Proteinase 3 (PR3), which is also called myeloblastin, the target autoantigen for antineutrophil cytoplasmic antibodies (ANCA) in Wegener’s granulomatosis, is a serine proteinase stored in azurophil granules of human neutrophils. We have previously shown that, in contrast to elastase or myeloperoxidase, PR3 is also expressed at the plasma membrane of a subset of unactivated neutrophils and that a high proportion of neutrophils expressing membrane PR3 is a risk factor for vasculitis. The present study demonstrates that the association of PR3 with the plasma membrane is not an ionic interaction and seems to be covalent. Fractionation of neutrophils shows that, besides the azurophil granules, PR3 could be detected both in specific granules and in the plasma membrane-enriched fraction containing secretory vesicles, whereas elastase and myeloperoxidase were exclusively located in azurophil granules. Electron microscopy confirms that PR3 is present along with CR1 in secretory vesicles as well as in some specific granules. In neutrophils stimulated with an increasing dose of FMLP, membrane PR3 expression increased with the degranulation of secretory vesicles, followed by specific granules, and culminated after azurophil granules mobilization. The presence of a readily plasma membrane-mobilizable pool of PR3 contained in the secretory vesicles might play a relevant role in the pathophysiological mechanisms of ANCA-associated vasculitis.
PROTEINASE 3 (PR3), which is also called myeloblastin,1,2 belongs to the family of neutrophil-derived serine proteases together with human neutrophil elastase (HNE), cathepsin G, and the enzymatically inactive azurocidin, which are all contained in azurophil granules.3-5 Like HNE, PR3 has the ability to degrade extracellular matrix proteins6,7 and to induce emphysema when injected into hamsters.8 The demonstration of specific biological activities, such as potentiation of platelet aggregation,9processing of cytokines (eg, the tumor necrosis factor-α [TNF-α] precursor10 and interleukin-8 [IL-8]11), and induction of IL-8 synthesis by endothelial cells,12 has pointed to a special role for PR3 in the inflammatory process.
PR3 differs from other neutrophil serine proteinases by 2 biological features. First, PR3 is an important factor in myeloid differentiation.2,13 Second, PR3 is the main target autoantigen in Wegener’s granulomatosis, a systemic necrotizing granulomatous vasculitis characterized by high titers of antineutrophil cytoplasmic antibodies (ANCA).14-16 ANCA have been shown to trigger neutrophil superoxide production and degranulation.17 The current hypothesis as to how ANCA gain access to the intracellular autoantigen is based on the concept that priming agents such as TNF-α, IL-8, or lipopolysaccharide (LPS) induce the translocation of PR3 from the azurophil granules to the plasma membrane.18 However, this hypothesis does not take into account our recent observation that PR3 is expressed at the membrane of a subset of isolated neutrophils in the absence of stimulating agents.19 Flow cytometry analysis using different conformational monoclonal antibodies (MoAbs) or IgG from Wegener’s patients has allowed us to clearly define 2 subsets of neutrophils in a given individual according to the presence (membrane PR3-positive neutrophils [mPR3+]) or the absence (membrane PR3-negative neutrophils [mPR3−]) of PR3 surface labeling. The proportion of mPR3+ neutrophils ranges from 0% to 95% of the total neutrophil pool and is strikingly stable for a given individual, even after in vitro neutrophil activation. Family studies have strongly suggested that the mPR3 phenotype is genetically controlled in the normal population, independently of neutrophil activation state,20 and is not related to apoptosis (our personal data). Most importantly, we have recently demonstrated that a high proportion of mPR3+ neutrophils is a risk factor for vasculitis and rheumatoid arthritis, thus pointing out that PR3 availability at the neutrophil plasma membrane is clinically relevant.20
Neutrophils are equipped with a wide variety of cytoplasmic granules that have been individualized according to their biogenesis occurring at different steps of myeloid differentiation and according to their protein content.3,4,5,21 PR3 is localized in azurophilic granules,1,6,7,22 the peroxidase-positive granules characterized by the presence of myeloperoxidase (MPO), which are acquired at the myeloblast/promyelocyte stage. Specific granules are formed at the myelocyte/metamyelocyte stage and contain lactoferrin and other functional membrane proteins such as adhesion molecules (CD11b/CD18), receptors for chemoattractants (FMLP receptor) or cytochrome b558.5 Tertiary granules are characterized by their high gelatinase content,23 and secretory vesicles are characterized by the presence of membrane latent alkaline phosphatase and by their albumin content.24 These latter vesicles are described as the intracellular reservoir of complement receptor 1 (CR1 or CD35)25 and are most easily exocytosed. Sequential degranulation experiments have clearly demonstrated a strict control of exocytosis in neutrophils submitted to increasing intracellular calcium concentrations or increasing doses of FMLP, starting with secretory vesicles and followed sequentially by gelatinase, specific, and azurophil granules, which are the most difficult to mobilize.26
We show here (1) that PR3 association with the plasma membrane appears to be covalent; (2) that, in addition to its main localization in the azurophil granules, PR3 is also localized in the specific granules, in the plasma membrane, and in secretory vesicles because it colocalizes with CR1; and (3) that membrane PR3 expression can be increased with the sole mobilization of secretory vesicles in response to nanomolar concentrations of FMLP.
MATERIALS AND METHODS
Human neutrophil isolation and in vitro activation.
Human neutrophils were isolated from EDTA-anticoagulated blood from healthy donors by centrifugation on Polymorphprep (Nycomed, Oslo, Norway), and contaminating erythrocytes were lyzed as previously described.19 Cells were washed in Hank’s balanced salt solution (HBSS) without Ca2+/Mg2+ (GIBCO/BRL, Gaithersburg, MD) and resuspended in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and 0.1% sodium azide for immediate incubation with antibodies for flow cytometry at 4°C. For in vitro cell activation, neutrophils in HBSS with Ca2+/Mg2+ (106/mL; GIBCO/BRL) were incubated in polypropylene tubes in the presence of the indicated concentration of FMLP (Sigma Chemical Co, St Louis, MO) for 15 minutes. When indicated, neutrophils were preincubated with 5 μg/mL cytochalasin B (Sigma) for 5 minutes. Neutrophils were then centrifuged and resuspended in PBS/BSA/azide either for flow cytometry analysis or measurement of PR3, HNE, or MPO release in the supernatant using specific sandwich enzyme-linked immunosorbent assay (ELISA) as previously described.27 28 When released PR3, HNE, and MPO were measured in the supernatants, protease inhibitors (1 mg/mL soybean trypsin inhibitor, 200 μg/mL eglin C) and radical scavengers (100 μmol/L methionine) were added to incubation medium.
Immunofluorescence flow cytometry.
Antibodies used for flow cytometry were: murine MoAb anti-PR3 CLB 12.8 (CLB, Amsterdam, The Netherlands), which was used to measure PR3 surface labeling and to define the mPR3+subset19 20; anti-CD16, anti-CD35, anti-CD11b, antiCD66, anti-CD63, control mouse Ig IgG1, and fluorescein isothiocyanate (FITC)-conjugated F(ab′)2 fragment of goat antimouse IgG or antirabbit were from Immunotech (Marseille, France); and anti-CD43 was from Becton Dickinson Immunocytometry Systems (Mountain View, CA).
Isolated neutrophils (106 cells/mL in PBS/BSA/azide) were first incubated for 30 minutes at 4°C with 10 mg/mL heat-aggregated goat IgGs (Sigma) to block Fcγ receptors. Cells were then treated with dilutions of MoAbs for 30 minutes at 4°C, followed by 2 washes in PBS/BSA/azide and incubation with FITC-conjugated F(ab′)2 fragments of goat antimouse IgG for 30 minutes at 4°C. For double-labeling experiments, neutrophils were first incubated with biotinylated MoAb anti-PR3 CLB 12.8 (NHS-LCbiotin; Pierce, Rockford, IL) and the other FITC-conjugated MoAb. After 2 washes, neutrophils were then incubated for 30 minutes with phycoerythrin (PE)-coupled streptavidin to show PR3 labeling. Neutrophils were fixed with 1% formaldehyde and analyzed for fluorescence on a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems) with a light scatter gate set.
Biochemical analysis of PR3 association with the plasma membrane on isolated neutrophils.
Acid, basic, and neuraminidase treatments to release membrane PR3 by modification of charge interactions were performed before antigen labeling. Isolated neutrophils (106 cells/mL) were treated either by acidic pH (incubation with 50 mmol/L glycine, 150 mmol/L NaCl, pH 3, for 7 minutes at 4°C), by basic pH (incubation with 100 μmol/L protamine in PBS, pH 10.7, for 10 minutes at 25°C), or by neuraminidase to cleave surface sialic acid residues: incubation for 1 hour at 4°C with 50 mU/mL neuraminidase from Vibrio cholerae (Boehringer Mannheim, Indianapolis, IN) and 250 mU/mL neuraminidase from Clostridium perfringens (Sigma), then 2 washes in PBS/BSA/azide. For surface glysosyl phosphatidyl inositol (GPI)-linked molecules cleavage, isolated neutrophils (106cells/mL) were treated with phosphatidylinositol-specific phospholipase C (PIPLC; Sigma) at 5 U/mL at 37°C for 30 minutes and then washed in PBS/BSA/azide. After treatment, neutrophils were labeled with anti-PR3 CLB 12.8, as described above, and analyzed by flow cytometry.
Neutrophil subcellular fractionation.
Human neutrophils were isolated on a ficoll gradient after dextran sedimentation of erythrocytes and fractionated.29 Briefly, neutrophils resuspended at 3 × 107 cells/mL in the relaxation buffer (100 mmol/L KCl, 3 mmol/L NaCl, 1 mmol/L Na2ATP, 3.5 mmol/L MgCl2, 0.5 mmol/L, 10 mmol/L PIPES, pH 7.2) containing antiproteinases (0.5 μmol/L aprotinin [Sigma], 1 mmol/L pefabloc [Boehringer Mannheim], 1 mmol/L chymostatin [Sigma], and 1 μmol/L leupeptin [Boehringer Mannheim]) were disrupted by nitrogen cavitation. Nuclei and unbroken cells were sedimented by centrifugation at 400g for 15 minutes and 10 mL of the postnuclear supernatant was loaded on top of a 28-mL 2-layer percoll density gradient (1.05/1.12 g/mL) and centrifuged at 37,000 for 30 minutes. Four subcellular fractions were then clearly identified, eg, the bottom band (α-band) containing azurophil granules, the intermediate band (β-band) containing specific and gelatinase granules, the top band (γ-band) containing plasma membrane and secretory vesicles, and the supernatant containing cytosol. Each band was aspirated, resuspended in PBS, and ultracentrifuged at 100,000g for 2 hours. Each fraction was solubilized in 1% triton to measure PR3, HNE, and MPO by specific sandwich ELISA as previously described.27,28 To determine the molecular mass of plasma membrane PR3, an aliquot of the membrane fraction was subjected to Western blot analysis in comparison with azurophil granules. After 2 washes in 3 mol/L NaCl, 50 mmol/L Tris, pH 7.8, to remove proteins bound through charge interaction, followed by a single wash in 50 mmol/L TRIS, pH 7.8, the membrane fraction was boiled in reducing Laemmli sample buffer. Samples were run on a 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane, and PR3 was detected by Western blot using a polyclonal rabbit anti-PR3 (gift of J. Gabay, Columbia University, New York, NY), as previously described,30followed by the detection with a secondary antibody F(ab′)2 of goat IgG antirabbit IgG conjugated with horseradish peroxidase (HRP) using the ECL detection kit (Amersham Corp, Arlington Heights, IL).
Electron microscopy.
Neutrophils from 2 individuals having 80% of neutrophils labeled for membrane PR3 as evaluated by flow cytometry were fixed in 1% glutaraldehyde in 0.1 mol/L phosphate buffer, pH 7.4, and pelleted in 10% gelatin in PBS. Cryosections were made on an ultracryomicrotome (Reichert Ultracut S.), and ultrathin sections mounted on Formvar-coated gold grids were prepared. During incubations at room temperature, the grids were floated on the surface of droplets as previously described.31 Briefly, the sections were incubated for 15 minutes with PBS and 15% glycine; for 5 minutes with PBS, 15% glycine, and 0.1% BSA; and for 20 minutes with PBS, 15% glycine, 0.1% BSA, and 10% normal goat serum followed by 1 hour of incubation with the primary antibody (or the mixture of the mouse monoclonal anti-PR3 with 1 rabbit polyclonal antibody for double labeling). The mouse monoclonal anti-PR3 CLB 12.8 diluted at 1/200, the rabbit polyclonal anti-CR132 diluted at 1/50, the rabbit polyclonal anti-MPO (Dako, Glostrup, Denmark) diluted at 1:2,000, and the rabbit polyclonal anti-lactoferrin (Cappel Laboratory, Downington, PA) diluted at 1/100 in PBS, 15% glycine, 0.1% BSA, 4% normal goat serum were incubated for 1 hour. After extensive rinsing in PBS, 15% glycine, and 0.1% BSA, sections were incubated for 30 minutes with gold-labeled secondary goat antimouse or goat antirabbit antibody, or both in a case of double-labeling, with a gold particle size of 10 nm (GAM 10) or 5 nm (GAR5), respectively (British BioCell, Cardiff, Wales). Sections were then washed for 30 minutes with PBS, 15% glycine, stained with 2% uranyl acetate for 10 minutes, and air-dried. Examination was performed in a Philips CM 10 electron microscope. Each labeling was performed in triplicate on 3 different grids and at least 10 neutrophils per grid were observed.
RESULTS
Plasma membrane-associated PR3 on isolated resting neutrophils is not bound through charge interactions.
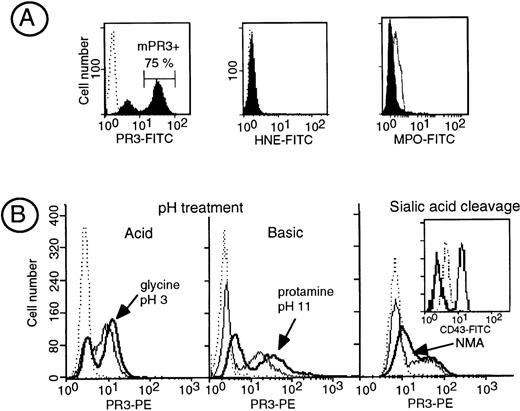
In contrast to HNE and MPO, PR3 is expressed at the plasma membrane of a subset of resting neutrophils called the mPR3+ subset (Fig 1A). Although we could not detect free soluble PR3 into the HBSS buffer used upon neutrophil isolation (data not shown), we assessed whether PR3, which is a cationic protein, could originate from intracellular stores and be subsequently bound to the cell membrane through ionic interactions. We thus studied its possible elution from the membrane by drastic pH changes. We first submitted neutrophils to either acid (50 mmol/L glycine, pH 3) or basic (100 μmol/L protamine, pH 10.7) pH. Neither acid nor basic treatment modified anti-PR3 membrane binding in the mPR3+ neutrophils subset (Fig 1B). We then studied the effect of modifying the overall negative charge of neutrophils surface by neuraminidase treatment to remove membrane sialic acid residues. No modification in the level of surface PR3 labeling was obtained after this treatment, except for a slight increase in PR3 fluorescence likely due to increased access of the antibodies to the cell surface after the removal of sialic acid. The efficiency of neuraminidase treatment was ascertained by the disappearance of anti-CD43 labeling using a MoAb that recognizes a sialic acid-dependent epitope. Thus, the interaction between PR3 and the plasma membrane is not ionic and does not seem to result from the binding of soluble PR3 to the negatively charged plasma membrane. Treatment of neutrophils with PIPLC triggered a significant increase in the mean fluorescent intensity of PR3 surface labeling (+39.1% ± 3.5%), whereas that of CD16, a known GPI-linked protein, was decreased by 48% ± 4.5%, thus demonstrating that PR3 anchorage was unlikely to be via a GPI link (data not shown).
Biochemical characterization of the interaction of PR3 with the plasma membrane. (A) Flow cytometry analysis of membrane expression of PR3, HNE, and MPO in isolated resting neutrophils. Neutrophils were stained with the MoAb anti-PR3 CLB 12.8, 2 HNE MoAbs, or a polyclonal anti-MPO shown by FITC-conjugated antibodies to visualize PR3, HNE, or MPO surface labeling, respectively. In this representative experiment performed in a given individual, flow cytometry analysis shows that 75% of the neutrophils are labeled with the MoAb anti-PR3 (mPR3+), whereas no surface labeling was observed for HNE and MPO under the same conditions. (B) Effect of modification of membrane charge on membrane PR3 expression: isolated neutrophils having a mPR3+ subset of 70% and 30% were treated either with acid pH (50 mmol/L glycine, 150 mmol/L Tris, pH 3) or with basic pH (100 μmol/L protamine, pH 10.7), respectively. The treatment resulted in an increase in PR3 surface labeling (bold line) as compared with untreated neutrophils (plain line) with reference to control IgG1 (dotted line). On the right, isolated neutrophils from an individual having a 25% mPR3+ subset (control IgG1 in dotted line) were treated with neuraminidase, resulting in an increase in PR3 surface labeling (bold line) as compared with untreated neutrophils (plain line); the insert shows the positive control for neuraminidase activity on CD43 expression shown by an MoAb whose epitope is sialic acid dependent.
Biochemical characterization of the interaction of PR3 with the plasma membrane. (A) Flow cytometry analysis of membrane expression of PR3, HNE, and MPO in isolated resting neutrophils. Neutrophils were stained with the MoAb anti-PR3 CLB 12.8, 2 HNE MoAbs, or a polyclonal anti-MPO shown by FITC-conjugated antibodies to visualize PR3, HNE, or MPO surface labeling, respectively. In this representative experiment performed in a given individual, flow cytometry analysis shows that 75% of the neutrophils are labeled with the MoAb anti-PR3 (mPR3+), whereas no surface labeling was observed for HNE and MPO under the same conditions. (B) Effect of modification of membrane charge on membrane PR3 expression: isolated neutrophils having a mPR3+ subset of 70% and 30% were treated either with acid pH (50 mmol/L glycine, 150 mmol/L Tris, pH 3) or with basic pH (100 μmol/L protamine, pH 10.7), respectively. The treatment resulted in an increase in PR3 surface labeling (bold line) as compared with untreated neutrophils (plain line) with reference to control IgG1 (dotted line). On the right, isolated neutrophils from an individual having a 25% mPR3+ subset (control IgG1 in dotted line) were treated with neuraminidase, resulting in an increase in PR3 surface labeling (bold line) as compared with untreated neutrophils (plain line); the insert shows the positive control for neuraminidase activity on CD43 expression shown by an MoAb whose epitope is sialic acid dependent.
Determination of subcellular localization of PR3 after neutrophil fractionation.
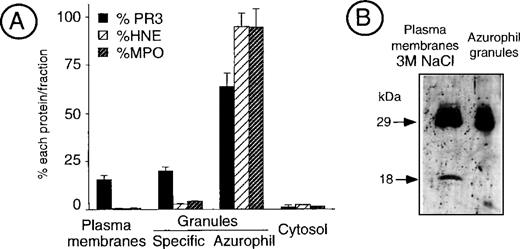
Measurement of PR3, HNE, and MPO in the different subcellular fractions of cavitated neutrophils showed that, beside its main localization in azurophil granules, significant amounts of PR3 were present within the plasma membrane-enriched fraction as well as within specific granules. In contrast, both HNE and MPO were almost exclusively located in azurophil granules (Fig 2A).
Analysis of PR3 subcellular localization by neutrophil fractionation. (A) Measurement of PR3, HNE, and MPO in fractionated neutrophils. Resting neutrophils from an individual having an 80% mPR3+ subset were fractionated into the plasma membrane-enriched fraction that contains secretory vesicles, the specific granules, the azurophil granules, and the cytosol. Double sandwich ELISA were used to specifically quantify PR3, HNE, and MPO in an aliquot of each fraction equivalent to 50 × 106neutrophils. The histogram depicts the percentage of each protein in the different fractions. Data are the mean ± SEM of 4 determinations obtained in a representative fractionation experiment. (B) Western blot analysis of the membrane-enriched fraction as compared with azurophil granules. The neutrophil membrane-enriched fraction (100 × 106 neutrophils) washed with high salt concentration buffer (50 mmol/L Tris, 3 mol/L NaCl) before analysis was compared with purified azurophil granules (10 × 106 neutrophils).
Analysis of PR3 subcellular localization by neutrophil fractionation. (A) Measurement of PR3, HNE, and MPO in fractionated neutrophils. Resting neutrophils from an individual having an 80% mPR3+ subset were fractionated into the plasma membrane-enriched fraction that contains secretory vesicles, the specific granules, the azurophil granules, and the cytosol. Double sandwich ELISA were used to specifically quantify PR3, HNE, and MPO in an aliquot of each fraction equivalent to 50 × 106neutrophils. The histogram depicts the percentage of each protein in the different fractions. Data are the mean ± SEM of 4 determinations obtained in a representative fractionation experiment. (B) Western blot analysis of the membrane-enriched fraction as compared with azurophil granules. The neutrophil membrane-enriched fraction (100 × 106 neutrophils) washed with high salt concentration buffer (50 mmol/L Tris, 3 mol/L NaCl) before analysis was compared with purified azurophil granules (10 × 106 neutrophils).
To characterize the molecular mass of PR3 detected at the surface of unstimulated neutrophils, the plasma membrane-enriched fraction was extensively washed with high salt concentration buffers (3 mol/L NaCl, 50 mmol/L Tris, pH 7.8) to remove proteins bound via ionic interactions and analyzed by Western blot. It showed that membrane PR3 appears as a triplet, with the prominent band being at 29 kD, similar to the azurophil granule enzyme (Fig 2B). In addition, a further band at 18 kD was found in all of our membrane preparations. This may be due to proteolysis during membrane preparations, despite the addition of proteinase inhibitors.
Analysis of subcellular localization of PR3 by immunoelectron microscopy.
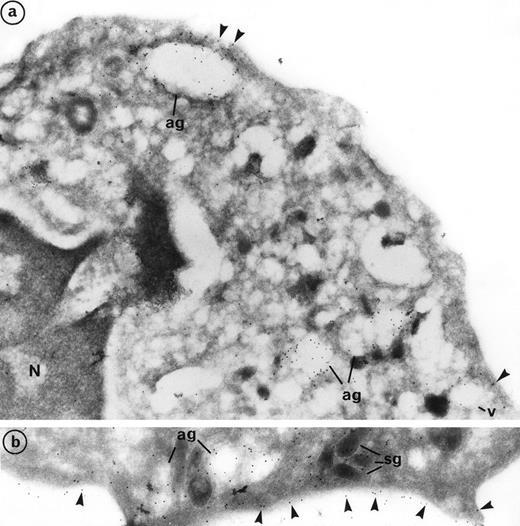
Labeling of PR3 with the mouse monoclonal CLB 12.8 showed immunogold grains on the plasma membrane as well as within intracellular granular compartments. PR3 labeling was also observed at the periphery of large extracted granules, the azurophil granules (ag), and empty vesicles (v) (Fig 3a). Plasma membrane labeling of PR3 was homogeneous on the cell surface and was not restricted to certain areas (Fig 3b). However, the intensity of this labeling varies from one neutrophil to another, thus confirming the heterogeneity of PR3 membrane labeling measured by flow cytometry using the same antibody CLB12.8. In the donor that we present in Fig 3, 80% of neutrophils were positive for PR3 membrane labeling and 20% were negative. However, all neutrophils showed azurophil granule labeling. Figure 3a shows a neutrophil with weak PR3 membrane labeling, although azurophil granule labeling was present. Figure 3b shows a neutrophil with intense PR3 membrane labeling.
Subcellular localization of PR3 by electron microscopy on neutrophils thin frozen sections labeled with anti-PR3. Electron micrograph of resting neutrophils from the same individual stained for localization of PR3 with the MoAb anti-PR3 CLB 12.8 followed by incubation with 10-nm gold particles-conjugated goat antimouse (GAM 10). (a) Gold-labeled antibody is present at the periphery of large extracted granules identified as the azurophil granules (ag) as well as in the membrane of intracellular empty vesicles (v). Plasma membrane labeling is shown by arrow heads (original magnification × 35,200). (b) The immunogold label (arrowheads) indicates the presence of PR3 on the plasma membrane in a random distribution head (original magnification × 42,550).
Subcellular localization of PR3 by electron microscopy on neutrophils thin frozen sections labeled with anti-PR3. Electron micrograph of resting neutrophils from the same individual stained for localization of PR3 with the MoAb anti-PR3 CLB 12.8 followed by incubation with 10-nm gold particles-conjugated goat antimouse (GAM 10). (a) Gold-labeled antibody is present at the periphery of large extracted granules identified as the azurophil granules (ag) as well as in the membrane of intracellular empty vesicles (v). Plasma membrane labeling is shown by arrow heads (original magnification × 35,200). (b) The immunogold label (arrowheads) indicates the presence of PR3 on the plasma membrane in a random distribution head (original magnification × 42,550).
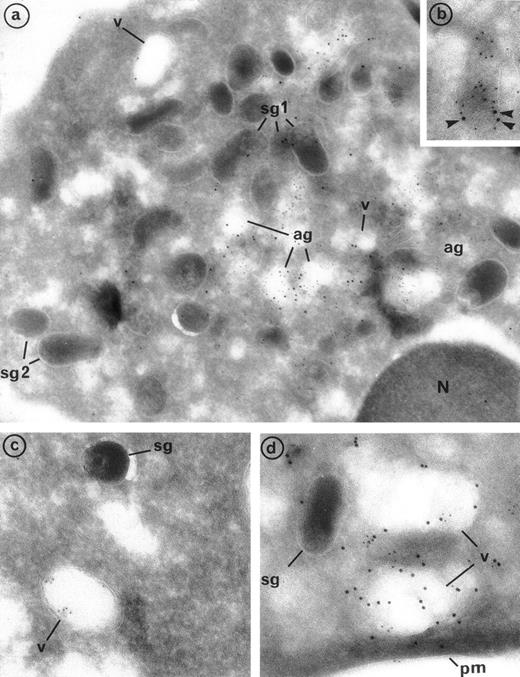
Double labeling of PR3 with MPO confirmed that PR3 is located with MPO in the azurophil granules (ag) that represent the major intracellular store of PR3 (Fig 4a). Azurophil granules appeared as large empty extracted granules with strong intragranular MPO labeling. Previous electron microscopy studies have pointed out the particular trend of azurophil granules to be extracted, which made them look lighter than the other granules.33 Azurophil granules were abundant and in clusters within neutrophil cytoplasm. PR3 labeling was mainly restricted to the periphery of granules. In contrast to MPO, PR3 labeling also appears in the membrane of small empty vesicles (v). PR3 labeling can be detected in the limiting membrane of some dense and small granules, with an elongated form characteristic of specific granules (sg) (Figs 3b and 4a, b, and d). This labeling was weak but appeared to be significantly higher than background labeling on mitochondria taken as control. Double-labeling PR3 with lactoferrin confirmed that PR3 is present in the membrane of lactoferrin-containing granules (Fig 4b).
Evidence of the colocalization of PR3 with MPO in azurophil granules, PR3 with lactoferrin in specific granules, and PR3 with CR1 in secretory vesicles using double immunolabeling electron microscopy. (a) Double immunolabeling with the MoAb anti-PR3 CLB 12.8 coupled with a 10-nm gold particles-conjugated goat antimouse (GAM 10) and the polyclonal anti-MPO coupled with a 5-nm gold particles-conjugated goat antirabbit (GAR 5). The presence of 5-nm gold grains in large empty granules indicates that MPO is localized exclusively in azurophil granules. The colocalization of gold grains of both sizes within these granules indicates that PR3 is located with MPO in these granules. However, in contrast to MPO, PR3 is also localized at the periphery of empty vesicles (v) and in some specific granules (sg1) (original magnification × 54,250). (b) Double immunolabeling with the MoAb anti-PR3 CLB 12.8 coupled with a 10-nm gold particles-conjugated goat antimouse (GAM 10) and the polyclonal antilactoferrin coupled with a 5-nm gold particles-conjugated goat antirabbit (GAR 5) showing the presence of PR3 (arrowheads) along the limiting membrane of an elongated specific granule identified as such thanks to its prominent lactoferrin content (original magnification × 86,800). (c) Visualization of secretory vesicles with CR1 labeling. Immunolabeling of CR1 was performed with the polyclonal anti-CR1 coupled with GAR 5 and shows the localization of CR1 in the membrane of the secretory vesicles, which appear as empty organelles, but not in specific granules (original magnification × 98,700). (d) Double immunolabeling with the MoAb anti-PR3 CLB 12.8 coupled with GAM 10 and the polyclonal anti-CR1 coupled with GAR 5. Both sizes of grains are detected in the membrane of secretory vesicles identified by the presence of CR1 (original magnification × 98,700).
Evidence of the colocalization of PR3 with MPO in azurophil granules, PR3 with lactoferrin in specific granules, and PR3 with CR1 in secretory vesicles using double immunolabeling electron microscopy. (a) Double immunolabeling with the MoAb anti-PR3 CLB 12.8 coupled with a 10-nm gold particles-conjugated goat antimouse (GAM 10) and the polyclonal anti-MPO coupled with a 5-nm gold particles-conjugated goat antirabbit (GAR 5). The presence of 5-nm gold grains in large empty granules indicates that MPO is localized exclusively in azurophil granules. The colocalization of gold grains of both sizes within these granules indicates that PR3 is located with MPO in these granules. However, in contrast to MPO, PR3 is also localized at the periphery of empty vesicles (v) and in some specific granules (sg1) (original magnification × 54,250). (b) Double immunolabeling with the MoAb anti-PR3 CLB 12.8 coupled with a 10-nm gold particles-conjugated goat antimouse (GAM 10) and the polyclonal antilactoferrin coupled with a 5-nm gold particles-conjugated goat antirabbit (GAR 5) showing the presence of PR3 (arrowheads) along the limiting membrane of an elongated specific granule identified as such thanks to its prominent lactoferrin content (original magnification × 86,800). (c) Visualization of secretory vesicles with CR1 labeling. Immunolabeling of CR1 was performed with the polyclonal anti-CR1 coupled with GAR 5 and shows the localization of CR1 in the membrane of the secretory vesicles, which appear as empty organelles, but not in specific granules (original magnification × 98,700). (d) Double immunolabeling with the MoAb anti-PR3 CLB 12.8 coupled with GAM 10 and the polyclonal anti-CR1 coupled with GAR 5. Both sizes of grains are detected in the membrane of secretory vesicles identified by the presence of CR1 (original magnification × 98,700).
CR1 labeling was used to identify the compartment of secretory vesicles. These appear as empty vesicles with heterogeneous size. CR1 labeling was localized within the membrane of secretory vesicles (Fig4c). We also observed CR1 labeling on the plasma membrane of resting neutrophils. We found that approximately one third of the immunogold grains were on the plasma membrane and that two thirds of CR1 labeling were within the membrane of these vesicles. The double-labeling PR3 and CR1 experiments clearly demonstrated localization of PR3 and CR1 in the membrane of secretory vesicles. The intensity of PR3 and CR1 labeling was similar (Fig 4d). Furthermore, in contrast to PR3, no CR1 was found in specific granules (Fig 4c and d).
In conclusion, electron microscopy study labeling confirmed the localization of PR3 both in the plasma membrane and in the membrane of granules distinct from the azurophil granules, eg, the secretory vesicles, and some specific granules.
Membrane PR3 expression increases after exocytosis of secretory vesicles.
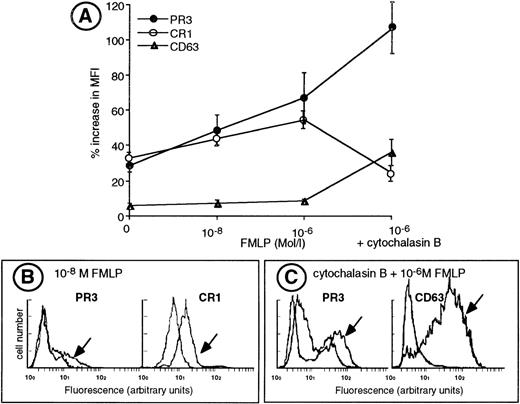
We performed sequential degranulation of isolated neutrophils using increasing doses of FMLP from 10−8 mol/L to 10−6 mol/L.25 26 As shown in Fig 5, 10−8 mol/L FMLP triggered exocytosis of secretory vesicles as ascertained by an increased CR1 expression concomittant with an increased membrane PR3 expression. Double-labeling experiments demonstrated that the heterogeneity of PR3 surface labeling was not related to a difference in the ability of neutrophils to mobilize their secretory vesicles, because CR1 labeling was homogeneous and thus similar in both the mPR3+ and the mPR3− neutrophil subsets (Fig 5B). Further stimulation of isolated neutrophils with 10−6 mol/L FMLP led to the mobilization of specific granules and to a further increase in membrane PR3 expression in the mPR3+ subset. Likewise, membrane PR3 upregulation (+134% ± 15%) paralleled that of CD11b (+152% ± 16%), which is localized both in the membrane of secretory vesicles and in specific granules and that of CD66 (+122% ± 17%) localized in the membrane of specific granules.
Upregulation of membrane PR3 expression during sequential degranulation of isolated neutrophils. (A) Isolated neutrophils were adjusted to a concentration of 106 cells/mL and labeled for flow cytometry analysis with the specific MoAb before (HBSS) or after activation with various concentrations of FMLP in the absence or in the presence of cytochalasin B. Membrane expression of CR1 and CD63 are expressed as the mean fluorescence index (MFI). For PR3 membrane expression, results are expressed as the MFI of the mPR3+subset. Results are given as the percentage increase in MFI defined as (MFI MoAb − MFI control IgG1) ± SEM from 6 independent experiments. (B) Representative experiment of double-labeling PR3 and CR1, a marker of secretory vesicle mobilization, in resting neutrophils and in neutrophils stimulated with 10−8 mol/L FMLP (indicated with an arrow) from an individual having a 28% mPR3+subset. Labeling with the MoAb anti-CR1 shows an homogeneous population. (C) Representative experiment of double-labeling PR3 and CD63, a marker of azurophil degranulation in resting neutrophils and in neutrophils stimulated with 10−6 mol/L FMLP in the presence of cytochalasin B (indicated with an arrow), from an individual having a 43% mPR3+ subset. Labeling with the MoAb anti-CD63 shows a homogeneous population.
Upregulation of membrane PR3 expression during sequential degranulation of isolated neutrophils. (A) Isolated neutrophils were adjusted to a concentration of 106 cells/mL and labeled for flow cytometry analysis with the specific MoAb before (HBSS) or after activation with various concentrations of FMLP in the absence or in the presence of cytochalasin B. Membrane expression of CR1 and CD63 are expressed as the mean fluorescence index (MFI). For PR3 membrane expression, results are expressed as the MFI of the mPR3+subset. Results are given as the percentage increase in MFI defined as (MFI MoAb − MFI control IgG1) ± SEM from 6 independent experiments. (B) Representative experiment of double-labeling PR3 and CR1, a marker of secretory vesicle mobilization, in resting neutrophils and in neutrophils stimulated with 10−8 mol/L FMLP (indicated with an arrow) from an individual having a 28% mPR3+subset. Labeling with the MoAb anti-CR1 shows an homogeneous population. (C) Representative experiment of double-labeling PR3 and CD63, a marker of azurophil degranulation in resting neutrophils and in neutrophils stimulated with 10−6 mol/L FMLP in the presence of cytochalasin B (indicated with an arrow), from an individual having a 43% mPR3+ subset. Labeling with the MoAb anti-CD63 shows a homogeneous population.
Evaluation of azurophil granule mobilization by measuring CD63 expression at the plasma membrane showed that no significant azurophil degranulation was detectable in freshly isolated neutrophils or in neutrophils stimulated with up to 10−6 mol/L FMLP (Fig 5A). In contrast, cytochalasin B, together with 10−6 mol/L FMLP, mobilized azurophil granules to the plasma membrane and resulted in a huge increase in CD63 membrane expression (+480% ± 35%). This azurophil degranulation coincided with a significant increase in membrane PR3 expression (+253% ± 45%; Fig 5A). Double-labeling of neutrophils for CD63 and PR3 showed that CD63 expression on neutrophils stimulated with 10−6 mol/L FMLP in the presence of cytochalasin B was homogeneous and similar in the mPR3+ and mPR3− subsets (Fig 5C). Moreover, azurophil degranulation triggered a clearcut decrease in CR1 expression (−24%; Fig 5A) indicative of the release of azurophil granule-derived proteinases that are known to induce the shedding of CR1.34
Comparison between membrane expression and extracellular release of PR3, HNE, and MPO after stimulation with 10−6 mol/L FMLP in the absence or in presence of cytochalasin B to mobilize either specific or azurophil granules, respectively, clearly demonstrates that, upon activation, PR3 remained mainly membrane-bound and was released in minute amounts into the extracellular medium (Fig 6). In contrast, upon azurophil degranulation, HNE and MPO could be detected at the plasma membrane but were mainly released into the extracellular medium, thus providing additional evidence that PR3 mobilization is different from that of HNE and MPO.
Effect of neutrophil activation on secretion and membrane expression of PR3, HNE, and MPO. Isolated neutrophils were adjusted to a concentration of 106 cells/mL and stimulated for 15 minutes with 10−6 mol/L FMLP in the absence or in the presence of cytochalasin B to mobilize specific or azurophil granules, respectively. (A) The concentrations of secreted PR3, HNE, or MPO were measured in the supernatant of stimulated cells using specific sandwich ELISA and are expressed in micrograms per milliliter. Results are given as the mean ± SEM from 9 independent experiments. (B) Neutrophils were labeled for flow cytometry analysis with specific antibodies before (HBSS) or after activation. Membrane expression of PR3, HNE, or MPO is expressed as the percentage of mean fluorescence index (MFI) increase above baseline and is calculated as ([MFI activation] − [MFI HBSS]/[MFI HBSS]) × 100. Results are given as the mean ± SEM from 9 independent experiments.
Effect of neutrophil activation on secretion and membrane expression of PR3, HNE, and MPO. Isolated neutrophils were adjusted to a concentration of 106 cells/mL and stimulated for 15 minutes with 10−6 mol/L FMLP in the absence or in the presence of cytochalasin B to mobilize specific or azurophil granules, respectively. (A) The concentrations of secreted PR3, HNE, or MPO were measured in the supernatant of stimulated cells using specific sandwich ELISA and are expressed in micrograms per milliliter. Results are given as the mean ± SEM from 9 independent experiments. (B) Neutrophils were labeled for flow cytometry analysis with specific antibodies before (HBSS) or after activation. Membrane expression of PR3, HNE, or MPO is expressed as the percentage of mean fluorescence index (MFI) increase above baseline and is calculated as ([MFI activation] − [MFI HBSS]/[MFI HBSS]) × 100. Results are given as the mean ± SEM from 9 independent experiments.
DISCUSSION
Interest in the investigation of PR3 stems from the fact that PR3 appears critical in ANCA-related vasculitis, because it is both a target for autoantibodies and an effector molecule through its proteolytic activity. Our observations that PR3 is expressed at the membrane of a stable subset of freshly isolated neutrophils and that a high mPR3+ subset may constitute a risk factor for inflammatory disease such as vasculitis have led us into having a particular interest in membrane-associated PR3.20 Our long-term goal is to elucidate the molecular and the genetic basis of PR3 membrane expression. In the present report, we addressed the following questions. What is the biochemical nature of PR3 association with the plasma membrane? Is this membrane PR3 expression compatible with an exclusive localization of PR3 in azurophil granules or is there another intracellular pool of PR3? What are the relationships between intracellular and plasma membrane PR3?
Biochemical characterization of membrane PR3.
Plasma membrane PR3 in resting neutrophils is not released after treatment with (1) high salt concentrations, which can dissociate protein/proteoglycan complexes35; (2) acidic or basic pH, which can release MPO and HNE from the membrane of activated neutrophils36,37; and (3) neuraminidase, which removes cell surface negative charge. In addition, PR3 interaction with the plasma membrane is unlikely to occur through a GPI anchor given its insensitivity to PIPLC treatment or to comply with an interaction such as ligand/receptor. PR3 association with the plasma membrane appears to be covalent and may involve lipid interactions. It is different from that occurring with MPO or HNE exclusively due to interaction and observed after neutrophil degranulation. Although there is no clear evidence of transmembrane domains in PR3 sequence,2,38 our previous observation that recombinant PR3 produced in a baculovirus system remains insoluble strongly suggests that PR3 has a high affinity for membranes and may contain hydrophobic stretches able to interact with them.30
Subcellular localization of PR3.
Although subcellular fractionation of resting neutrophils shows that the major intracellular store of PR3 remains the azurophil granules, PR3 is also present in the plasma-enriched fraction containing secretory vesicles and in the specific granules. In contrast, the only intracellular pool of HNE and MPO is the azurophil granules. Western blot analysis of the plasma membrane fraction demonstrates that plasma membrane-associated PR3 has the same apparent molecular mass (29 kD) as azurophil granule PR3.30
Immunoelectron microscopy further confirms the localization of PR3 at the plasma membrane in resting neutrophils as well as its localization in the membrane of other organelles. One important feature of PR3 membrane labeling is its heterogeneity among neutrophils, thus confirming the results that we have obtained using flow cytometry.19,20 Plasma membrane of PR3 was observed in the absence of azurophil degranulation, because no membrane-bound MPO could be detected. Indeed, the presence of PR3 at the plasma membrane of mature resting neutrophils has already been described in a previous electron microscopy study.39 However, the investigators attributed this to neutrophil artefactual azurophil degranulation during neutrophil preparation. We provide the first direct evidence of the colocalization of PR3 with CR1 in the membrane of secretory vesicles that appear as empty vesicles that are heterogeneous in size. We found that CR1-positive vesicles are not abundant and that their number represents only approximately 5% of the granules, which is in agreement with a previous electron microscopy study using HRP-coupled antibody to detect intracellular stores of CR1.40As has been described for CR1, membrane expression of PR3 can be increased after the mobilization of secretory vesicles induced by isolation procedure.41 Immunoelectron microscopy confirms the findings of subcellular fractionation, because PR3 is also localized in the membrane of some specific granules. We confirm that azurophil granules represent the major intracellular store of PR3. In contrast to MPO, PR3 is mainly localized at the periphery of the granule. The localization of PR3 in the crystalloid structure of azurophil granules has already been described in azurophil granules from promyelocytes.42 This latter study, focusing on ultrastructural azurophil granules, was performed in immature cells that did not contain specific granules and secretory vesicles. This may explain why no PR3 was observed at the plasma membrane of promyelocytes. Our results suggest a continuum in PR3 biosynthesis from the promyelocytic stage to mature neutrophils.
Functional analysis of subcellular localization of PR3.
We have studied the relationships between plasma membrane PR3 expression and neutrophil degranulation assuming that proteins that are mobilized together also localize together. Indeed, PR3 localization in secretory vesicles leads to its translocation in response to nanomolar concentrations of FMLP together with CR1. In contrast to other neutrophil membrane proteins stored in intracellular granules (examples being CR1 in secretory vesicles and CD11b/CD18, FMLP receptor, or cytochrome b558 in specific granules) that are expressed at low levels on neutrophil plasma membranes within whole blood,5 it remains unclear as to whether plasma membrane PR3 is similarily expressed. Indeed, we have previously shown that, within whole blood, PR3 is inaccessible to antibodies because of the presence of inhibitors. Removal of the plasma is insufficient in itself to expose membrane PR3 on neutrophils, which requires incubation of washed cells at 37°C.20 Similar conditions have been used to mobilize alkaline phosphatase from secretory vesicles to plasma membrane.24 Sequential degranulation experiments show that, on isolated neutrophils, membrane PR3 expression further increases after specific granule mobilization and culminates after azurophil granule mobilization ascertained by strong CD63 membrane expression. It is important to stress that, in contrast to HNE and MPO, upon azurophil degranulation, PR3 remains mainly membrane bound.
In conclusion, subcellular fractionation, immunoelectron microscopy, and degranulation studies all converge to the conclusion that azurophil granules are not the only intracellular store of PR3 in neutrophils and that PR3 is mainly membrane-bound. We have demonstrated the presence of PR3 at the neutrophil plasma membrane as well as in the highly mobilizable secretory vesicles and in some specific granules. Consequently, PR3 should be considered not only as a bactericidal serine proteinase stored in the azurophil granule compartment, but also as a membrane protein that may serve other functional roles in neutrophils and in the inflammatory process, especially in ANCA-associated vasculitis.
ACKNOWLEDGMENT
The authors thank Dr Béatrice Descamps-Latscha for helpful discussion and comments, Dr Michelle Webb for critically reviewing the manuscript, and Gilles Bessou for excellent technical assistance.
Supported by grants from the Association Française de Lutte contre la Mucoviscidose (AFLM), the Association pour l’Aide à la Recherche contre la Mucoviscidose et l’Assistance aux Malades, and the Association de Recherche sur la Polyarthrite (ARP).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Véronique Witko-Sarsat, PhD, INSERM U507, Hôpital Necker, 161, rue de Sèvres, 75015 Paris, France; e-mail: witko-sarsat@necker.fr.

![Fig. 6. Effect of neutrophil activation on secretion and membrane expression of PR3, HNE, and MPO. Isolated neutrophils were adjusted to a concentration of 106 cells/mL and stimulated for 15 minutes with 10−6 mol/L FMLP in the absence or in the presence of cytochalasin B to mobilize specific or azurophil granules, respectively. (A) The concentrations of secreted PR3, HNE, or MPO were measured in the supernatant of stimulated cells using specific sandwich ELISA and are expressed in micrograms per milliliter. Results are given as the mean ± SEM from 9 independent experiments. (B) Neutrophils were labeled for flow cytometry analysis with specific antibodies before (HBSS) or after activation. Membrane expression of PR3, HNE, or MPO is expressed as the percentage of mean fluorescence index (MFI) increase above baseline and is calculated as ([MFI activation] − [MFI HBSS]/[MFI HBSS]) × 100. Results are given as the mean ± SEM from 9 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/7/10.1182_blood.v94.7.2487.419k07_2487_2496/6/m_blod41907006x.jpeg?Expires=1769302524&Signature=PYsYUbiUMl8FnuLbq2Z8AyoGC-VS7207JyWktuFkcMok-0Z~Nn9mPqNYCgdxSEzFhwjYa3OGqFhkRPmRJBpxIDnQ8Ns~i335ZAxE~PZ1kjsEo5pO-nv3RskSPsJvRpsI6xIf3sQyixufAv4uyqxYD7R4AmmG13mdb0tRUPzs2~LKb7bRQZuw~mVec1auMxOmdfgRJj9OeIDn1A1llSXvsed4jhDQuJhPXBXRzJuNDPNQ4ugldahudFEPgHUOnUAnI~cY6G077xPXL8nUc2xMbK6dtxzFwvVw1p01Hcsn0BKPUdPKHnobU94-EzkY9Q7pFtiAFh2GQfaF03KnioxxEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal