Several angiogenesis inhibitors are fragments of larger proteins that are themselves not active as angiogenesis inhibitors. Vasostatin, the N-terminal domain of calreticulin inclusive of amino acids 1-180, is an angiogenesis inhibitor that exerts antitumor effects in vivo. In the present study, we examined whether the full-length calreticulin molecule shares the antiangiogenic and antitumor activities of vasostatin. Similar to vasostatin, calreticulin selectively inhibited endothelial cell proliferation in vitro, but not cells of other lineages, and suppressed angiogenesis in vivo. When inoculated into athymic mice, calreticulin inhibited Burkitt tumor growth comparably with vasostatin. Calreticulin lacking the N-terminal 1-120 amino acids inhibited endothelial cell proliferation in vitro and Burkitt tumor growth in vivo comparably with vasostatin. An internal calreticulin fragment encompassing amino acids 120-180 also inhibited endothelial cell proliferation in vitro and angiogenesis in vivo comparably with calreticulin and vasostatin. These results suggest that the antiangiogenic activities of vasostatin reside in a domain that is accessible from the full-length calreticulin molecule and localize to calreticulin N-terminal amino acids 120-180. Thus, calreticulin and calreticulin fragments are inhibitors of angiogenesis that directly target endothelial cells, inhibit angiogenesis, and suppress tumor growth. This information may be critical in designing targeted inhibitors of pathological angiogenesis that underlies cancer and other diseases.
TUMOR GROWTH and metastasis formation are dependent on the existence of adequate blood supply. As tumors grow larger, adequate blood supply to the tumor tissue is often ensured by new vessel formation, a process named angiogenesis.1,2 In preclinical models, agents that target the tumor vasculature have been shown to prevent or delay tumor growth and even to promote tumor regression or dormancy.3-14 For example, antibodies to αvβ3, an integrin molecule that is expressed at high levels on proliferating vessels, inhibited angiogenesis and tumor formation on the chick chorioallantoid membrane.8,15 Antibodies to vascular endothelial growth factor (VEGF), a stimulator of angiogenesis that is produced by a variety of tumor cells, reduced tumor growth in experimental models.6,14 Thrombospondin, an extracellular matrix protein secreted by endothelial cells and other cells, inhibited endothelial cell chemotaxis, neovascularization, and tumor growth.3,16Angiostatin, a fragment of plasminogen, and endostatin, a fragment of collagen XVIII, suppressed angiogenesis and the growth of a variety of experimental tumors.9 13
Recent experiments have suggested distinct potential advantages of tumor treatment with angiogenesis inhibitors. Resistance, commonly observed with chemotherapeutic agents, was not noted even after extended experimental treatment with the antiangiogenic protein endostatin, suggesting that targeting the tumor vasculature can be useful in the context of tumor cell resistance to chemotherapeutic agents.17,18 In addition, treatment of experimental tumors with the angiogenesis inhibitor angiostatin combined with radiotherapy displayed synergistic antitumor effects, suggesting that antiangiogenic agents may be useful in combination with agents that target more directly the tumor cells.19
Efforts in our laboratory aimed at identifying novel inhibitors of endothelial cell proliferation in the culture supernatants of Epstein-Barr virus (EBV)-immortalized cells resulted in the isolation of vasostatin, the N-terminal domain of calreticulin, which includes calreticulin amino acids 1-180.20 Vasostatin is a specific inhibitor of basic fibroblast growth factor (bFGF)-induced endothelial cell proliferation in vitro and a suppressor of bFGF-induced angiogenesis in vivo.20 When inoculated into athymic mice, vasostatin prevented or significantly reduced experimental tumor growth.20 Because other inhibitors of angiogenesis, such as certain internal domains of prolactin and fibronectin, angiostatin, and endostatin, are fragments of larger proteins that are themselves not active as angiogenesis inhibitors,5,9,13 21 we sought to examine whether the full-length calreticulin molecule displays the antiangiogenic and antitumor properties of vasostatin. Additionally, we wished to define further the vasostatin domain that is active in endothelial cell and tumorigenesis assays. To this end, we have compared vasostatin, calreticulin, a fragment of calreticulin lacking the N-terminal amino acids 1-120, and an internal calreticulin peptide, including amino acids 120 to 180 (Fig 1), for their ability to inhibit endothelial cell proliferation and angiogenesis.
Schematic representation of full-length calreticulin and calreticulin fragments expressed in E coli as fusion proteins with MBP. MBP is depicted as a shaded box. The N-terminal (N), proline-rich (P), and C-terminal (C) domains of calreticulin are noted. Numbers above each box denote the amino acid numbers from mature calreticulin.
Schematic representation of full-length calreticulin and calreticulin fragments expressed in E coli as fusion proteins with MBP. MBP is depicted as a shaded box. The N-terminal (N), proline-rich (P), and C-terminal (C) domains of calreticulin are noted. Numbers above each box denote the amino acid numbers from mature calreticulin.
MATERIALS AND METHODS
Cell proliferation assays.
Fetal bovine heart endothelial cells (American Type Culture Collection [ATCC], Manassas, VA) were grown through passage 12 in Dulbecco’s Modified Eagle’s Medium (DMEM) culture medium (BioWittaker, Walkersville, MD) containing 10% heat inactivated fetal bovine serum (BioWittaker), 25 ng/mL bFGF (R&D Systems, Minneapolis, MN), and 5 μg/mL gentamicin (Sigma, St Louis, MO). For proliferation assays, cells were trypsinized (Trypsin/EDTA; GIBCO BRL, Gaithersburg, MD), washed, suspended in culture medium (DMEM containing 10% heat inactivated fetal bovine serum and 5 μg/mL gentamicin), plated (800 cells/well in 0.2 mL culture medium) in triplicate onto 96-well plates, and incubated for 5 days. DNA synthesis was measured by 3H thymidine deoxyribose uptake (0.5 mCi/well, 6.7 Ci/mmol; New England Nuclear, Boston, MA) during the last 20 to 23 hours of culture; cells were detached from wells by freezing and thawing.22
Matrigel angiogenesis assay.
The Matrigel assay was performed as described.22 23Matrigel, a crude extract of the Englebreth-Holm-Swarm tumor was obtained from Collaborative Biomedical Products. (Becton Dickinson Labware, Bedford, MA.) An aliquot (0.5 mL) of Matrigel alone or with desired additives was injected subcutaneously (sc) into the midabdominal region of female BALB/c nude mice, 6 to 8 weeks old. Five mice were injected with each mixture. After 5 to 7 days, the animals were killed, Matrigel plugs were removed, fixed in 10% neutral buffered formalin solution (Sigma), and embedded in paraffin. Tissues were sectioned (5 μ thickness) and slides stained with Masson’s trichrome (American Histolabs, Gaithersburg, MD). Quantitative analysis of angiogenesis in Matrigel plugs used a computerized semi-automated digital analyzer (40-10 System; Optomax, Hollis, NH). The instrument was adjusted to evaluate a circular area measuring 1.26 × 105 mm2 of Matrigel, and within this area, to measure the area occupied by cells. For each plug, 12 to 15 distinct fields were evaluated. The fields were randomly selected from each plug, and the operator was blind to the experimental design. The average area occupied by cells/1.26 × 105mm2 Matrigel field was calculated. Results are expressed as the mean area occupied by cells/Matrigel field.
Production of recombinant calreticulin and calreticulin fragments.
The expression of human calreticulin, the calreticulin N-terminal domain (amino acids 1-180), and the calreticulin N-terminal deletion fragment lacking amino acids 1-120 (Δ120-calreticulin) fused to maltose-binding protein (MBP) in Escherichia coli was reported.24,25 For expression of the calreticulin fragment encompassing amino acids 120 to 180 calreticulin), the coding region for this fragment was amplified by polymerase chain reaction and then cloned (confirmed by sequencing) as an N-terminal translational fusion protein with the MBP gene for expression in E coli. E coli were grown in Luria-Bertani broth (Advanced Biotechnologies, Inc, Columbia, MD) with 0.2% glucose and 100 μg/mL ampicillin (Sigma) to an OD600 of approximately 1.0, after which fusion protein expression was induced with 0.3 mmol/L IPTG (GIBCO/BRL) for 2 to 2.5 hours. After lysis (1 μg/mL lysozyme in 10 mmol/L Tris; pH 7.5; 5% glycerol; 100 mmol/L EDTA; 5 mmol/L B-ME), sonication, and centrifugation (30 minutes at 8,360g) of the bacterial suspension, the supernatant was loaded onto a preequilibrated (20 mmol/L Tris; pH 7.5; 200 mmol/L NaCl; and l mmol/L EDTA) 15 mL amylose resin column (New England Biolabs, Beverly, MA). Bound material was eluted with 10 mmol/L maltose. Protein-containing fractions were ultracentrifuged (2 hours at 104,000g); supernatant was retained. Separation of MBP from calreticulin and vasostatin was accomplished by cleavage with factor Xa, as described.24 Purification of cleaved calreticulin or vasostatin from MBP was achieved by anion exchange chromatography using a preequilibrated (20 mmol/L Tris; pH 8.0; 25 mmol/L NaCl) Resource Q column (Amersham Pharmacia Biotech, Arlington Heights, IL). Bound material was eluted by a step-wise gradient where MBP elutes at 100 to 150 mmol/L NaCl; Factor Xa elutes at approximately 400 mmol/L NaCl; and calreticulin or vasostatin elute at approximately 250 mmol/L NaCl. For construction of the glutathione S transferase (GST)-calreticulin fusion construct, the coding region for the mature calreticulin protein was cloned as a C-terminal translational fusion with the GST gene for expression in E coli. The coding region of human calreticulin was amplified and cloned in frame with GST protein (confirmed by sequencing). The growth of E coli and the induction and release of GST-calreticulin from the bacteria was similar to that described for MBP-calreticulin, except for concentration of ITPG (0.6 mmol/L). Purification of GST-calreticulin was achieved by lysis of the bacteria, followed by sonication and centrifugation. The supernatants (adjusted to pH 7.0) were mixed with prewashed Glutathione Sepharose 4B (Bulk GST purification module; Amersham Pharmacia Biotech) in phosphate-buffered saline (PBS) with 1.0% Triton X-100. After 30 minutes incubation and washing the beads, bound protein was eluted with a 50 mmol/L Tris-HCl buffer containing 10 mmol/L Glutathione, pH 8.0. Eluted material was ultracentrifuged (2 hours at 104,000g), and supernatant retained. All protein lots for in vivo and in vitro experiments (GST-calreticulin, control GST, MBP-calreticulin, MBP-vasostatin, MBP, cleaved calreticulin, and vasostatin) were tested for endotoxin by the Limulus Amebocyte Lysate (LAL) kinetic-QCLTM assay (BioWittaker) and were found to contain less than 5 U/10 μg protein.
Protein sequencing.
Protein bands were excized from Coomassie-stained gel and destained. The proteins were digested with trypsin (Promega, Madison, WI) in the gel26 and the resulting fragments were separated by microcapillary high performance liquid chromatography and analyzed in-line by ion-trap mass spectrometry (Finnigan model LCQ, San Jose, CA).27
Mouse tumor model.
BALB/c nu/nu female mice, 6 weeks of age (National Cancer Institute, Frederick, MD) maintained in pathogen-limited conditions, received 400 rad (1 rad = 0.01 Gy) total body irradiation and 24 hours later were injected sc in the right abdominal quadrant with 107exponentially growing human Burkitt lymphoma cells (CA46 cell line11) in 0.2 mL PBS. Immediately after the Burkitt cells were inoculated sc, and continuing daily thereafter 6 d/wk, the mice received sc injections proximal to the site of original cell inoculation of test samples or appropriate controls. These included purified GST-calreticulin, control GST, MBP-calreticulin, MBP-vasostatin, MBP, or formulation buffer used to dilute test proteins (sterile saline solution containing 50 mg/mL human albumin and 5 mg/mL mannitol; endotoxin <5 U/mL). Tumor size was estimated (in mm2) twice weekly as the product of 2-dimensional caliper measurements (longest perpendicular length and width). A subcutaneous mass appearing at or proximal to the site of cell inoculation was considered a tumor when it measured at least 0.16 cm2 in surface area and increased in size by at least 30% over the following week.
Histology and immunohistochemistry.
Tumors and Matrigel plugs were fixed in 10% neutral buffered formalin solution (Sigma), embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin, Masson’s trichrome, or elastin van Gieson reagent by standard methods. Immunohistochemical staining of tumor vessels was performed as described28 on paraffin-embedded samples that had been deparaffinized and rehydrated. After treatment with trypsin for 10 minutes or heat for 25 minutes, tissue sections were blocked with 3% goat serum in Tris-buffered saline, after incubation (overnight at 4°C) with primary antibody. Purified rabbit anti–von Willebrand factor (1:50 dilution) and mouse anti-smooth muscle actin monoclonal antibody (clone 1A4, 0.25 μg/mL) were purchased from DAKO (Carpinteria, CA). After washing, biotynilated goat anti-rabbit or horse anti-mouse secondary antibody (2 μmol/L; Vector Labs, Burlingame, CA) was applied followed by VECTASTAIN ABC peroxidase complex (Elite ABC-kit; Vector Labs). The sections were developed using peroxidase 3,3′-diaminobenzidine (DAB) substrate and counterstained with hematoxylin.
Statistical analysis.
Student’s t-test was used to evaluate the significance of group differences; Wilcoxon Rank Sums test was used to evaluate differences in tumor growth rates; Kruskal-Wallis Rank Sums test was used to evaluate group differences in 3-way comparisons.
RESULTS
Effects of calreticulin on endothelial cell proliferation.
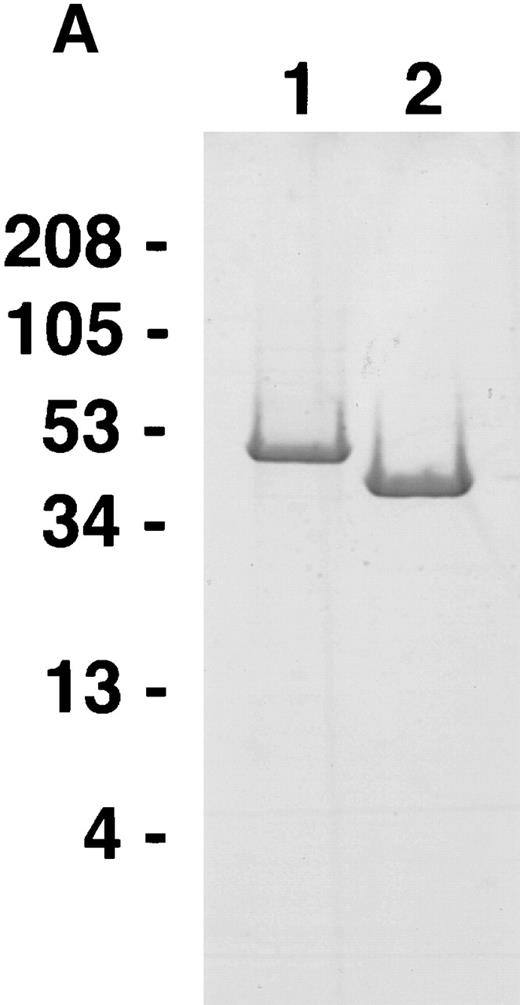
In initial experiments, we tested full-length calreticulin and a truncated form of calreticulin lacking the N-terminal 1 to 120 amino acids for their ability to inhibit endothelial cell proliferation in vitro and compared with vasostatin. To this end, in addition to purifying the recombinant N-terminal domain of calreticulin (amino acids 1 to 18020), we purified human calreticulin fromE coli expressing the recombinant protein fused to either MBP or GST. We also purified a truncated form of calreticulin, Δ120-calreticulin, from E coli expressing the protein fused to MBP. When analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig 2), GST-calreticulin (lane 1), GST (lane 2), MBP-calreticulin (lane 3), MBP (lane 5), and MBP-Δ120-calreticulin (lane 6) resolved as discrete bands migrating at the expected relative positions. Calreticulin, cleaved from MBP-calreticulin by treatment with factor Xa and subsequently purified by anion exchange chromatography, resolved as a doublet with a relative molecular weight of approximately 50 and 55 kD (lane 4). On Western blotting, both bands were recognized by a rabbit antiserum against human calreticulin (not shown). The lower band was subjected to trypsin digestion and the tryptic fragments were sequenced by ion-trap mass spectrometry. By this method, this band was identified as a cleavage fragment of calreticulin encompassing at least amino acids 49 to 321.
SDS-PAGE of recombinant purified proteins. 1: GST-calreticulin; 2: GST; 3: MBP-calreticulin; 4: calreticulin cleaved from MBP-calreticulin; 5: MBP; 6: MBP–▵120-calreticulin.
SDS-PAGE of recombinant purified proteins. 1: GST-calreticulin; 2: GST; 3: MBP-calreticulin; 4: calreticulin cleaved from MBP-calreticulin; 5: MBP; 6: MBP–▵120-calreticulin.
When tested in functional assays, recombinant purified MBP-calreticulin inhibited the proliferation of fetal bovine heart endothelial cells induced by bFGF (Fig 3). At a concentration of 1 μg/mL, MBP-calreticulin inhibited fetal bovine heart endothelial cell growth by 67%, whereas control MBP had minimal effects. Similar inhibition was noted with 1 μg/mL GST-calreticulin (Fig4) and with vasostatin.20Recombinant calreticulin that had been cleaved and purified from MBP-calreticulin also inhibited the proliferation of fetal bovine heart endothelial cells, whereas control MBP displayed minimal inhibition (Fig 4). Furthermore, recombinant purified MBP–Δ120-calreticulin inhibited the proliferation of fetal bovine heart endothelial cells, and the degree of inhibition was comparable with that of calreticulin (Fig 5).
Inhibition of endothelial cell proliferation by MBP-calreticulin. Fetal bovine heart endothelial cells (800 cells/well) were incubated for 5 days either in medium alone or medium supplemented with bFGF (25 ng/mL), with or without recombinant purified MBP-calreticulin or MBP (both at 1 μg/mL). The results of 16 experiments are expressed as mean cpm (±SD).
Inhibition of endothelial cell proliferation by MBP-calreticulin. Fetal bovine heart endothelial cells (800 cells/well) were incubated for 5 days either in medium alone or medium supplemented with bFGF (25 ng/mL), with or without recombinant purified MBP-calreticulin or MBP (both at 1 μg/mL). The results of 16 experiments are expressed as mean cpm (±SD).
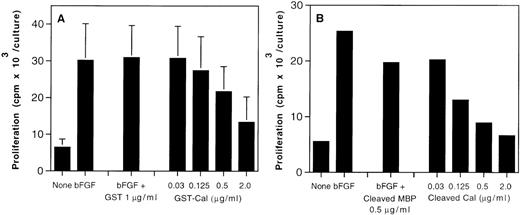
Dose-dependency of inhibition of endothelial cell proliferation by calreticulin. Fetal bovine heart endothelial cells (800 cells/well) were cultured for 5 days in medium alone or medium supplemented with bFGF (25 ng/mL). Recombinant purified GST-calreticulin (1 μg/mL), control recombinant GST (0.03 to 1 μg/mL), calreticulin cleaved and purified from MBP-calreticulin (0.03 to 2 μg/mL), and MBP cleaved and purified from MBP-calreticulin (0.5 μg/mL) were added to endothelial cell cultures with bFGF (25 ng/mL). Proliferation was measured by 3H thymidine incorporation during the final 20 to 23 hours of culture; the results reflect mean cpm/culture. (A) Reflects the mean of 9 experiments, each performed in triplicate. (B) Reflects the mean of 2 experiments, each performed in triplicate.
Dose-dependency of inhibition of endothelial cell proliferation by calreticulin. Fetal bovine heart endothelial cells (800 cells/well) were cultured for 5 days in medium alone or medium supplemented with bFGF (25 ng/mL). Recombinant purified GST-calreticulin (1 μg/mL), control recombinant GST (0.03 to 1 μg/mL), calreticulin cleaved and purified from MBP-calreticulin (0.03 to 2 μg/mL), and MBP cleaved and purified from MBP-calreticulin (0.5 μg/mL) were added to endothelial cell cultures with bFGF (25 ng/mL). Proliferation was measured by 3H thymidine incorporation during the final 20 to 23 hours of culture; the results reflect mean cpm/culture. (A) Reflects the mean of 9 experiments, each performed in triplicate. (B) Reflects the mean of 2 experiments, each performed in triplicate.
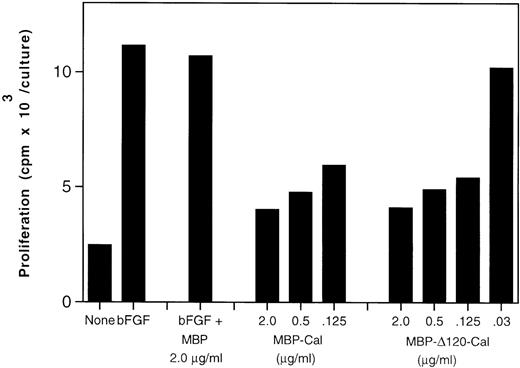
Inhibition of endothelial cell proliferation by ▵120-calreticulin. Fetal bovine heart endothelial cells (800 cells/well) were cultured for 5 days in medium alone or medium supplemented with bFGF (25 ng/mL). Recombinant purified MBP-calreticulin, MBP–▵120-calreticulin, or MBP were added to bFGF-supplemented cultures. Proliferation was measured by3H thymidine incorporation during the final 20 to 23 hours of culture. The results reflect the mean of triplicate cultures; SDs within 12% of the mean.
Inhibition of endothelial cell proliferation by ▵120-calreticulin. Fetal bovine heart endothelial cells (800 cells/well) were cultured for 5 days in medium alone or medium supplemented with bFGF (25 ng/mL). Recombinant purified MBP-calreticulin, MBP–▵120-calreticulin, or MBP were added to bFGF-supplemented cultures. Proliferation was measured by3H thymidine incorporation during the final 20 to 23 hours of culture. The results reflect the mean of triplicate cultures; SDs within 12% of the mean.
Because vasostatin (calreticulin N-domain, encompassing amino acids 1-180), full-length calreticulin, and Δ120-calreticulin (calreticulin missing the N-terminal 1-120 amino acids) inhibited bFGF-induced endothelial cell proliferation to a similar degree at similar concentrations, we investigated whether the 61 amino acid calreticulin fragment encompassing amino acids 120-180 (120-180 calreticulin) was also active. To this end, we purified an MBP 120-180 calreticulin fragment from E coli expressing the recombinant protein fused to MBP (Fig 6A). When tested for inhibition of endothelial cell proliferation, recombinant purified MBP 120-180 calreticulin fragment inhibited the proliferation of bovine heart endothelial cells induced by bFGF (Fig 6B). At a concentration of 0.5 μg/mL, the 61-amino acid calreticulin fragment inhibited fetal bovine heart endothelial cell proliferation by 65%. A side-by-side comparison of full-length MBP-calreticulin, MBP-vasostatin (1-180 calreticulin), MBP-Δ120-calreticulin and MBP 120-180 calreticulin fragment revealed that, on a molar basis, the 4 proteins display similar endothelial cell growth inhibitory activity in vitro. Control MBP was not inhibitory (Fig 7). These results suggest that the antiangiogenic activity of calreticulin resides in a domain between amino acids 120 and 180.
(A) SDS-PAGE of recombinant purified proteins. 1: MBP 120-180 calreticulin fragment; 2: MBP. (B) Inhibition of endothelial cell proliferation by MBP 120-180 calreticulin fragment. Fetal bovine heart endothelial cells (800 cells/well) were incubated for 5 days either in medium alone or medium supplemented with bFGF (15 ng/mL), with or without recombinant purified MBP 120-180 calreticulin peptide (1 μg/mL). The results of 8 experiments are expressed as mean cpm (±SD).
(A) SDS-PAGE of recombinant purified proteins. 1: MBP 120-180 calreticulin fragment; 2: MBP. (B) Inhibition of endothelial cell proliferation by MBP 120-180 calreticulin fragment. Fetal bovine heart endothelial cells (800 cells/well) were incubated for 5 days either in medium alone or medium supplemented with bFGF (15 ng/mL), with or without recombinant purified MBP 120-180 calreticulin peptide (1 μg/mL). The results of 8 experiments are expressed as mean cpm (±SD).
Comparative analysis of endothelial cell growth inhibition by MBP calreticulin (□), MBP–▵120-calreticulin (○), MBP-vasostatin (◊), MBP 120-180 calreticulin (▵) fragment, and MBP (⊞). Fetal bovine heart endothelial cells (800 cells/well) were cultured for 5 days in medium alone or medium supplemented with bFGF (15 ng/mL). Recombinant purified fusion proteins were added to culture at 0.4 to 32 nmol/L concentrations to bFGF-supplemented cultures. Proliferation was measured by 3H thymidine incorporation during the final 20 to 23 hours of culture. The results reflect the mean of triplicate cultures; SDs within 15% of the mean. The mean response of endothelial cells was 2,217 cpm when cultured in medium alone, and 23,377 cpm when cultured with bFGF alone.
Comparative analysis of endothelial cell growth inhibition by MBP calreticulin (□), MBP–▵120-calreticulin (○), MBP-vasostatin (◊), MBP 120-180 calreticulin (▵) fragment, and MBP (⊞). Fetal bovine heart endothelial cells (800 cells/well) were cultured for 5 days in medium alone or medium supplemented with bFGF (15 ng/mL). Recombinant purified fusion proteins were added to culture at 0.4 to 32 nmol/L concentrations to bFGF-supplemented cultures. Proliferation was measured by 3H thymidine incorporation during the final 20 to 23 hours of culture. The results reflect the mean of triplicate cultures; SDs within 15% of the mean. The mean response of endothelial cells was 2,217 cpm when cultured in medium alone, and 23,377 cpm when cultured with bFGF alone.
In contrast to its inhibitory effect on endothelial cell growth, calreticulin at concentrations of 0.5 to 10 μg/mL had minimal effect on the proliferation of a variety of other primary cells and cell lines (not shown), including human peripheral blood mononuclear cells either not stimulated or stimulated with phytohemagglutinin; B-cell–enriched peripheral blood mononuclear cells stimulated with EBV; T-cell–enriched populations stimulated with pokeweed mitogen; human foreskin fibroblasts (H5 68); Burkitt lymphoma cells (CA46, BL41, KK124, Ag876, SHO); lymphoblastoid cells (VDS-O line); T-cell line (Molt-4); neuroblastoma cells (SK-N-MC); lung carcinoma cells (A-549); breast adenocarcinoma cells (MDA-MB-468); acute promyelocytic leukemia cells (HL-60); prostate adenocarcinoma cells (Tsu-Pr1, PC-3, Dul45); Hodgkin’s lymphoma cells (Hs445); colon adenocarcinoma cells (SW-480); Wilms tumor cells (SK-NEP-1); and melanoma cells (A-375). The 61-amino acid calreticulin fragment (120-180 calreticulin) at concentrations of 0.5 to 10 μg/mL had minimal effect on the proliferation of lymphoblastoid and Burkitt lymphoma cells (not shown). These results are similar to those derived with vasostatin.20 Thus, calreticulin and vasostatin can selectively inhibit the proliferation of endothelial cells in vitro.
Calreticulin and calreticulin fragments inhibit angiogenesis.
The murine Matrigel assay22 23 was used to evaluate the effects of calreticulin and calreticulin fragments on angiogenesis in vivo. When added to Matrigel at concentrations of 1.25 to 5 μg/mL, GST-calreticulin displayed a concentration-dependent inhibition of bFGF-induced neovascularization (Table 1, experiment 1). When tested in the same experiment, MBP-calreticulin and MBP-vasostatin used at the same concentration (5 μg/mL) inhibited bFGF-induced neovascularization to a similar degree (Table 1, experiment 2). In another experiment, calreticulin cleaved and purified from MBP, MBP–Δ120-calreticulin, and MBP 120-180 calreticulin fragment similarly inhibited bFGF-induced neovascularization (Table 1, experiment 3). These results show that full-length calreticulin, the N-terminal domain of calreticulin (amino acids 1-180, vasostatin), and a calreticulin fragment encompassing amino acids 120-180 can all function as angiogenesis inhibitors in vivo.
Effects of Calreticulin and Calreticulin Fragments on Angiogenesis In Vivo
| Additions to Matrigel . | Mean Surface Area Occupied by Cells (mm2/ 1.26 × 105 mm2) . | Inhibition (%) . |
|---|---|---|
| 1. None | 671 | |
| bFGF | 17,732 | |
| bFGF + GST-calreticulin (5 μg/mL) | 4,616 | 74 |
| bFGF + GST-calreticulin (2.5 μg/mL) | 6,387 | 64 |
| bFGF + GST-calreticulin (1.25 μg/mL) | 9,870 | 44 |
| 2. None | 487 | |
| bFGF | 14,472 | |
| bFGF + MBP-calreticulin (5 μg/mL) | 5,112 | 65 |
| bFGF + MBP-vasostatin (5 μg/mL) | 4,989 | 66 |
| 3. None | 1,329 | |
| bFGF | 8,320 | |
| bFGF + cleaved calreticulin (5 μg/mL) | 3,464 | 58 |
| bFGF + MBP–Δ120-calreticulin (15 μg/mL) | 4,291 | 48 |
| bFGF + MBP 120-180 calreticulin (5 μg/mL) | 2,458 | 70 |
| Additions to Matrigel . | Mean Surface Area Occupied by Cells (mm2/ 1.26 × 105 mm2) . | Inhibition (%) . |
|---|---|---|
| 1. None | 671 | |
| bFGF | 17,732 | |
| bFGF + GST-calreticulin (5 μg/mL) | 4,616 | 74 |
| bFGF + GST-calreticulin (2.5 μg/mL) | 6,387 | 64 |
| bFGF + GST-calreticulin (1.25 μg/mL) | 9,870 | 44 |
| 2. None | 487 | |
| bFGF | 14,472 | |
| bFGF + MBP-calreticulin (5 μg/mL) | 5,112 | 65 |
| bFGF + MBP-vasostatin (5 μg/mL) | 4,989 | 66 |
| 3. None | 1,329 | |
| bFGF | 8,320 | |
| bFGF + cleaved calreticulin (5 μg/mL) | 3,464 | 58 |
| bFGF + MBP–Δ120-calreticulin (15 μg/mL) | 4,291 | 48 |
| bFGF + MBP 120-180 calreticulin (5 μg/mL) | 2,458 | 70 |
Mice were injected sc with Matrigel alone, Matrigel plus bFGF (150 ng/mL), Matrigel plus bFGF (150 ng/mL) plus GST-calreticulin, MBP-calreticulin, MBP-vasostatin, calreticulin cleaved and purified from MBP-calreticulin, MBP–Δ120-calreticulin, MBP-calreticulin 120-180 peptide, or MBP. Plugs were removed after 5 to 7 days, and histological sections were stained with Masson’s trichrome. The results reflect the mean surface area (expressed in mm2) occupied by cells within a circular surface area of 1.26 × 105 mm2; 12 to 15 nonoverlapping fields were scanned in each plug; there were 5 plugs/group. Determinations of surface area were performed by a semi-automated digitalized analyzer.
Calreticulin inhibits tumor growth in mice.
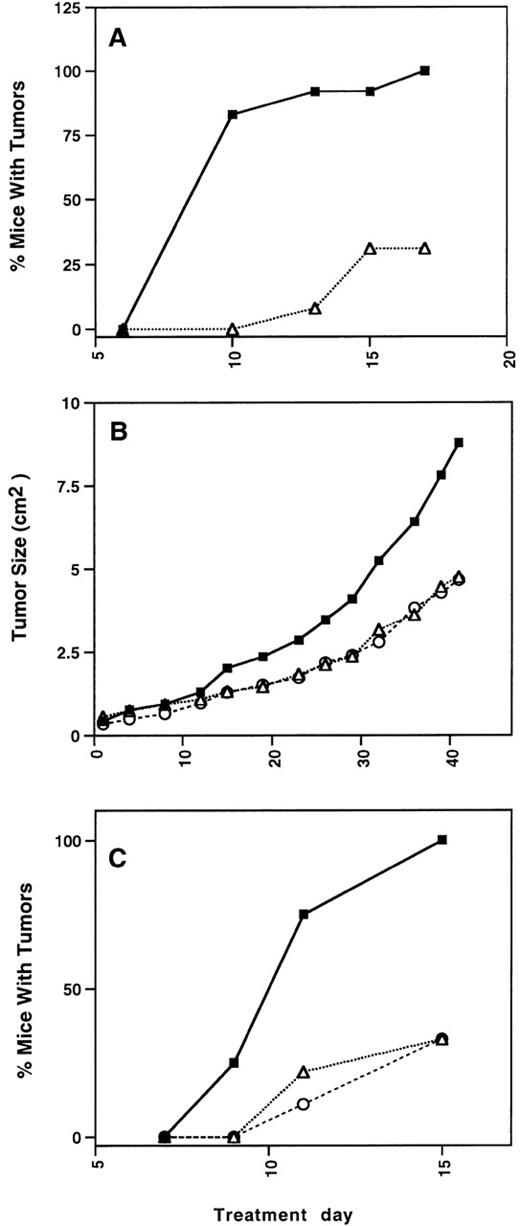
In previous experiments, vasostatin prevented or significantly reduced Burkitt tumor growth in nude mice.20 Using the same experimental system, we now tested calreticulin for its ability to prevent the growth of Burkitt lymphomas in athymic mice. Sublethally irradiated, 6-week-old female BALB/c nude mice were inoculated sc with 10 × 106 Burkitt cells (CA46 cell line). Beginning at the time of cell inoculation, the mice received daily, 6 days/week for 14 days, sc injections (100 μL volume) of either control GST protein (20 μg/mouse) or GST-calreticulin (60 μg/mouse), adjacent to the site of cell injection (Fig 8A). As expected, 12/12 mice injected with control GST protein developed a tumor by day 17. By contrast, only 4/13 mice injected with GST-calreticulin developed a tumor by day 17 (P = .005). The tumor-bearing mice were killed, and the remaining 9 nontumor-bearing mice were maintained untreated. Tumors eventually developed in 8 of 9 mice that had received initial calreticulin treatment. The latest tumor developed on day 36, 22 days after treatment had ended. One mouse remains tumor free (>200 days).
Calreticulin and calreticulin fragments inhibit tumor growth. Burkitt lymphoma cells (CA46 cell line, 1 × 107cells) were inoculated sc into BALB/c athymic mice, 6 weeks of age. (A) Beginning on the day of cell inoculation and continuing thereafter daily, 6 days/week, 12 mice were inoculated with control GST protein (▪, 20 μg/d × 14 days), and 13 mice were inoculated with GST-calreticulin (▵, 60 μg/d × 14 days). (B) Beginning on the day of tumor appearance, 5 mice were inoculated with control MBP protein (▪, 12.5 μg/d), 4 mice were inoculated with MBP-calreticulin (▵, 12.5 μg/d), and 4 mice were inoculated with MBP-vasostatin (○, 12.5 μg/d). (C) Beginning on the day of cell inoculation and continuing thereafter daily, 6 d/wk, 8 mice were inoculated with control MBP (▪, 20 μg/d × 14 days), 9 mice were inoculated with MBP-vasostatin (○, 30 μg/d × 14 days), and 9 mice were inoculated with ▵120 calreticulin (▵, 30 μg/d × 14 days).
Calreticulin and calreticulin fragments inhibit tumor growth. Burkitt lymphoma cells (CA46 cell line, 1 × 107cells) were inoculated sc into BALB/c athymic mice, 6 weeks of age. (A) Beginning on the day of cell inoculation and continuing thereafter daily, 6 days/week, 12 mice were inoculated with control GST protein (▪, 20 μg/d × 14 days), and 13 mice were inoculated with GST-calreticulin (▵, 60 μg/d × 14 days). (B) Beginning on the day of tumor appearance, 5 mice were inoculated with control MBP protein (▪, 12.5 μg/d), 4 mice were inoculated with MBP-calreticulin (▵, 12.5 μg/d), and 4 mice were inoculated with MBP-vasostatin (○, 12.5 μg/d). (C) Beginning on the day of cell inoculation and continuing thereafter daily, 6 d/wk, 8 mice were inoculated with control MBP (▪, 20 μg/d × 14 days), 9 mice were inoculated with MBP-vasostatin (○, 30 μg/d × 14 days), and 9 mice were inoculated with ▵120 calreticulin (▵, 30 μg/d × 14 days).
We then examined the effects of calreticulin on established Burkitt tumors and compared with vasostatin (Fig 8B). In a representative experiment, the rate of tumor growth appeared to be reduced in the group of mice (4 mice) treated with MBP-calreticulin or MBP-vasostatin (both at the dose of 25 μg/mouse) compared with the control group (5 mice) treated with MBP (12.5 μg/mouse), but the difference did not achieve overall significance (P = .089), in part because of the small sample size. Growth rates were similar in the 2 groups of mice treated with either MBP-calreticulin or MBP-vasostatin. All tumors were removed on day 41 of treatment. The mean (±SD) weight of tumors in the control group (5.55 ± 2.93 g) was greater than the weight of tumors from mice treated with either MBP-calreticulin (2.74 ± 0.95 g) or MBP-vasostatin (3.01 ± 1.22 g).
In an additional experiment (Fig 8C), we tested MBP–Δ120-calreticulin (30 μg/mouse) for its ability to prevent Burkitt tumor growth and compared its effects with those of MBP-vasostatin (30 μg/mouse). After 14 days of treatment, all mice (8 of 8) inoculated with control MBP (20 μg/mouse) developed a tumor. By contrast, only 3 of 9 mice inoculated with MBP–Δ120-calreticulin, and 3 of 9 mice inoculated with MBP-vasostatin developed a tumor (P = .009). After treatment was suspended on day 14, the mice were observed. One mouse from the group treated with MBP–Δ120-calreticulin and one mouse from the group treated with MBP-vasostatin remain tumor free as of day 60. Thus, similar to vasostatin, MBP–Δ120-calreticulin can prevent Burkitt tumor growth.
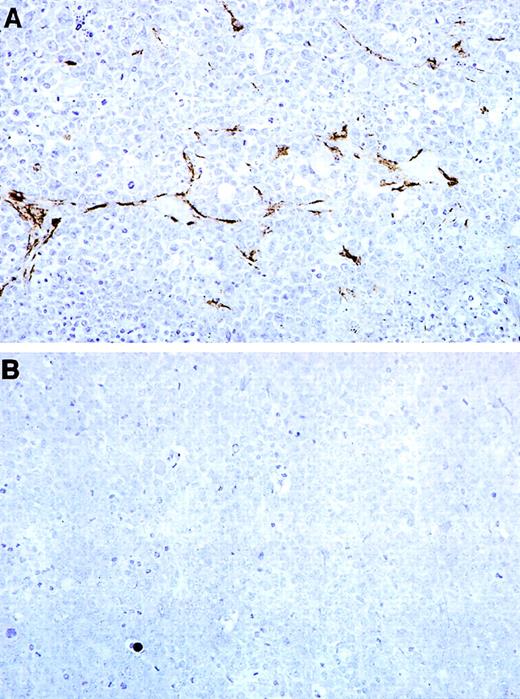
The histology of Burkitt tumors treated with MBP-calreticulin (Fig9), MBP–Δ120-calreticulin (not shown), and MBP-vasostatin20 was indistinguishable from controls with respect to the morphology of the tumor cells and the number of tumor cell mitoses. Immunohistochemical staining of vessels using antibodies against smooth muscle actin (Fig 9) and factor VIII-related antigen (not shown) revealed that Burkitt tumors treated with MBP-calreticulin displayed fewer vessels than control tumors. To quantitatively assess tumor vascularization, we counted all visible microvessels at a magnification of 200 (ie, 0.74 mm2/field) in tumor tissue sections. In 3 in vivo experiments, the number of intratumoral and peritumoral microvessels was significantly (P < .05) reduced in animals treated with GST-calreticulin, MBP-calreticulin, MBP–Δ120-calreticulin, or MBP-vasostatin compared with controls. Occasionally, calreticulin-treated tumors displayed vascular alterations, including medial thickening, fibrinoid necrosis and neutrophil infiltration of the vessel wall that were not noted in control tissues (not shown). These vascular alterations were not different from those noted in vasostatin20 or MBP–Δ120-calreticulin (not shown) treated mice. Similar to our previous findings in vasostatin-treated animals,20 gross and histological examination of liver, spleen, kidneys, heart, lung, and lymph nodes from mice treated with either calreticulin or MBP–Δ120-calreticulin detected no abnormalities.
Immunohistochemical detection of the tumor vasculature in representative Burkitt tumors treated with MBP or MBP-calreticulin. Paraffin-embedded tumor sections were stained with mouse anti-alpha smooth muscle actin monoclonal antibody and counterstained with hematoxylin. (A) Representative tumor tissue from a mouse treated with MBP alone. (B) Representative tumor tissue from mouse treated with MBP-calreticulin (original magnification ×20).
Immunohistochemical detection of the tumor vasculature in representative Burkitt tumors treated with MBP or MBP-calreticulin. Paraffin-embedded tumor sections were stained with mouse anti-alpha smooth muscle actin monoclonal antibody and counterstained with hematoxylin. (A) Representative tumor tissue from a mouse treated with MBP alone. (B) Representative tumor tissue from mouse treated with MBP-calreticulin (original magnification ×20).
DISCUSSION
Recently, we have reported that vasostatin, the N-domain of calreticulin composed of amino acids 1 to 180, can inhibit endothelial cell proliferation, angiogenesis and tumor growth.20 Here we show that full-length calreticulin, a protein of 417 amino acids deduced from its cDNA, a deletion fragment of calreticulin lacking the N-terminal 1-120 amino acids (Δ120 calreticulin), and an internal calreticulin fragment composed of amino acids 120-180 can inhibit endothelial cell growth in vitro. When compared with vasostatin for their ability to inhibit endothelial cell growth in vitro, calreticulin, Δ120 calreticulin, and the 120-180 calreticulin fragment inhibited endothelial cell growth to a similar degree at similar concentrations. Like vasostatin, calreticulin, Δ120 calreticulin, and the 120-180 calreticulin fragment inhibited endothelial cell growth specifically and directly, without a requirement for other cell types or extracellular matrix proteins and with minimal effects on cell viability. When compared in Matrigel-based angiogenesis assays, calreticulin, the 120-180 calreticulin fragment and vasostatin displayed similar inhibition. Like vasostatin, calreticulin and Δ120 calreticulin could prevent or delay significantly the development of Burkitt lymphoma. The similarities of biological activities displayed by calreticulin, vasostatin, Δ120 calreticulin, and the 120-180 calreticulin fragment in endothelial cell proliferation and neovascularization assays suggest that the antiangiogenic activity of calreticulin resides in a domain within amino acids 120-180.
Calreticulin, a highly conserved and ubiquitous protein, serves as one of the major storage depots for calcium within the endoplasmic reticulum and participates in calcium signaling.29,30Structure-function analyses have distinguished the molecule in 3 domains. The highly acidic C-domain contains the KDEL signal sequence for calreticulin retention in the endoplasmic reticulum and binds calcium with high capacity.31 It can also associate with the blood clotting factors IX, X, and prothrombin.32 The proline-rich P domain has extensive sequence homology to calnexin, a protein of the endoplasmic reticulum proposed to serve as a chaperon, and contains the high-affinity, calcium-binding site of calreticulin.33 The N-domain, the most conserved domain among calreticulins cloned so far, does not bind calcium but can bind the cytoplasmic domain of alpha subunits of integrins and the nuclear receptors for glucocorticoid, androgen, and retinoic acid.34-36 It also binds C1q, the recognition subunit of the first component of the classical complement pathway.37Additional activities of the N-domain of calreticulin that we have recently recognized include inhibition of angiogenesis and tumor growth.20 Here we show that full-length calreticulin, Δ120 calreticulin, and an internal calreticulin fragment composed of amino acids 120 to 180 can also inhibit endothelial cell growth, angiogenesis, or tumor growth.
A number of previously identified inhibitors of angiogenesis are fragments of larger proteins, which are not themselves active as angiogenesis inhibitors. An internal 16-kD fragment of prolactin was reported to be an inhibitor of angiogenesis, but prolactin was found to be inactive.5 Similarly, 2 heparin-binding fragments of fibronectin inhibited endothelial cell growth, whereas intact fibronectin did not.21 Angiostatin, a fragment of plasminogen, and endostatin, a fragment of collagen XVIII, are previously recognized angiogenesis inhibitors. However, neither plasminogen nor collagen XVIII were found to be active or to interfere with the inhibitory action of their internal fragments.9,13In contrast to these angiogenesis inhibitors, thrombospondin, a high molecular weight extracellular matrix glycoprotein, displayed antiangiogenic activity, as did some of its internal fragments.38
Like vasostatin, calreticulin inhibited endothelial cell proliferation directly and specifically, raising the possibility that it may bind to a receptor on endothelial cells. Previously, calreticulin was reported to bind specifically and reversibly to endothelial cells in vitro with a kd of approximately 7.4 nmol/L.32Endothelial cells were reported to release nitric oxide in response to calreticulin.32 When injected in mice, calreticulin localized selectively to the vascular endothelium, particularly in the lungs.32 We do not know the mechanism by which calreticulin or vasostatin inhibit angiogenesis and tumor growth. Although nitric oxide was reported to suppress endothelial cell migration and angiogenesis,39 40 we have found no evidence that nitric oxide release mediates the effects of calreticulin or vasostatin based on in vitro experiments with inhibitors of nitric oxide synthase (not shown). In addition, we have found no evidence that C1q binding is important to the antiangiogenic effects of calreticulin and vasostatin, because C1q was not measurable in endothelial cell cultures (not shown). We believe, however, that inhibition of endothelial cell growth is central to the inhibition of angiogenesis and tumor growth induced by calreticulin and vasostatin.
We do not know whether calreticulin and its fragments are effective inhibitors of all types of angiogenic endothelial cells or the full spectrum of tumors that are inhibited by these agents. The observation that full-length calreticulin, vasostatin, Δ120 calreticulin, and the 120-180 calreticulin fragment are all active inhibitors of endothelial cell growth suggests that the active site resides within a 60-amino acid internal fragment of calreticulin between amino acids 120-180. This observation raises the possibility that we may be able to identify active fragments of calreticulin even smaller than vasostatin or the 61 amino acid internal calreticulin peptide that might be biologically active as angiogenesis inhibitors. These molecules may be easier to produce and deliver. There is considerable enthusiasm for the potential application of inhibitors of angiogenesis to cancer treatment.41 Calreticulin, Δ120 calreticulin, and vasostatin hold promise as novel therapeutics for the treatment of pathological angiogenesis in cancer and many other diseases.
ACKNOWLEDGMENT
We thank Drs Greg Pogue, Rob Duncan, Andrea Tenner, Malcolm Moos, Bob Boykins, Venkatesha Basrur, Hynda Kleinman, Doug Roberts, Robert Yarchoan, and Yoshi Aoki for their help in various aspects of this work.
S.E.P. and L.Y. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
This is a US government work. There are no restrictions on its use.
REFERENCES
Author notes
Address reprint requests to Giovanna Tosato, MD, Center for Biologics Evaluation and Research, 1401 Rockville Pike, Rockville, MD 20852; e-mail: Tosato@A1.CBER.FDA.GOV.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal