabolic defect in TPI-deficient lymphoblastoid cells from patient A (Table 1). (a) Reduction in accumulated intracellular DHAP. (b) Intracellular TPI activity. (c) TPI activity in culture medium. Cells were cultured in serum free medium with (▪) or without (•) normal human plasma as described in Materials and Methods.
Reversal of metabolic defect in TPI-deficient lymphoblastoid cells from patient A (Table 1). (a) Reduction in accumulated intracellular DHAP. (b) Intracellular TPI activity. (c) TPI activity in culture medium. Cells were cultured in serum free medium with (▪) or without (•) normal human plasma as described in Materials and Methods.
Cycloheximide-treated lymphoblastoid cells similarly showed increased intracellular TPI activity (255 ± 45 U/μg protein) and a reduced DHAP level (531 ± 105 μmol/L/μg protein) in the presence of FFP when compared with untreated cells (TPI activity, 25 ± 10 U/μg protein; DHAP, 1,606 ± 255 μmol/L/μg protein). This indicates that reversal of the metabolic block in deficient cells is not dependent on de novo protein synthesis.
Culture of TPI-deficient cells with purified TPI.
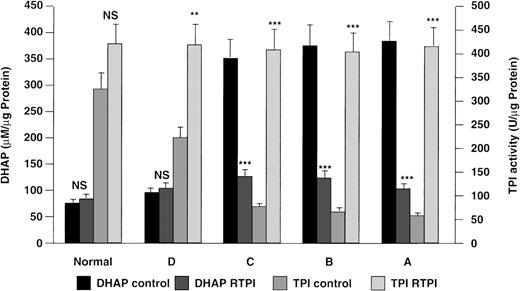
The ability of purified enzyme to reverse the metabolic defect in TPI-deficient lymphoblastoid cells and primary skeletal muscle myoblasts cultured in the presence of purified rabbit muscle TPI was evaluated by estimating intracellular accumulation of DHAP and TPI activity. After incubation with exogenous TPI, the intracellular DHAP level and TPI activity observed in cell lines from patients A, B, and C were comparable to those in cells derived from normal and heterozygous subjects (Fig 2). The alterations in intracellular TPI activity and DHAP level for lymphoblastoid cells cultured in the presence of rabbit muscle TPI were highly significant when compared with controls cultured in serum-free medium alone (Fig2). When TPI-deficient primary skeletal muscle myoblasts were cultured in the presence of purified rabbit muscle TPI, a 4- to 5-fold increase in intracellular TPI activity (340 ± 65 U/μg protein), accompanied by a reciprocal decrease in DHAP level (105 ± 30 μmol/L/μg protein), was observed. These figures are highly significant (P < .001) when compared with control values (TPI activity, 75 ± 25 U/μg protein; DHAP, 1,600 ± 315 μmol/L/μg protein), and equivalent to those in normal human primary skeletal muscle myoblasts (TPI activity, 550 ± 60 U/μg protein; DHAP, 80 ± 25 μmol/L/μg protein; n = 2).
Reversal of metabolic block in TPI deficiency by exogenous rabbit muscle TPI (RTPI). Lymphoblastoid cells derived from TPI-deficient patients A, B, and C, a heterozygote, D, and a normal subject were cultured in serum-free medium with or without exogenous RTPI (50 U/mL) for 24 hours. Changes in intracellular enzyme activity and DHAP level were measured as described in Materials and Methods. Statistical significance: *P < .05, **P < .01, ***P < .001; NS, not significant.
Reversal of metabolic block in TPI deficiency by exogenous rabbit muscle TPI (RTPI). Lymphoblastoid cells derived from TPI-deficient patients A, B, and C, a heterozygote, D, and a normal subject were cultured in serum-free medium with or without exogenous RTPI (50 U/mL) for 24 hours. Changes in intracellular enzyme activity and DHAP level were measured as described in Materials and Methods. Statistical significance: *P < .05, **P < .01, ***P < .001; NS, not significant.
Uptake of TPI by other human cell lines.
Table 2 shows that human skeletal muscle and astrocytoma cell lines cultured in the presence of exogenous rabbit muscle TPI have an increased TPI activity when compared with their respective basal level.
Uptake of Exogenous Purified Rabbit Muscle TPI by Human Muscle and Brain Cell Lines
| Cell Line . | TPI Activity (U/μg protein) . | |
|---|---|---|
| Cultured in Serum-Free Medium . | Cultured in Serum-Free Medium + Rabbit Muscle TPI . | |
| Skeletal muscle | 26.7 ± 3 | 47.6 ± 6 |
| Brain (astrocytoma) | 10 ± 0.5 | 32 ± 6 |
| Cell Line . | TPI Activity (U/μg protein) . | |
|---|---|---|
| Cultured in Serum-Free Medium . | Cultured in Serum-Free Medium + Rabbit Muscle TPI . | |
| Skeletal muscle | 26.7 ± 3 | 47.6 ± 6 |
| Brain (astrocytoma) | 10 ± 0.5 | 32 ± 6 |
Cell lines were cultured in serum-free medium with or without (controls) rabbit muscle TPI. Rabbit muscle TPI (50 U in 1 mL medium) was added medium to muscle (1 × 104 cells) and brain (1 × 104 cells) cell lines and incubated as described in the Methods. Data represent means ± SD of 3 experiments in triplicate.
Coculture of TPI-deficient cells and K562 cells.
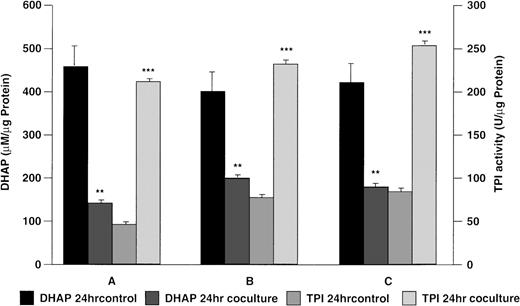
To investigate whether sustained correction of the metabolic defect in TPI-deficient cells in vitro may be feasible, deficient lymphoblastoid cells or primary skeletal muscle myoblasts were cocultured with K562 cells. Figure 3 shows results obtained when TPI-deficient lymphoblastoid cells from patients A, B, and C (Table 1) were cocultured with K562 cells. After 24 hours in coculture, there was a significant reduction in DHAP and a significant increase in intracellular TPI activity when compared with lymphoblastoid cells cultured in the absence of K562 cells (Fig 3). Cocultured deficient primary skeletal muscle myoblasts showed a significant increase (P< .001) in intracellular TPI activity (328 ± 75 U/μg protein) with a significant reduction (P < .001) in DHAP level (93 ± 45 μmol/L/μg protein) when compared with control values (TPI activity, 89 ± 30 U/μg protein; DHAP, 1,491 ± 250 μmol/L/μg protein).
Reversal of metabolic block in TPI deficiency using a coculture model. Lymphoblastoid cells derived from patients A, B, and C were cocultured for 24 hours in the presence or absence (control) of human K562 erythroleukemia cells in serum free-medium as described in Materials and Methods. Statistical significance: *P < .05, **P < .01, ***P < .001.
Reversal of metabolic block in TPI deficiency using a coculture model. Lymphoblastoid cells derived from patients A, B, and C were cocultured for 24 hours in the presence or absence (control) of human K562 erythroleukemia cells in serum free-medium as described in Materials and Methods. Statistical significance: *P < .05, **P < .01, ***P < .001.
DHAP concentration in deproteinized extracts.
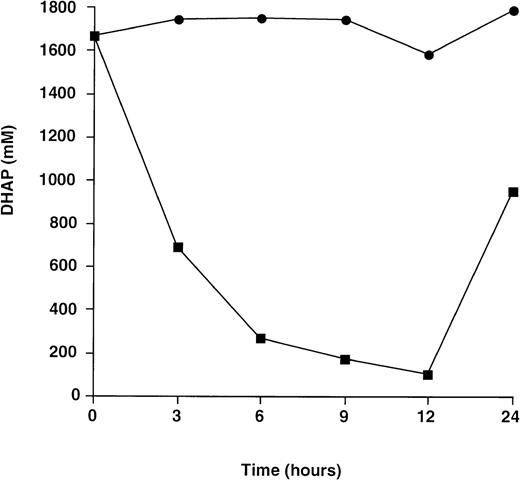
Fig 4 shows results obtained when lymphoblastoid cells from patient A (Table 1) cultured in the presence of rabbit muscle TPI were immediately deproteinized in perchloric acid. There was a marked reduction in DHAP concentration for cells cultured in the presence of exogenous TPI when compared to controls cultured in serum free medium alone. These results demonstrate conclusively that metabolic block is reversed through uptake of active enzyme by TPI-deficient cells.
Change in DHAP level for TPI-deficient lymphoblastoid cells immediately deproteinized in 5% perchloric acid. Cells were cultured in serum-free medium with (▪) or without (•) exogenous rabbit muscle TPI (50 U/mL) and processed as described in Materials and Methods. Each point represents the mean of 1 experiment performed in triplicate.
Change in DHAP level for TPI-deficient lymphoblastoid cells immediately deproteinized in 5% perchloric acid. Cells were cultured in serum-free medium with (▪) or without (•) exogenous rabbit muscle TPI (50 U/mL) and processed as described in Materials and Methods. Each point represents the mean of 1 experiment performed in triplicate.
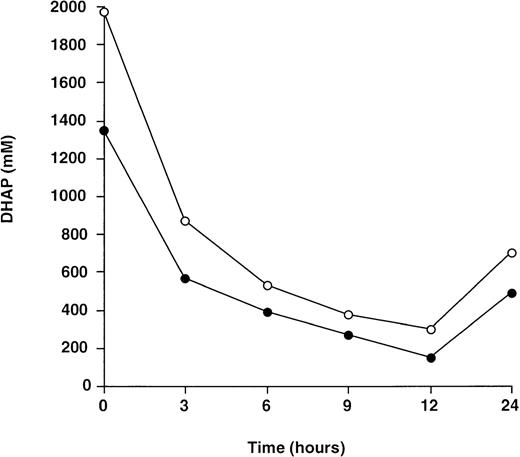
To further eliminate the possibility that membrane adherent or entrapped enzyme might contribute to the reduction in intracellular DHAP level in deficient cells cultured in the presence of TPI, DHAP concentration in deproteinized cell extracts and cell lysates was compared. No significant difference between the DHAP level in cell lysates and that from deproteinized cell extracts was observed (Fig5).
Comparison between the change in DHAP level for TPI-deficient lymphoblastoid cells immediately deproteinized in perchloric acid (•) and cell lysates (○). Cells were cultured in the presence of exogenous rabbit muscle TPI (50 U/mL) and protein-free extracts processed as described in Materials and Methods. Each point represents the mean of 1 experiment performed in triplicate.
Comparison between the change in DHAP level for TPI-deficient lymphoblastoid cells immediately deproteinized in perchloric acid (•) and cell lysates (○). Cells were cultured in the presence of exogenous rabbit muscle TPI (50 U/mL) and protein-free extracts processed as described in Materials and Methods. Each point represents the mean of 1 experiment performed in triplicate.
DISCUSSION
Specific enzyme replacement therapy has hitherto not been attempted in TPI deficiency or other inborn errors of glycolytic metabolism. TPI deficiency is the most clinically severe defect of glycolysis and typically results in death before the age of 6 years. In patients with severe clinical phenotypes, no form of therapy has been shown to modify the natural history of the disorder. Efforts to bypass the enzymatic defect by methylene blue stimulation of the hexose monophosphate shunt have proved unsuccessful and splenectomy did not alter the course of the disease in one patient.2 The prospect for somatic correction of TPI deficiency by enzyme replacement therapy was therefore studied. Alterations in intracellular DHAP concentration and TPI activity were monitored to evaluate the capacity of exogenous enzyme to reverse metabolic block in TPI-deficient cells. The results clearly demonstrate increased intracellular TPI activity with a marked reduction in DHAP accumulation in deficient cells treated with exogenous TPI or cocultured with donor cells as a renewable source of functional enzyme.
While TPI localizes in the cytosol, enzyme activity is detectable in plasma.7,8 17 The origin of extracellular TPI is uncertain, but is likely to reflect diffusion or secretion from blood cells. This prompted examination of the potential role of human plasma as a vehicle for delivery of exogenous enzyme to TPI-deficient lymphoblastoid cells and whether correction of TPI deficiency in vitro could be achieved at a concentration within the physiologic range found in FFP.
TPI-deficient lymphoblastoid cells cultured in the presence of FFP showed a nadir in intracellular DHAP level coincidental with maximal TPI activity. Partial reversal of this effect with time was seen due to gradual depletion of enzyme in medium (Fig 1). This suggests frequent or continuous delivery of enzyme may be necessary to achieve therapeutic benefit in vivo.22 Functional restoration of glycolysis in vitro was confirmed in TPI-deficient lymphoblastoid cells cultured in the presence of rabbit muscle TPI (Fig 2). DHAP was reduced to a level comparable to that seen in nondeficient cells.Notably, reversal of metabolic block in lymphoblastoid cells was observed for each of the 3 TPI-deficient patients studied, irrespective of the causative mutation. Reaccumulation of DHAP and reversion of TPI activity to basal levels were observed when medium to which exogenous enzyme had been added was replaced with medium lacking the enzyme.
Patients homozygous for TPI deficiency have less than 20% residual enzyme activity in muscle cells.23 The consequence of impaired glycolysis and defective mitochondrial metabolism has been directly linked to the severe myopathy seen in homozygous TPI deficiency.23 This study provides evidence for correction of the metabolic defect in TPI-deficient primary skeletal muscle myoblasts by exogenous enzyme. Furthermore, other cell types, including nondeficient human muscle and astrocytoma cell lines (Table 2), as well as lymphoblastoid cell lines from normal and heterozygous subjects (Fig2), exhibited uptake of TPI from culture medium containing enzyme. These observations imply existence of a transport mechanism that permits the transfer of functional enzyme across the cell membrane. The basis for this remains to be elucidated. Taken in conjunction, these results establish the feasibility of intracellular enzyme replacement therapy in TPI deficiency.
The mechanism underlying the neuropathic effects of TPI deficiency, which may include central and peripheral components, is unknown. Disturbance of lipid metabolism secondary to accumulation of DHAP, a precursor of ether lipids which are essential components of myelin, has been postulated.24 25 Despite evidence that the metabolic defect in TPI-deficient myoblasts and lymphoblastoid cells is reversible, it remains to be determined whether enzyme replacement would correct the prominent neurological manifestations of TPI deficiency. Inability of functional enzyme to traverse the blood-brain barrier and its relatively short half-life are likely to present significant obstacles in vivo.
Provision of a deficient enzyme to neural cells by bone marrow derived microglial cells after hemopoietic stem-cell transplantation ameliorates or arrests neurologic progression in some lysosomal and peroxisomal disorders.10-15 Evidence of correction from in vitro models of secretion and recapture of functional enzyme generally correlates with a favorable outcome after bone marrow transplantation. Coculture of TPI-deficient primary myoblasts and lymphoblastoid cells with human K562 erythroleukemia cells led to functional restoration of glycolysis. Recent studies in which myeloid colonies (colony-forming unit granulocyte-macrophage [CFU-GM]) from a TPI-deficient patient were cultured in methyl cellulose–containing feeder leukocytes derived from a normal human leukocyte antigen (HLA)-matched related donor confirm hematopoietic cells are a viable source of functional enzyme (Ationu A, Humphries A, Gordon M, et al, unpublished observations, December 1997). Collectively, these results have important implications for the possible role of allogeneic bone marrow transplantation as a means of sustained delivery of TPI to neural and other somatic cells, and provide a rationale for development of therapeutic approaches for correction of the defect in TPI deficiency and potentially other metabolic disorders of glycolysis associated with a severe clinical phenotype.
ACKNOWLEDGMENT
We are grateful to David McGonigle for technical assistance with biochemical studies.
Supported by grants from the James Stewardson TPI Research Trust and Medical Research Council.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Art Ationu, MSc, PhD, Department of Haematological Medicine, Guy’s, King’s, and St Thomas’ School of Medicine, Bessemer Rd, London, SE5 9PJ, UK.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal