Abstract
Primary CD30+ cutaneous T-cell lymphomas (CTCLs) represent a spectrum of non-Hodgkin’s lymphomas (NHLs) that have been well defined at the clinical, histologic, and immunologic level. This group, which includes 2 main entities (large cell lymphoma and lymphomatoid papulosis [LyP]) and borderline cases, is characterized by the expression of CD30 antigen by neoplastic large cells at presentation, possible spontaneous regression of the skin lesions, and generally favorable clinical course. Although the functional relevance of CD30 and its natural ligand (CD30L) expression in most cases of NHL is presently undefined, previous studies indicate that CD30L is likely to mediate reduction of proliferation in CD30+ anaplastic large-cell NHL. No information is currently available concerning the expression of CD30L in primary CD30+ CTCLs. In this study, we investigated the immunophenotypic and genotypic expression of CD30 and CD30L in different developmental phases of skin lesions (growing v spontaneously regressing). By immunohistochemistry, CD30L expression was detected in regressing lesions only; by molecular analysis, the expression of CD30L was clearly higher in regressing lesions than in growing ones. CD30L, while expressed by some small lymphocytes, was most often coexpressed by CD30+neoplastic large cells, as demonstrated by 2-color immunofluorescence and by immunohistochemistry on paraffin sections. Taken together, these data suggest that CD30-CD30L interaction may play a role in the pathobiology of primary cutaneous CD30+lymphoproliferative disorders. In particular, CD30L (over)expression might have a major role in the mechanism of self-regression of skin lesions, the most distinctive clinical feature of this cutaneous lymphoma subtype.
RECENTLY, DIFFERENT FORMS OF cutaneous T-cell lymphoma (CTCL) other than mycosis fungoides (MF) have been defined on the basis of well-defined clinical, histologic, immunologic, and molecular features.1-3 This group includes a spectrum of diseases known as CD30+ lymphoproliferative disorders of the skin4-7: primary cutaneous CD30+ large T-cell lymphoma (CD30+ large-cell CTCL) and lymphomatoid papulosis (LyP). CD30+ large-cell CTCL is characterized clinically by presentation with solitary or localized skin lesions, possible spontaneous regression (partial to complete), good and rapid response to local radiotherapy, and favorable prognosis, despite frequent cutaneous relapses. LyP, historically defined as “a continuing, self-healing eruption, clinically benign, histologically malignant,”8 is characterized clinically by an intermittent or continuous eruption of usually multiple, papulonodular lesions, with ulceration, crusting, and self-healing, sometimes with scarring and/or depigmentation. CD30+ atypical cells occur in a background of inflammatory cells.3,4,6,7 It is worthy of emphasis that CD30 expression by neoplastic T cells has a special diagnostic and favorable prognostic significance when expressed at presentation and correlated with the above typical clinicopathologic features.2,5,7 Conversely, CD30 expression occurring in the transformation of MF into large-cell lymphoma tumor stage does not influence the prognosis, which is invariably poor.9
CD30 antigen is a member of the tumor necrosis factor (TNF) receptor superfamily,10,11 which was identified as a cell surface antigen on primary and cultured Hodgkin’s and Reed-Sternberg cells by the monoclonal antibody Ki-1.12,13 CD30 antigen is normally expressed by a subset (15% to 20%) of CD45RO+ T cells after activation by a variety of T-cell stimuli.14 CD30 is also expressed at variable levels in different non-Hodgkin’s lymphomas (NHLs), as well as in several virally transformed T- and B-cell lines.15,16 In particular, CD30 is a specific marker of a subset of peripheral T-cell NHL, ie, anaplastic large cell (ALC) lymphoma.17 More recently, CD30 preferential expression has been detected on subset of tissue and circulating CD4+ or CD8+ T cells producing type 2 T-helper (Th2) cytokines in healthy and immunoreactive conditions.18,19 The biologic significance of CD30 molecule is related to the existence of a natural ligand (CD30L), a member of the TNF ligand superfamily recently identified in murine T cells and in human peripheral blood T cells and mainly expressed on activated T cells and monocytes and, constitutively, on granulocytes and some Burkitt-like lymphoma cell lines.10,20,21 CD30 and CD30L are involved in the regulation of cell proliferation, activation, and differentiation, including control of cell survival or death by apoptosis or cytotoxicity.21
Recent data demonstrate pleiotropic biologic activities of CD30L on different CD30+ lymphoma cells lines and indicate that a CD30-CD30L interaction might have a pathophysiologic role in Hodgkin’s lymphoma and in specific subsets of NHL, particularly ALC lymphoma.20,21 CD30L is capable of transducing signals leading to either cell death or proliferation through its specific cognate molecule CD30. Although previous studies indicate that CD30L plays a key role as a paracrine- or autocrine-acting surface molecule in the pathophysiology of Hodgkin’s lymphoma16 and in CD30+ NHL,22 no information is currently available concerning the expression of CD30L in primary cutaneous lymphomas. By the combined use of different methods, including immunohistochemistry and 2-color immunofluorescence, reverse-transcriptase polymerase chain reaction (RT-PCR), quantitative PCR, and Southern blot, we have analyzed the phenotypic and genotypic expression of CD30 and CD30L in cutaneous lymphoproliferative CD30+ disorders, to investigate the correlation between CD30 and CD30L expression in different developmental phases of skin lesions (growing v spontaneously regressing) and its possible significance in the pathophysiology of clinical regression of skin lesions in these disorders.
MATERIALS AND METHODS
Patients and Skin Samples
The diagnosis of patients enrolled in this study (Table1) was based on clinical, histologic, and immunologic criteria described earlier.1-3 The definitions of “growing” and “regressing,” used below, concern the evolutive phase of the lesion biopsy. Growing lesions are characterized by the absence of necrosis, ulceration, and/or crusting. This is typical of florid, diagnostic lesions in CD30+ large-cell CTCL (nodules or plaques histologically composed by >75% of large, CD30+ neoplastic cells). In LyP, conversely, those defined as growing are newly developing, early lesions before they undergo the necrosis, ulceration, crusting, and self-regression that are the hallmark of the disease. Histologically, growing lesions are composed of large, CD30+ neoplastic cells and inflammatory cells in variable proportions. Regressing lesions are characterized by epidermal damage (necrosis and ulceration, eventually with crusting). This is typical of diagnostic lesions in LyP, histologically characterized by sparse large, CD30+ neoplastic cells within a heavy inflammatory infiltrate. In CD30+ large-cell CTCL, some of the infiltrative lesions undergo partial to total regression, histologically characterized by the presence of an inflammatory cell component heavier than in growing lesions. The 13 skin samples, consisting of 6-mm punch biopsies, were obtained under local anaesthesia from 6 patients with CD30+ large-cell CTCL (3 were taken from growing and 3 from regressing lesions; in the latter, the histologic diagnosis was made on growing lesions) and from 6 patients with LyP (2 from growing and 5 from regressing lesions; in 1 patient, 2 skin samples were taken at the same time, 1 from a growing and 1 from a regressing lesion). Control skin samples were obtained from 5 healthy subjects (patients who underwent cosmetic surgery). Each tissue specimen was in part formalin-fixed and paraplast-embedded for routine histologic examination, in part embedded in OTC (Tissue Tek; Miles Scientific, Naperville, IL), snap-frozen, and stored at −80°C until sectioning and preparing for immunohistochemistry (APAAP method). A portion of biopsies from 6 skin lesions were prepared for molecular analysis. Each tissue specimen was put in RNase-free microfuge and immediately frozen in liquid nitrogen until RNA extraction. All skin biopsies were taken after informed consent was obtained.
Clinical Data, Methods of Investigation Used, and Semiquantitative Scoring of Immunohistochemical Staining in the Series of 10 Patients
| Samples . | Age/Sex . | Diagnosis . | Skin Lesion . | Method . | CD30 (%) . | CD30L (%) . |
|---|---|---|---|---|---|---|
| 1 | 35/F* | LyP | Growing | IHC, MA, TCI | 47 | Neg |
| 2 | 76/M | LCL | Growing | IHC, MA, TCI | 83 | Neg |
| 3 | 35/F* | LyP | Regressing | IHC, MA, TCI | 22 | 19 |
| 4 | 64/F | LCL | Regressing | IHC, MA, TCI | 41 | 44 |
| 5 | 26/M | LCL | Growing | IHC, MA, TCI | 91 | Neg |
| 6 | 55/M | LCL | Regressing | IHC, MA, TCI | 43 | 37 |
| 7 | 47/M | LCL | Regressing | IHC, TCI | 49 | 40 |
| 8 | 32/F | LyP | Regressing | IHC, TCI | 15 | 18 |
| 9 | 49/F | LyP | Regressing | IHP | 30 | 52 |
| 10 | 49/M | LyP | Regressing | IHP | 23 | 25 |
| 11 | 40/F | LyP | Regressing | IHP | 19 | 20 |
| 12 | 78/M | LCL | Growing | IHC, TCI | 80 | Neg |
| 13 | 42/F | LyP | Growing | IHC, TCI | 41 | Neg |
| Samples . | Age/Sex . | Diagnosis . | Skin Lesion . | Method . | CD30 (%) . | CD30L (%) . |
|---|---|---|---|---|---|---|
| 1 | 35/F* | LyP | Growing | IHC, MA, TCI | 47 | Neg |
| 2 | 76/M | LCL | Growing | IHC, MA, TCI | 83 | Neg |
| 3 | 35/F* | LyP | Regressing | IHC, MA, TCI | 22 | 19 |
| 4 | 64/F | LCL | Regressing | IHC, MA, TCI | 41 | 44 |
| 5 | 26/M | LCL | Growing | IHC, MA, TCI | 91 | Neg |
| 6 | 55/M | LCL | Regressing | IHC, MA, TCI | 43 | 37 |
| 7 | 47/M | LCL | Regressing | IHC, TCI | 49 | 40 |
| 8 | 32/F | LyP | Regressing | IHC, TCI | 15 | 18 |
| 9 | 49/F | LyP | Regressing | IHP | 30 | 52 |
| 10 | 49/M | LyP | Regressing | IHP | 23 | 25 |
| 11 | 40/F | LyP | Regressing | IHP | 19 | 20 |
| 12 | 78/M | LCL | Growing | IHC, TCI | 80 | Neg |
| 13 | 42/F | LyP | Growing | IHC, TCI | 41 | Neg |
Abbreviations: LyP, lymphomatoid papulosis; LCL, large cell lymphoma; IHC, immunohistochemistry on cryostat sections; IHP, immunohistochemistry on paraffin sections; MA, molecular analysis (RT-PCR, Southern blot); TCI, two-color immunofluorescence; Neg, negative or <2%.
Samples 1 and 3 were taken from the same patient.
Immunohistochemistry
Immunohistochemical staining was performed on 7-μm cryostat sections using the APAAP method, as previously described.23 The monoclonal antibodies (MoAbs) used were mouse anti-human CD3, CD4, CD8, CD19, CD22, CD25, CD45RO, CD30 (clone Ber-H2 IgG1) (Dako A/S, Glostrup, Denmark) and CD30L, clone IgG2b (Serotec, Oxford, England). Normal human lymph nodes were stained in parallel as positive controls. Sections incubated with an irrelevant, isotype-matched mouse antibody were used as negative controls. The step section method was used to evaluate results; serial sections of each tissue specimen were carefully and blindly evaluated by 2 of the authors (N.P. and M.M.). For quantitative analysis, the stained cells in the dermal infiltrate were counted in 5 consecutive microscopic fields (×250). The results were expressed as the mean number of stained cells per 100 cells observed. Only cells whose nuclei were contained in the plane of the section were considered. The results were scored independently by the 2 observers, and the resulting figures were averaged.
Two-Color Immunofluorescence
Surface expression of CD30, CD30L, Fas (CD95), and FasL (CD95L) was analyzed by 2-color immunofluorescence method using 4 different experimental protocols: (1) fluorescein isothiocyanate (FITC)-conjugated MoAb mouse anti-human Ki-1 (CD30), clone Ber-H2 IgG1, MoAb mouse anti-human CD30L, clone IgG2b, and phycoerythrin (PE)-conjugated goat anti-mouse IgG2b polyclonal antibody (Southern Biotechnology, Birmingham, AL); (2) FITC-conjugated MoAb mouse anti-human CD30, biotin-conjugated MoAb mouse anti-human FAS CD95, clone DX2 IgG1 (Pharmigen, San Diego, CA), and PE-conjugated streptavidin (Southern Biotechnology); (3) FITC-conjugated monoclonal mouse anti-CD30, biotin-conjugated MoAb mouse anti-human FAS Ligand (CD95) clone NOK-1 IgG1 (Pharmigen), and PE-conjugated streptavidin; and (4) FITC-conjugated MoAb mouse anti-human FasCD95, clone DX2 IgG1, biotin-conjugated anti-CD95, and PE-conjugated streptavidin. Negative control stains were performed by replacing the primary antibody with an irrelevant isotype-matched (IgG2b) mouse MoAb and with chromogen only.
RNA Isolation and Reverse Transcriptase
Total mRNA was extracted from skin biopsies by a commercial kit (Ambion, Austin, TX). All samples used in these experiment clearly gave 18S and 28S bands in 0.8% agarose gels, indicating the integrity of RNA. Following extraction, 1 μg of RNA was reverse-transcribed from an oligo (dT) primer using a M-MLV reverse transcriptase (GIBCO-BRL, Gaithersberg, MD).
Competitive PCR for Beta-Actin
Competitive PCR for beta-actin was performed by using PCR MIMIC Protocol (Clontech Lab, Palo Alto, CA) according to manufacturer’s instructions. In this method, a competitor control fragment (PCR MIMIC) is used together with sample cDNA in the reaction mixture; sample and control cDNA are amplified with the same primers in the same reaction, but they are distinguished on gel electrophoresis by differences in length. By knowing the amount of PCR MIMIC added to the reaction, it is possible to determine the amount of target cDNA, and thus the initial mRNA levels. Each sample was subject to 25 cycles of amplification according to the manufacturer’s instructions. This method allowed us to use a constant number of molecules of mRNA for beta-actin for each of the following experiments.
PCR
Different amounts of cDNA for each sample (corresponding to the same amount of mRNA molecules for beta-actin) were amplified in a 10-μL volume of final reaction mix in an Idaho Technology (Idaho Falls, ID) thermal cycler with capillary glass with 0.25 U of Taq DNA polymerase (Perkin-Elmer-Cetus, Branchburg, NJ) and 10 pmol/L of primers specific for CD30 (sense, 5’-TGA CAA GGC TGT CAG GAG GTG CTG TTA CCG, region 333-362 and antisense, 3’-CCT CGT CAG TTT AGA AGC AGC TTC CTG GGC, region 852-823), CD30L (sense, 5’-CCC CTC AAA GGA GGA AAT TGC TCA GAA GAC, region 353-382, and antisense, 3’-ATT GAC TGA TAT GGT GGT GTT GAC CTG CAG, region 748-719), and beta-actin (sense, 5’-ATC TGG CAC CAC ACC TTC TAC AAT GAG CTG CG, region 294-324, and antisense, 3’-CGT CAT ACT CCT GCT TGC TGA TCC ACA TCT GC, region 1131-1100). Primers for CD30 and CD30L were selected using Oligo Primer Analysis Software Version 5.0 (National Bioscience, Plymouth, MN) and were purchased from Genset (Paris, France); primers for beta-actin were purchased from Clontech Laboratories. PCR conditions for CD30 and beta-actin were 30 seconds at 94°C, followed by 30 cycles of 10 seconds at 96°C, 20 seconds at 67°C, and 30 seconds at 72°C. PCR conditions for CD30L were 30 seconds at 94°C, followed by 30 cycles of 10 seconds at 96°C, 20 seconds at 66°C, and 30 seconds at 72°C. After the last cycle, samples were incubated for 50 seconds at 72°C to ensure completion of the final extension step. To monitor for carry-over contamination, a negative control (without template) was included in each PCR amplification. Following amplification, the PCR cocktail was sized in a 1.5% agarose gel at 100 V for 1 hour and counter-stained in a ethidium bromide solution (10 mg/mL). The gel was visualized under UV light and the size of any bands present was compared with molecular weight markers run in a parallel lane.
Analysis of Amplified DNA by Southern Blot for CD30L
An aliquot (5 or 10 μL) of the amplified DNA was fractionated on a 1.5% agarose gel and transferred to a nitrocellulose membrane filter as described by Southern.26 Blots were washed twice for 1 hour at 65°C in 1x SSPE (0.15 mol/L NaCL, 10 mmol/L NaH2PO4, l mmol/L EDTA) containing 0.1% sodium dodecyl sulfate. Southern blot analysis was performed with an internal probe designed to recognize intervening sequence between primers (sense, 5’-CCT ACC TCC AAG TGG CAA AGC ATC TAA ACA, region 426-455, and antisense, 3’-GTG TTT CGT TTG CAT TCC AGA CTC ACA CAC, region 683-653). This probe were obtained by PCR amplification. Primers were selected using Oligo Primer Analysis Software Version 5.0 and were purchased from Genset. The DNA fragment obtained by PCR was subcloned using the p-GEM-T Vector System (Promega, Madison, WI) according to the manufacturer’s instructions, and sequenced. Sequencing of the subcloned product was performed using the Sequenase version 2.0 DNA Sequencing kit (USB, Cleveland, OH). Southern blot was performed as previously described.26 The probe was labeled with (32P)dCTP using the Megaprime DNA labeling System (Amersham, Buckinghamshire, UK).
Image Analysis (quantitative analysis)
The intensity of bands obtained by Southern blot was measured by a CCD video camera (C3077/01; Hamamatsu Photonics, Hamamatsu, Japan) connected to a video frame-grabber M4476 (Hamamatsu Photonics), in a Macintosh computer Iisi (Apple Europe, Korch, Holland). Acquisition of the image was obtained with Imagequest IQB software by Hamamatsu Photonics. Image processing and analysis was performed with the free software IMAGE by Wayne Rasband, National Institutes of Health Research Services Branch, NIMH, version 1.28.
RESULTS
Immunohistochemistry
Frozen sections.
CD30+ cells, invariably of the T-helper cell phenotype (CD3+, CD4+), were found in all examined specimens in different proportions (Table 1). In particular, CD30+ cell numbers were greater than 75% in samples no. 2, 5, and 12 (CD30+ large-cell CTCL, growing lesions); 40% to 75% in samples no. 1 and 13 (LyP, growing lesion), and in 4, 6, and 7 (CD30+ large-cell CTCL, regressing lesions); and 15% to 40% in samples no. 3 and 8 (LyP, regressing lesion). CD30L+ cells were found in regressing lesions only (Table1). By step-section analysis, CD30L+ cells showed a distribution similar or identical to that of CD30+neoplastic cells.
No CD30+ and/or CD30L+ cells were found in clinically healthy subjects’ control skin samples (data not shown).
Occasional isolated, presumably reactive, CD19+CD22+ B cells were found in both growing and regressing lesions. In fact, neither CD30+ nor CD30L+ cells coexpressed B-cell markers.
Paraffin sections.
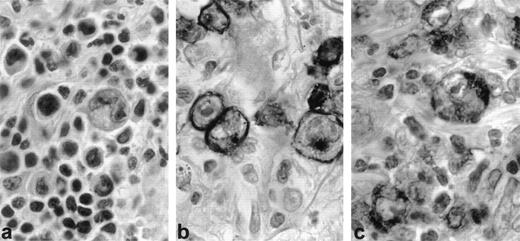
Three LyP cases with regressing lesions studied in paraffin sections showed 10% to 20% CD30+ cells (Fig1a and b). Most of the same large cells and some of the smaller cells, including mast cells, also stained for CD30L (Fig 1c).
CD30 and CD30 ligand (CD30L) expression in regressing lesion (LyP, patient no. 1). Immunohistochemistry on paraffin sections. (a) Typical histologic feature of LyP, type A: large, atypical blasts admixed with small, reactive lymphocytes (H&E, original magnification × 400). (b) CD30 staining is restricted to large neoplastic cells (anti-CD30, PAP method, original magnifica- tion × 400). (c) CD30L is mostly expressed by large neoplastic cells (anti-CD30L, PAP method, original magnification × 400).
CD30 and CD30 ligand (CD30L) expression in regressing lesion (LyP, patient no. 1). Immunohistochemistry on paraffin sections. (a) Typical histologic feature of LyP, type A: large, atypical blasts admixed with small, reactive lymphocytes (H&E, original magnification × 400). (b) CD30 staining is restricted to large neoplastic cells (anti-CD30, PAP method, original magnifica- tion × 400). (c) CD30L is mostly expressed by large neoplastic cells (anti-CD30L, PAP method, original magnification × 400).
Two-Color Immunofluorescence
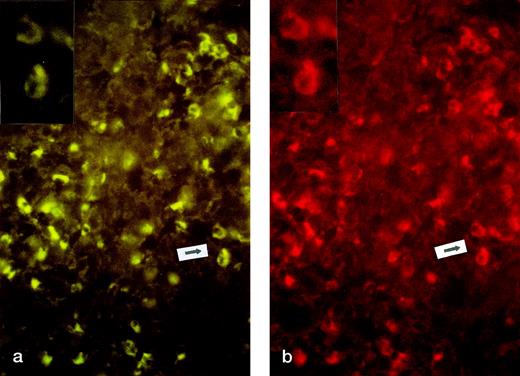
CD30L expression was not detected in the 3 biopsies from growing lesions. On the contrary, CD30L expression was found in all biopsies (5/5) from regressing lesions. The expression of CD30L was mostly restricted to cells expressing CD30 antigen (Fig2a and b). CD30+ cells coexpressed Fas and FasL in all examined specimens, independent of regression (data not shown).
CD30 and CD30L expression in regressing lesion (LyP, patient no. 4). Two-color immunofluorescence on frozen sections. (a) CD30 staining (FTIC, see Materials and Methods, original magnification × 200). Inset: particular of the area indicated by arrow. (b) CD30L staining (PE, see Materials and Methods, original magnification × 200). Inset: particular of the area indicated by arrow.
CD30 and CD30L expression in regressing lesion (LyP, patient no. 4). Two-color immunofluorescence on frozen sections. (a) CD30 staining (FTIC, see Materials and Methods, original magnification × 200). Inset: particular of the area indicated by arrow. (b) CD30L staining (PE, see Materials and Methods, original magnification × 200). Inset: particular of the area indicated by arrow.
RT-PCR and Southern Blot for CD30L
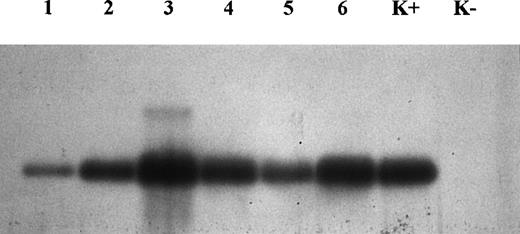
At partial variance from immunohistochemistry, molecular analysis demonstrated the presence of CD30L expression in all samples examined (Fig 3). Nevertheless, the image analysis of the intensity of bands obtained by Southern blot showed a different level of expression of CD30L, despite the same amount of target cDNA, relative to the same mRNA levels obtained for beta-actin (quantitative PCR). Our results indicate that the intensity of bands from regressing lesions is clearly higher than that from growing lesions (Fig4). In particular, this was evident in 2 samples (growing v regressing lesions) obtained from the same patient (Fig 3, lanes 1 and 3, respectively, growing and regressing lesion of the same patient).
Analysis of amplified DNA by Southern blot for CD30L. Note that lanes 3, 4, and 6 (regressing lesions) are thicker than lanes 1, 2, and 5 (growing lesions). Lanes 1 and 3 concern 2 different lesions (growing and regressing, respectively) from the same patient. K+, positive control; K−, negative control.
Analysis of amplified DNA by Southern blot for CD30L. Note that lanes 3, 4, and 6 (regressing lesions) are thicker than lanes 1, 2, and 5 (growing lesions). Lanes 1 and 3 concern 2 different lesions (growing and regressing, respectively) from the same patient. K+, positive control; K−, negative control.
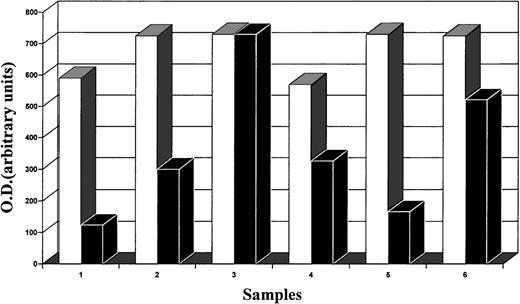
Image analysis (quantitative analysis) of the intensity of bands obtained by Southern blot analysis of DNA (CD30, □; CD30L, ▪). The intensity of CD30L bands from regressing lesions is clearly higher than that from growing lesions. In particular, this is evident in 2 samples (1 v 3, growing v regressing lesions) obtained from the same patient (see also Fig 3).
Image analysis (quantitative analysis) of the intensity of bands obtained by Southern blot analysis of DNA (CD30, □; CD30L, ▪). The intensity of CD30L bands from regressing lesions is clearly higher than that from growing lesions. In particular, this is evident in 2 samples (1 v 3, growing v regressing lesions) obtained from the same patient (see also Fig 3).
Summary of Results
In this study, we have analyzed the phenotypic and genotypic expression of CD30 and CD30L in this typical CTCL subset, in order to investigate CD30-CD30L interaction in different developmental phases of skin lesions (growing v spontaneously regressing). At the immunohistochemical level, CD30L expression was detected in regressing lesions only, whereas it was not found in growing ones. The 2-color immunofluorescence analysis of frozen tissue showed that CD30L was mostly coexpressed by CD30+ neoplastic cells. Colocalization of CD30L with CD30 was also demonstrated by immunohistochemistry on paraffin sections from an additional 3 LyP patients, in which some smaller lymphocytes also appeared to express CD30L. Molecular analysis by PCR and Southern blot demonstrated the presence of CD30L expression in all samples. Nonetheless, the intensity of bands shown by image analysis of Southern blots from regressing lesions was clearly higher than that from growing lesions. In particular, this was evident in 2 samples (growing v regressing lesion) obtained from the same patient. Although the significance of the above findings (higher expression of CD30L in regressing vgrowing lesions) could be hampered by the obvious consideration that small reactive cells are much fewer in growing lesions as compared with regressing ones, it has to be stressed CD30L staining was totally negative in growing lesions, irrespective of the presence of small cells. In fact, growing lesions from patients with LyP—notwithstanding they always contained small cells—did not stain for CD30L. In contrast, the expression of CD30L was mostly restricted to large CD30+ cells in regressing lesions, where a few small cells also stained for CD30L.
DISCUSSION
Recently, several studies have indicated that CD30L plays a key role as a paracrine- or autocrine-acting surface molecule involved in the pathobiology of Hodgkin’s lymphoma and NHL.15 It has been shown that the interaction of CD30L with its cognate receptor is able to induce either a proliferative or nonproliferative effect, depending on the cell type and/or on different intracellular signaling pathways.27-29 In particular, it has been demonstrated that recombinant CD30L exerts a potent antiproliferative effect on CD30+ ALC lymphoma cell lines.21 Primary cutaneous CD30+ lymphoma, characterized by frequent regression of skin lesions, seems a suitable in vivo model for the investigation of a possible pathophysiologic role of CD30/CD30L interaction. Indeed our results, showing CD30L at higher levels in regressing than nonregressing skin lesions, suggest that CD30L may be a key mediator of tumor regression in the spectrum of CD30+cutaneous lymphomas.
We found evidence of CD30L expression in both regressing and nonregressing skin lesions, but not in healthy skin. Moreover, semiquantitative studies using image analysis on Southern blot of PCR products showed a higher level of expression of CD30L in regressing lesions when compared with growing skin lesions. This observation was confirmed by examining both growing and regressing lesions from an individual patient.
CD30L may be necessary but insufficient to cause regression in advanced skin lesions of CD30+ cutaneous lymphomas in which activation of certain oncogenes or loss of tumor-suppressor genes may contribute to the progression of disease. For example, we found low levels of bcl-2 in regressing lesions of LyP, but high levels of bcl-2 in tumor cells of CD30+ cutaneous lymphomas.30We also found inactivation of receptors for tumor growth factor-beta, a potent growth inhibitor of normal lymphocytes, in the progression of LyP to CD30+ ALC lymphoma.31 These findings may explain the limited regression of CD30+cutaneous lymphomas that have detectable CD30L.
CD30 activation by CD30L has been shown to have pleiotropic effects in cell lines derived from Hodgkin’s lymphoma and ALC lymphoma.20 29 Since CD30L was shown to induce cytolytic cell death in CD30+ ALC lymphoma cell lines, and is expressed at higher levels in regressing than growing skin lesions, it is likely that CD30L inhibits the growth of CD30+ atypical cells and thereby mediates the regression of skin lesions in LyP and CD30+ cutaneous lymphomas. This hypothesis is now being tested by treatment of CD30+ cells isolated from patients with LyP and CD30+ cutaneous lymphomas.
In large part, CD30L appeared to be colocalized with CD30 antigen within the cytoplasm of large atypical cells. However, we have not been able to detect CD30L protein by immunohistochemistry and flow cytometry, nor CD30L transcripts by PCR amplification of cDNA from CD30+ ALC lymphoma cell lines derived from 2 patients with LyP who progressed to ALCL (M.E.K., unpublished observation, June 1998). It is possible that progression to lymphoma in these 2 cases was associated with loss of CD30L. Our paraffin sections studies (Fig 1c) and other studies suggest that T cells, macrophage/histiocytes, and granulocytes, often numerous in regressing skin lesions of LyP, can also express CD30L.20 21However, a major role of CD30L+ small reactive T cells in the induction of clinical regression in our series of CD30+ CTCL was not proven for 2 reasons: the expression of CD30L was mostly restricted to large CD30+ cells in regressing lesions, and fewer small cells stained for CD30L. In addition, growing lesions from patients with LyP—notwithstanding they contained small cells—did not stain for CD30L. These data suggest that small reactive lymphocytes are not the only or even the main source of CD30L. Of course, we cannot exclude that the lesions are regressing because of a combination of 2 mechanisms: coexpression of CD30 receptor and ligand by neoplastic cells, and CD30L signaling provided by small reactive lymphocytes.
In the hypothesis that an apoptotic cell death mechanism might underlie the clinical regression of skin lesions in CD30+ CTCL, we considered the possible role played by the interaction between FasL and its receptor Fas/APO-1 (CD95). However, we did not find any differences in Fas/FasL coexpression between regressing and nonregressing skin lesions from CD30+ CTCL patients (invariably detected). This finding is consistent with the observation of uniform expression of Fas in regressing and nonregressing lesions of LyP and CD30+ cutaneous ALC lymphomas.30 Moreover, it has recently been reported that Fas/FasL coexpression by neoplastic cells is not per se sufficient to activate the apoptotic cell death mechanism.32,33 Therefore, the lack of difference in Fas/FasL coexpression between regressing and nonregressing lesions in CD30+ CTCL does not exclude apoptosis as the possible crucial mechanism for clinical regression. Indeed, CD30L expression by both neoplastic and reactive cells, possibly enhancing the tumor cell sensitivity to FasL-mediated cell death, might represent the triggering mechanism for apoptosis, which is frequent in regressing lesions.34 Interestingly, the putative CD4+cytotoxic nature of neoplastic CD30+ cells in CD30+ CTCL, recently suggested by some groups35-37 on the basis of the expression of specific cytotoxic markers, ie, granzyme B, perforin, and T-cell–restricted intracellular antigen (TIA-1), represents a further element to support apoptosis as the possible mechanism of clinical regression. In fact, it has been reported that cytotoxic proteins (granzyme B) are required for the induction of apoptosis.38 Even in this regard, a triggering factor seems necessary: granzyme B is invariably detected in CD30+ CTCL lesions,37 apparently irrespective of the type of lesion (regressing or not) investigated.
In conclusion, the data presented here suggest that CD30-CD30L interactions may play a role in the pathobiology of primary cutaneous CD30+ lymphoproliferative disorders. In particular, CD30L overexpression might have a major role in the mechanism of self-regression of skin lesions, the most distinctive clinical feature of this cutaneous lymphoma subtype.
Supported by grants from the bank Cassa di Risparmio dl Firenze S.p.A., Florence, Italy (Skin Tumor Project), from the Italian Ministry of University and Scientific and Technologic Research (University funds, 60%), from the Associazione Italiana per la Ricerca sul Cancro (AIRC), and from the Italian Ministry of Health (MPI 40% Oncology).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to N. Pimpinelli, MD, PhD, Institute of Dermatology and Venereology, University of Florence Medical School, Via degli Alfani 37, I-50121 Firenze, Italy; e-mail:pimpi@cesit1.unifi.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal