In adult bone marrow, hematopoietic stem cells are found in close association with distinctive stromal cell elements. This association is necessary for maintenance of hematopoiesis, but the precise mechanisms underlying the cross-talk between stromal cells and hematopoietic stem cells are poorly understood. In this study, we used a bone marrow stromal cell line (MS-5) that is able to support human long-term hematopoiesis. This hematopoietic-promoting activity cannot be related to expression of known cytokines and is abolished by addition of hydrocortisone. Using a gene trap strategy that selects genes encoding transmembrane or secreted proteins expressed by MS-5 cells, we obtained several insertions that produced fusion proteins. In one clone, fusion protein activity was downregulated in the presence of hydrocortisone, and we show that insertion of the trap vector has occurred into the neuropilin-1 gene. Neuropilin-1 is expressed in MS-5 cells, in other hematopoietic-supporting cell lines, and in primary stromal cells but not in primitive hematopoietic cells. We show that neuropilin-1 acts as a functional cell-surface receptor in MS-5 cells. Two neuropilin-1 ligands, semaphorin III and VEGF 165, can bind to these cells, and the addition of VEGF 165 to MS-5 cells increases expression of 2 cytokines known to regulate early hematopoiesis, Tpo and Flt3-L. Finally, we show that stromal cells and immature hematopoietic cells express different neuropilin-1 ligands. We propose that neuropilin-1 may act as a novel receptor on stromal cells by mediating interactions between stroma and primitive hematopoietic cells.
DURING ADULT HEMATOPOIESIS, proliferation and differentiation of hematopoietic progenitors occur within the bone marrow. Stromal cells, which form the backbone of the bone marrow microenvironment, consist of myofibroblasts, endothelial cells, adipocytes, osteoclasts, and macrophages. These cells provide a complex array of extracellular matrix proteins that facilitate cell-cell interactions. In addition, stromal cells are instrumental in providing various soluble or resident cytokines for the controlled differentiation and proliferation of early hematopoietic progenitors. Molecules mediating interactions between hematopoietic cells and stromal cells (adhesion molecules, cytokines, and their receptors) are either transmembrane or secreted proteins.
Long-term bone marrow cultures have been developed to reproduce the bone marrow microenvironment in vitro. In these cultures, maintenance of hematopoiesis requires the prior formation of an adherent layer whose cellular complexity prevents the understanding of the relationship between stromal cells and hematopoietic cells.1,2 Some murine stromal cell lines have been shown to support growth and differentiation of human immature cells. One of them, the murine MS-5 cell line, was derived from the irradiated adherent layer of a Dexter-type long-term culture.3 MS-5 promotes the expansion of human hematopoietic cells selected for their high expression of CD34 antigen and lack of expression of CD38 antigen (CD34+/CD38−) for 5 to 10 weeks and without the addition of human growth factors.4 Futhermore, MS-5 cells also support the B-lymphoid potential of human CD34+/CD38− cells.5 No combination of murine cytokines, which are active on human cells, was able to mimic the hematopoietic-promoting activity of MS-5.6 Finally, addition of hydrocortisone in the coculture inhibits the hematopoietic-promoting activity of MS-5 cells,7 and exogenous supply of growth factors cannot reverse this effect. Altogether, these data strongly suggest that hydrocortisone may downregulate an MS-5–derived activity which is necessary for proliferation or differentiation of CD34+/CD38− cells or, alternatively, upregulate an MS-5–derived inhibitory activity.
In this study, we used a gene-trap strategy designed to clone secreted and transmembrane molecules expressed by MS-5 cells, and we have selected insertions that produced fusion proteins whose expression was modulated by hydrocortisone. We obtained 10 independent clones in which fusion protein activity was downregulated by hydrocortisone. In one of these clones, vector insertion has occurred into the neuropilin-1 gene. We show that neuropilin-1 acts as a functional receptor on stromal cells and that neuropilin-1 ligands are differentially expressed in stromal cells and in immature hematopoietic cells.
MATERIALS AND METHODS
Cell Lines
Stromal MS-5, 30E, 30W, and S17 cell lines.
These murine bone marrow–derived cell lines were maintained in α Modified Eagle’s Medium (αMEM) supplemented with 10% fetal calf serum (FCS; purchased from Stem Cell Technologies, Vancouver, Canada). Hydrocortisone (10−6 mol/L, hydrocortisone 21-hemisuccinate; Sigma, St Louis, MO) was added when indicated. 30E and 30W cells were kindly provided by Dr D. Rennick (DNAX Institute, Palo Alto, CA), MS-5 cells by Dr K. Mori (Niigata University, Niigata, Japan), and S17 cells by Dr A. Cumano (Pasteur Institute, Paris, France).
Chinese hamster ovary (CHO) cells.
Xylosyltransferase-deficient pgsA-745 CHO cells were cultured in DMEM-F12 medium supplemented with 10% FCS. Cells were transfected with 10 μg of expression vector carrying either the rat NP-1 cDNA8 or the human KDR cDNA and 1 μg of pSV2 expression vector carrying the neomycin resistance gene with the lipofectin reagent (GIBCO, Ceigy Pontoise, France). The KDR cDNA we used consists of coding sequences from position 1 to 24779and encodes a receptor that lacks the kinase domain. Stable integrants were selected using 500 μg/mL G418 (GIBCO). Cloning was performed by colony isolation using a Pasteur pipette and clones were further screened for their ability to specifically bind iodinated VEGF.10
293 cells.
The semaphorin III-AP secreting cell line was previously described.8
Human umbilical vein endothelial cells (HUVEC).
HUVEC were cultured as described.11 For proliferation assays, 10,000 HUVEC were seeded on gelatin-coated 12 multiwell plates in SFM medium (GIBCO) supplemented with 10% fetal calf serum.
Bone Marrow–Derived Stromal Cells and Hematopoietic Progenitors
Human bone marrow samples were obtained from patients undergoing hip surgery. Cells were extracted from the bone fragments as previously described.7
Culture of human marrow adherent cells.
Long-term bone marrow cultures were established in a mixture of FCS and horse serum and 10−6 mol/L hydrocortisone.7 After 4 to 6 weeks in culture at 33°C, nonadherent cells were discarded and adherent cells were detached with trypsin.
Selective culture of human fibroblasts.
To grow selectively fibroblastic cells, bone marrow cells were incubated in αMEM with 10% FCS (but without horse serum) and hydrocortisone in 25-cm2 flasks at a concentration of 106 cells/mL. After 24 hours, nonadherent cells were removed and fresh medium was added. After 3 to 4 weeks of culture at 37°C, cells were subcultured and collected after 3 passages as previously described.12 At this time, adherent cells were devoid of endothelial cells because no expression of von Willebrand factor could be detected by reverse transcription-polymerase chain reaction (RT-PCR) (data not shown).
Isolation of CD34+/CD38− cells.
Low-density mononuclear cells were subjected to a standard CD34 immunomagnetic bead separation using the miniMACS system following the manufacturer’s guidelines (Miltenyi Biotec, Auburn, CA). CD34+ cells obtained after bead separation were further purified by cell sorting either immediately or after overnight incubation at 4°C in high serum concentration. Before sorting, CD34+ cells were incubated with a 1/5 dilution of a Cy-5-phycoerythrin (PE)-anti-CD34 monoclonal antibody (MoAb) (Immunotech, Marseille, France) and PE-anti CD38 MoAb (Becton Dickinson, San Jose, CA). Sorting of CD34+CD38low fractions was performed using a FACS-Vantage equipped with an argon ion laser (Innova 70-4-Coherent radiation; Innova, Palo Alto, CA) tuned to 488 nm and operating at 500 mW. A morphological gate including all the CD34+ cells was determined on 2-parameter histogram side scatter (SSC) versus forward scatter (FSC). Limit for CD38low cells was defined using control cells labeled with PE-CD34 (HPCA2; Becton Dickinson) and an irrelevant IgG1 MoAb. CD38low represented 10% to 15% of total CD34high cells. Compensation was set up as described above.
Culture of murine marrow adherent cells.
Bone marrow from C56Bl mice was obtained by flushing cells from 1 tibia and 1 femur. Undissociated cells were grown in a 25-cm2flask in LTC medium (purchased from Stem Cell Technologies) with 10−6 mol/L hydrocortisone. At weekly intervals, half of the medium containing nonadherent cells was collected and replaced by fresh medium. After 5 weeks, adherent cells were detached with trypsin.
Gene Trap Strategy
Transfection and selection conditions.
The gene-trap vector pGT1.8TM, provided by Dr William Skarnes (University of California, Berkeley), was introduced into MS-5 cells using Lipofectamine reagent (GIBCO) following the manufacturer’s instructions. A total of 108 cells divided in 50 100-mm Petri dishes were transfected with 600 μg of linearized plasmid DNA. After 3 days, the content of each Petri dish was split up into 5 dishes and cells were selected for resistance to G418 at a concentration of 250 μg/mL for 15 days. After G418 selection, 750 clones were tested for β-galactosidase activity.
For in situ staining, cells were washed in phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde for 10 minutes, and stained with X-gal solution (4 mmol/L K4 [Fe (CN)6]; 4 mmol/L K3 [Fe(CN)6]; 4 mmol/L MgCl2; 0.4 mg/mL of Xgal [Amersham, Pharmacia Biotech, Orsay, France] in PBS). For quantitative assays, MS-5 cells grown in 100-mm Petri dishes were collected, washed, and lysed in 100 μL of 250 mmol/L Tris pH 7.8, 0.2% TritonX-100, 5 mmol/L dithiothreitol (DTT), 10% glycerol. Thirty microliters of cell lysate was assayed with o-nitrophenyl-β-D-galactopyranoside (Pharmacia) as previously described.13
5′ RACE.
Total RNA from MS-5 clones was prepared using Trizol reagent (GIBCO). Cloning of 5′ cDNA ends was performed using the 5′ RACE system (GIBCO). First-strand cDNA was reverse transcribed in a volume of 25 μL where 1 μg of total RNA was annealed to 2.5 pmol of oligonucleotide 1 (5′ CCA GAA CCA GCA AAC TGA AGG G 3′, CD4 sequences located 364 bp from the splice site), as previously described.14 PCR of dC-tailed cDNA was performed using the Abridged Anchor Primer (AAP) from the kit and oligonucleotide 2 (5′ AGT AGA CTT CTG CAC AGA CAC C 3′, CD4 sequences located 281 bp from the splice site). This primary PCR product was diluted 500-fold following the manufacturer’s instructions and used as template in a nested amplification using the Abridged Universal Amplification Primer (AUAP) and oligonucleotide 3 (5′ TGC TCT GTC AGG TAC CTG TTG G 3′, engrailed-2 sequences located 130 bp from the splice site). PCR products were cloned in pCRII vector (Invitrogen, Carlsbad, CA) for sequencing.
RT-PCR Analysis
First-strand cDNA was reverse-transcribed in a volume of 30 μL. One microgram of total RNA was annealed to 300 ng of desoxyhexanucleosides [pd(N)6; Pharmacia] and incubated for 1 hour at 37°C with Superscript II (GIBCO) in buffer supplied by the manufacturer. One microliter of RT product was used as template in a 50-μL PCR reaction containing 100 ng of each oligonucleotide and 2.5 U of AmpliTaqGold (Perkin Elmer). Reactions were performed in a 2400 Gene Amp PCR System (Perkin Elmer) under the following conditions: 10 minutes at 94°C, 30 to 40 cycles composed of 10 seconds at 94°C, 30 seconds at annealing temperature, and 30 seconds at 72°C, and, finally, 7 minutes at 72°C.
Primer sequences are the following. Mouse neuropilin-1 forward primer 5′GAA GTT TAT GGC TGC AAG 3′ and reverse primer 5′ CTT CTC TGT GGC CAG GAC 3′ (35 cycles, annealing at 50°C). Human neuropilin-1 forward primer 5′ ACG ATG AAT GTG GCG ATA CT 3′ and reverse primer 5′ AGT GCA TTC AAG GCT GTT GG 3′ (35 cycles, annealing at 50°C). S14forward primer 5′ GGC AGA CCG AGA TGA ATC CTC A 3′ and reverse primer 5′ CAG GTC CAG GGG TCT TGG TCC 3′ (30 cycles, annealing at 64°C). HPRT forward primer 5′ GCT GGT GAA AAG GAC CTC T 3′ and reverse primer 5′ CAC AGG ACT AGA ACA CCT GC 3′ (30 cycles, annealing at 50°C).Human semaphorin III forward primer 5′ ACT CAC TGT TCA GAC TTA C 3′ and reverse primer 5′ GAG CTG CAT GAA GTC TCT 3′ (35 cycles, annealing at 50°C). Human semaphorin IV forward primer 5′ GCG CAT GAA GTT GAT CAC 3′ and reverse primer 5′ ACC AGT GGA TGC CCT TCT 3′ (40 cycles, annealing at 54°C). Human semaphorin V forward primer 5′ CAA CTG GGC AGG GAA GGA CAT 3′ and reverse primer 5′ CGT CTG GGT TCT CGC TCT CCG 3′ (35 cycles, annealing at 60°C). Human and murine VEGF165 isoform forward primer 5′ TGG TCC CAG GCT GCA CCC A 3′ and reverse 5′ AAC AAA TGC TTT CTC CGC TCT G 3′ (40 cycles annealing at 60°C).Human and murine semaphorin E forward primer 5′ GCA AAA TGG CTG GCA AAG ATC C 3′ and reverse primer 5′ CCC ATG AAA TCT ATA TAC ATT CC 3′ (35 cycles, annealing at 60°C).Murine semaphorin III forward primer 5′ GGT CCC AAC TAT CAG TGG 3′ and reverse primer 5′ GGC ACA GTA AGG GTC CCG 3′. Murine semaphorin IV forward primer 5′ TGT GGG CAG CAA GGA CTA CG 3′ and reverse primer 5′ GGT AGA AGA TGT AAT CCT GTG C 3′ (35 cycles, annealing at 60°C). Murine semaphorin V forward primer 5′ GCA ACT GGG CAG GGA AGG A 3′ and reverse primer 5′ CGC ACA TCG TTC ATG CTG TAC 3′ (35 cycles, annealing at 60°C). Murine Tpo forward primer 5′ ACT TTA GCC TGG GAG AAT GGA AA 3′ and reverse primer 5′ AGG AGT AAT CTT GAC TGT GAA TC 3′ (32 cycles, annealing at 50°C). Murine Flt3-ligand forward primer 5′ AGT TGA CTG ACC ACC TGC TT 3′ and reverse primer 5′ AGG AGT AAT CTT GAC TGT GAA TC 3′ (36 cycles, annealing at 58°C).
Binding Experiments
Semaphorin III-AP.
MS-5 cells were cultured in 6-well plates. Subconfluent cells (5 × 105) were treated for 90 minutes with concentrated conditioned medium containing semaphorin III-AP. After incubation, cells were processed for AP chemistry.8 The cell content of 1 well was assayed in 1 mL. For competition assays, cells were incubated with conditionned medium containing 5 ng/mL of semaphorin III-AP and various dilutions of purified VEGF 165.
VEGF165.
Recombinant human VEGF (VEGF 165) was synthesized in Sf9 cells infected with a recombinant baculovirus containing the VEGF 165 cDNA. Native VEGF 165 was further cleaved by plasmin as already described10 and further purified by heparin affinity chromatography.15 The 110-amino acid (aa) fragment (V110) corresponding to the N-terminus was contained in the flow-through and the 55-aa carboxy-terminal fragment (V55) was eluted with 0.5 mol/L NaCl, whereas uncleaved VEGF was collected with 0.65 mol/L NaCl.
Four micrograms of VEGF was iodinated by the iodogen procedure according to Jonca et al.10 The specific activity averaged 200,000 cpm/ng. Cell cultures (106 cells per dish) were washed twice in ice-cold binding medium (DMEM-20 mmol/L HEPES, 1 mg/mL pH 7.3-gelatin, 0.5 μg/mL heparin) and incubated in the same medium with 0.5 ng/mL 125I-VEGF and various dilutions of VEGF 165, V110 and V55. The binding was performed for 2 hours at 4°C with gentle agitation. After 2 washes in PBS, the cell monolayers were dissolved with 0.2 mol/L NaOH and the radioactivity counted in a gamma counter.
For cross-linking experiments, the procedure was similar except that concentration of 125I-VEGF was 5 ng/mL and concentration of unlabeled VEGF 165, V55, and semaphorin III-AP was 1 μg/mL. Cross-linking was performed with 0.15 mmol/L disuccinimidyl suberate (DSS; Pierce, Rockford, IL) in PBS for 15 minutes at room temperature and stopped with 0.2 mol/L glycin pH 7.4. MS-5 cell pellets were resuspended in (0.1% sodium dodecyl sulfate [SDS], 0.3 mol/L NaCl, 10 mmol/L EDTA, 0.02 mol/L Tris-HCl pH 7.4, 1% Triton-X100, 0.05% Tween-20), centrifuged, and further incubated with 2 μg/mL of an antibody directed against the cytoplasmic domain of neuropilin-1 (a gift of Drs Zhigang He and Marc Tessier-Lavigne, University of California, San Francisco). The immunocomplexes were collected using protein A-Sepharose beads (Amersham, Pharmacia Biotech), washed, and dissolved in 1X sample buffer (Tris-HCl pH 6.9, 0.1 mol/L; SDS 0.2%; glycerol 10%). The CHO pgs A-745 cell pellets were directly dissolved in 1X sample buffer. Proteins were resolved on a 7% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Gels were stained with Coomassie blue, dried, and analyzed on a PhosphorImager (Amersham, Pharmacia Biotech).
RESULTS
A Gene Trap Approach to Identify Genes Encoding Transmembrane or Secreted Proteins Expressed by MS-5 Cells
To identify membrane or secreted proteins involved in MS-5 function, we used an original gene trap strategy called “secretory trap.”16 The pGT1.8TM gene trap vector (Fig 1) contains the CD4 transmembrane domain fused to βgeo, a chimeric protein that possesses both β-galactosidase (β-gal) and neomycin phosphotransferase (neoR) activities. This coding sequence is preceded by a splice acceptor cassette; insertion into an intron is predicted to generate a fusion mRNA which may, in some cases, code for a fusion protein containing βgeo at its C-terminus. In protein fusions that lack a signal peptide, the CD4 transmembrane domain acts as a signal anchor sequence that exposes βgeo to the lumen of the endoplasmic reticulum where β-galactosidase activity, but not neomycin phosphotransferase activity, is lost. In fusions that contain a signal peptide, β-galactosidase activity can be detected, presumably because βgeo does not enter the endoplasmic reticulum.16
Structure of the secretory trap vector, pGT1.8TM. En-2, murine engrailed-2 sequences; SA, splice acceptor site; TM, transmembrane domain; βgeo, fusion between LacZ and neomycin phosphotransferase sequences; polyA, polyadenylation signal.
Structure of the secretory trap vector, pGT1.8TM. En-2, murine engrailed-2 sequences; SA, splice acceptor site; TM, transmembrane domain; βgeo, fusion between LacZ and neomycin phosphotransferase sequences; polyA, polyadenylation signal.
We have transfected the pGT1.8TM plasmid into MS-5 cells, selected G418-resistant clones for 10 to 15 days, and stained purified clones for β-galactosidase activity. Thirty-two of a total of 750 clones selected for G418 resistance were stained blue with X-gal. β-Galactosidase activity was then measured in the absence and in the presence of hydrocortisone. In these conditions, 20 clones showed a constitutive activity of β-galactosidase, 2 clones an upregulated activity, and 10 clones a downregulated activity.
Gene Trap Insertion Into the Neuropilin-1 Gene
We focused our attention on the clones for which β-galactosidase was downregulated by hydrocortisone. Endogenous trapped sequences were cloned from the fusion transcripts using the 5′ RACE method. cDNA fragments derived from the fusion transcripts were cloned and sequenced. In the 15D3 clone, for which β-galactosidase activity was downregulated 2-fold in the presence of hydrocortisone (Fig 2), the sequence of the 5′ RACE product revealed an insertion into the coding region of the neuropilin-1 gene that codes for a type I cell-surface glycoprotein.17Sequence analysis showed a fusion to the neuropilin-1 coding sequence after the codon 642 (Fig 3A). The structure of the 15D3 cDNA was surprising. Fifty base pairs of vector DNA were found between the En-2 sequences and the neuropilin-1 sequences. These 3 sequences formed an in-frame fusion transcript (Fig 3B). Because only 1 band could be detected in Northern blot analysis using a LacZ probe (data not shown), neuropilin-1-βgeo must be the unique fusion transcript produced by the 15D3 clone.
β-Galactosidase activity is downregulated by hydrocortisone in 15D3 cells. Confluent 15D3 cells were grown in the absence (top panel) or in the presence (bottom panel) of 10−6 mol/L hydrocortisone. After 48 hours, cells were fixed with paraformaldehyde and stained for β-galactosidase activity.
β-Galactosidase activity is downregulated by hydrocortisone in 15D3 cells. Confluent 15D3 cells were grown in the absence (top panel) or in the presence (bottom panel) of 10−6 mol/L hydrocortisone. After 48 hours, cells were fixed with paraformaldehyde and stained for β-galactosidase activity.
Characterization of the fusion cDNA produced by 15D3 cells. (A) Predicted structure of the mutant fusion protein. The neuropilin-1 protein and the mutant protein are shown. SS, signal sequence; TM, transmembrane domain. a1, a2, b1, b2, and MAM are previously described neuropilin-1 sub-domains.42 (B) Portion of the sequence obtained by 5′ RACE, displaying the fusion between neuropilin-1 and gene trap vector sequences. 642 is the last codon of neuropilin-1 sequences and 220 is the first codon of En-2 sequences present in the trap vector.
Characterization of the fusion cDNA produced by 15D3 cells. (A) Predicted structure of the mutant fusion protein. The neuropilin-1 protein and the mutant protein are shown. SS, signal sequence; TM, transmembrane domain. a1, a2, b1, b2, and MAM are previously described neuropilin-1 sub-domains.42 (B) Portion of the sequence obtained by 5′ RACE, displaying the fusion between neuropilin-1 and gene trap vector sequences. 642 is the last codon of neuropilin-1 sequences and 220 is the first codon of En-2 sequences present in the trap vector.
The Neuropilin-1 Gene Is Expressed in Bone Marrow Adherent Cells But Not in Immature Hematopoietic Cells
Reports of neuropilin-1 expression have been limited predominantly to the nervous system of the developing embryo and, more recently, to some adult tissues.18 Because no data about its expression in hematopoietic and stromal cells have been reported so far, we performed RT-PCR assays. In addition to MS-5, neuropilin-1 transcripts were found in the murine 30E, 30W, and S17 stromal cell lines (Fig 4), which are derived from long-term bone marrow cultures19 but barely detectable in NIH3T3 cells (not shown). Neuropilin-1 was also expressed in both human and murine marrow adherent cells (Fig 4, lanes marked Hu Adh and Mo Adh), and also in cultures of human bone marrow–derived fibroblasts that are devoid of endothelial cells (Fig 4, lane marked Fibro). Interestingly, neuropilin-1 expression could not be detected in human primitive hematopoietic cells sorted on the basis of their high expression of CD34 and low expression of CD38 (Fig 4, lane 34+/38−).
Expression pattern of the neuropilin-1 gene. RT-PCRs were performed using total RNA isolated from stromal cell lines (MS-5, 30E, 30W, and S17), adherent layers of long-term murine and human cultures (Mo Adh and Hu Adh), human fibroblasts (Fibro), and human CD34+/CD38− (34+/38−) hematopoietic primitive cells. PCR products are 450 bp for human neuropilin-1 and 635 bp for murine neuropilin-1. As a control, amplification of RT products with either S14 primers (human samples) or HPRT primers (murine samples) is shown.
Expression pattern of the neuropilin-1 gene. RT-PCRs were performed using total RNA isolated from stromal cell lines (MS-5, 30E, 30W, and S17), adherent layers of long-term murine and human cultures (Mo Adh and Hu Adh), human fibroblasts (Fibro), and human CD34+/CD38− (34+/38−) hematopoietic primitive cells. PCR products are 450 bp for human neuropilin-1 and 635 bp for murine neuropilin-1. As a control, amplification of RT products with either S14 primers (human samples) or HPRT primers (murine samples) is shown.
Semaphorin III Binding to MS-5 Cells Is Displaced by VEGF 165
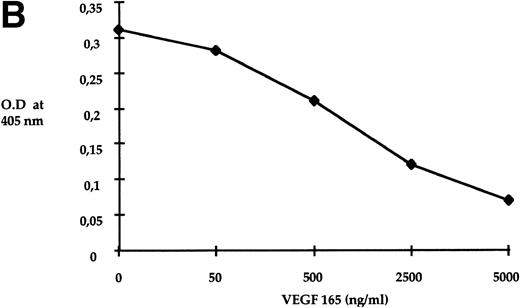
Neuropilin-1 was first described as a semaphorin III receptor.8,20 To ensure that neuropilin-1 acts as a cell-surface receptor in MS-5 cells, we first tested the binding of semaphorin III to MS-5 cells. The coding region of human semaphorin III was fused to that of alcaline phosphatase (AP), and the resulting chimeric protein (sema III-AP) was expressed in human embryonic kidney 293 cells.8 Conditioned medium from these cells was applied to MS-5 cells and AP activity was measured. The binding affinity of sema III-AP to MS-5 cells was measured in equilibrium binding experiments, based on the relative amounts of AP activity in the supernatant and bound to cells. Sema III-AP binding to MS-5 cells increased in a dose-dependent manner and reached saturation at approximately 100 ng/mL (data not shown). Scatchard analysis of sema III-AP binding showed a single class of semaphorin III binding sites with a kd of 125 pmol/L and 2,500 binding sites per cell (Fig 5A). The value for the dissociation constant for the interaction of semaphorin III with neuropilin-1 has been previously estimated at 325 pmol/L8 or 1,500 pmol/L.20
Semaphorin III binding to MS-5 cells. Sub-confluent MS-5 cells were treated for 90 minutes with different concentrations of semaIII-AP. Then, AP activity from bound semaIII-AP was measured colorimetrically (see Materials and Methods for details). Specific binding was determined by substraction of values obtained from binding to MS-5 cells and to COS cells. COS cells do not express neuropilin-1 and are unable to bind semaIII-AP.8 (A) Scatchard’s analysis. The values shown are an average of 3 different assays. Linear regression analysis of values showed that MS-5 cells express 2,500 binding sites per cell and bind Semaphorin III with a kd of 1.2 × 10−10 mol/L. (B) MS-5 cells were incubated with concentrated conditioned medium containing 5 ng/mL of semaIII-AP in the absence or in the presence of indicated concentrations of VEGF 165. Values shown are an average of 2 different assays. Reaction time for AP activity detection was 3 hours.
Semaphorin III binding to MS-5 cells. Sub-confluent MS-5 cells were treated for 90 minutes with different concentrations of semaIII-AP. Then, AP activity from bound semaIII-AP was measured colorimetrically (see Materials and Methods for details). Specific binding was determined by substraction of values obtained from binding to MS-5 cells and to COS cells. COS cells do not express neuropilin-1 and are unable to bind semaIII-AP.8 (A) Scatchard’s analysis. The values shown are an average of 3 different assays. Linear regression analysis of values showed that MS-5 cells express 2,500 binding sites per cell and bind Semaphorin III with a kd of 1.2 × 10−10 mol/L. (B) MS-5 cells were incubated with concentrated conditioned medium containing 5 ng/mL of semaIII-AP in the absence or in the presence of indicated concentrations of VEGF 165. Values shown are an average of 2 different assays. Reaction time for AP activity detection was 3 hours.
As VEGF 165 was recently described as a neuropilin-1 ligand, we tested whether VEGF 165 was able to displace sema III-AP binding to MS-5 cells. MS-5 cells were incubated with conditioned medium containing 5 ng/mL of sema III-AP in the absence or in the presence of purified VEGF 165. As shown in Fig 5B, sema III-AP binding was progressively displaced in the presence of increasing concentrations of VEGF 165. These results indicate that semaphorin III can bind to MS-5 cells and that this binding can be competed by VEGF 165.
Neuropilin-1 Is a VEGF 165 Receptor on MS-5 Cells
The active form of VEGF is a homodimer whose activities are mediated on endothelial cells by 2 specific tyrosine kinases receptors, the fms-like tyrosine kinase Flt-1 and the kinase insert domain-containing receptor KDR/Flk-1. Cell-surface receptor cross-linking experiments with 125I-VEGF have shown that additional VEGF receptors may exist that are neither KDR or Flt-1. Recently, a 130- to 135-kD VEGF receptor was identified as neuropilin-1.18 A striking feature of neuropilin-1 is that it binds VEGF 165 but not VEGF 121.
The structural difference between VEGF 165 and VEGF 121 is that VEGF 165 contains 44 additional aa encoded by exon 7 of the VEGF gene. A fusion protein containing the exon 7–encoded domain of VEGF 165 binds to neuropilin-1 directly and is able to compete for VEGF 165 binding.21 Thus, VEGF 165 binding to neuropilin-1 occurs through the VEGF 165 exon 7–encoded domain. By contrast, KDR and Flt-1 interact with domains of VEGF 165 that are encoded by exons 4 and 3, respectively.
MS-5 cells bound 125I-VEGF 165 in a saturable fashion. Scatchard’s analysis showed the presence of a single class of binding sites and a dissociation constant of 120 pmol/L (data not shown). To test the specificity of VEGF 165 binding, 125I-VEGF 165 was bound to MS-5 cells in the presence of an increasing excess of nonlabeled VEGF 165 proteolytic fragments. Both native VEGF 165 and V55, which consists of a peptide encoded by exons 7 and 8, competed VEGF 165 binding to MS-5 cells while V110, which consists of a peptide encoded by exons 1 to 5, was unable to displace VEGF 165 binding (Fig6A). Therefore, VEGF 165 binding by MS-5 cells is unlikely to be mediated by KDR or Flt-1.
VEGF 165 binding to MS-5 cells. (A) Left: Structure of native VEGF 165 (V165) and its proteolytic fragments (V110 and V55). Right: Confluent MS-5 cells were incubated with 0.5 ng/mL of125I VEGF 165 and various amounts of V165, V110 (domain encoded by exons 1 to 5 that do not bind neuropilin-1), and V55 (domain encoded by exons 7 and 8 that bind neuropilin-1). Nonspecific binding was measured in the presence of 2 μg/mL of unlabeled V165. The competitive displacement is expressed as the average of iodinated V165 specific binding in triplicate assays. Standard errors were less than 10%. (B) Subconfluent cells were washed twice in ice-cold binding medium and incubated with 5 ng/mL 125I-VEGF for 2 hours in the absence (lane 0) or in the presence of 1 μg/mL of V165 (lane V165), V55 (lane V55), or sema III-AP (lane sema III). For MS-5 cells, cross-linked complexes were incubated in the presence of 2 μg/mL of anti–neuropilin-1 antibodies. Proteins were resolved on a 7% SDS polyacrylamide gel, stained with Coomassie Blue, and autoradiographed. Position of molecular-weight markers is indicated on the right. pgs-A745: xylosyltransferase deficient pgs-745 CHO cells constitutively expressing either neuropilin-1 (pgs-A745 NP-1) or KDR (pgs-A745 KDR). The upper band corresponds to species that did not enter the gel. Arrowheads indicate cross-linked complexes.
VEGF 165 binding to MS-5 cells. (A) Left: Structure of native VEGF 165 (V165) and its proteolytic fragments (V110 and V55). Right: Confluent MS-5 cells were incubated with 0.5 ng/mL of125I VEGF 165 and various amounts of V165, V110 (domain encoded by exons 1 to 5 that do not bind neuropilin-1), and V55 (domain encoded by exons 7 and 8 that bind neuropilin-1). Nonspecific binding was measured in the presence of 2 μg/mL of unlabeled V165. The competitive displacement is expressed as the average of iodinated V165 specific binding in triplicate assays. Standard errors were less than 10%. (B) Subconfluent cells were washed twice in ice-cold binding medium and incubated with 5 ng/mL 125I-VEGF for 2 hours in the absence (lane 0) or in the presence of 1 μg/mL of V165 (lane V165), V55 (lane V55), or sema III-AP (lane sema III). For MS-5 cells, cross-linked complexes were incubated in the presence of 2 μg/mL of anti–neuropilin-1 antibodies. Proteins were resolved on a 7% SDS polyacrylamide gel, stained with Coomassie Blue, and autoradiographed. Position of molecular-weight markers is indicated on the right. pgs-A745: xylosyltransferase deficient pgs-745 CHO cells constitutively expressing either neuropilin-1 (pgs-A745 NP-1) or KDR (pgs-A745 KDR). The upper band corresponds to species that did not enter the gel. Arrowheads indicate cross-linked complexes.
Next we estimated the molecular weight of VEGF 165 cell-surface receptors on MS-5 cells using cross-linking assays. As size controls, we transfected CHO cells with either human KDR or rat neuropilin-1.125I VEGF 165 was bound to transfected CHO cells and cross-linked (Fig6B). The radiolabeled complexes formed in cells transfected with KDR (CHO-KDR cells) had an apparent molecular weight of 180 kD (Fig 6B, bottom panel). Subtraction of the molecular weight of the VEGF 165 dimer (45 kD) from these cross-linked complexes gave us 135 kD, the molecular weight of this form. In CHO cells transfected with neuropilin-1 (CHO-Np-1 cells), labeled complexes displayed a 165-kD molecular weight (Fig 6B, middle panel). This molecular weight was consistent with the binding of a VEGF 165 dimer (45 kD) to receptors of 120 kD. This value is in accordance with the molecular weight of neuropilin-1 detected on endothelial cells (130 to 135 kD).18 When 125I VEGF 165 was cross-linked to MS-5 cells in the same conditions, a faint complex was detected (data not shown). This suggested that neuropilin-1 was weakly expressed on the MS-5 cell surface. To confirm that VEGF 165 binds on MS-5 cells through neuropilin-1, cross-linked complexes were immunoprecipitated with anti–neuropilin-1 antibodies. As shown in Fig 6B (top panel), a 165-kD radiolabeled complex was detected. As in CHO-Np-1 cells, this molecular weight was consistent with the binding of a VEGF 165 dimer (45 kD) to receptors of 120 kD. As expected, the labeled complex was displaced in the presence of VEGF 165, V55, or sema III-AP.
We conclude that VEGF 165 can bind on MS-5 cells to receptors of 120 kD, which are specifically recognized by anti–neuropilin-1 antibodies. This binding was competed by V55 or sema III-AP but not by V110. Because sema III-AP binding was displaced by VEGF 165, we conclude that both VEGF 165 and semaphorin III compete for binding to 120-kD receptors that we identified as neuropilin-1.
VEGF 165 Has No Mitogenic Activity on MS-5 Cells but Increases Expression of Thrombopoietin (Tpo) and Flt-3 Ligand (Flt3-L)
To understand the role of neuropilin-1 on stromal cells, we first investigated whether growth of MS-5 cells could be influenced by VEGF 165. MS-5 cells were incubated in the absence or in the presence of 5 ng/mL VEGF 165 for 5 days. As shown in Table 1, VEGF 165 has no effect on MS-5 cell proliferation, although it is able to stimulate the growth of HUVEC. Then, we investigated whether VEGF 165 could modulate the expression of cytokines by MS-5 cells. MS-5 cells were incubated for 72 hours in the absence or in the presence of 50 ng/mL or 100 ng/mL VEGF 165. Expression of several cytokines known to influence the proliferation and differentiation of hematopoietic progenitors was determined by RT-PCR. Expression of stem cell factor (SCF), granulocyte colony-stimulating factor (G-CSF), hepatocyte growth factor (HGF),22 macrophage inhibitory factor-1α (MIP-1α), nerve growth factor (NGF),23 transforming growth factor-β1 (TGF-β1), tumor necrosis factor-α (TNF-α), and stromal cell–derived factor-1β (SDF-1β)24 was unaffected by the presence of VEGF 165 (not shown), but expression of Flt3-L and Tpo was increased 5-fold and 3-fold, respectively, in the presence of VEGF 165 (Fig 7).
Effect of VEGF 165 on the Growth of HUVEC and MS-5 Cells
| . | HUVEC . | MS-5 . |
|---|---|---|
| Without VEGF | 9,790 ± 51 | 93,510 ± 1,290 |
| With VEGF | 40,240 ± 2,140 | 94,330 ± 1,270 |
| . | HUVEC . | MS-5 . |
|---|---|---|
| Without VEGF | 9,790 ± 51 | 93,510 ± 1,290 |
| With VEGF | 40,240 ± 2,140 | 94,330 ± 1,270 |
HUVEC or MS5 cells were seeded at 10,000 cells per well in 12 multiwell plates. In half of the plates, 5 ng/mL VEGF 165 was added every other day; the cells were trypsinized and counted on day 5 in a Coulter counter (Beckman Coulter, Miami, FL). Data are presented as the mean cell number ± SEM of triplicate dishes. The results are representative of 3 distinct experiments.
VEGF 165 stimulates stromal Tpo and Flt-3 ligand mRNAs. Confluent MS-5 cells (7.5 × 105) were incubated for 72 hours in the absence (lane VEGF 0) or the the presence of either 50 ng/mL (lane VEGF 50) or 100 ng/mL (lane VEGF100) VEGF 165. RT-PCRs were performed using 1 μg of total RNA. Expected PCR products are 270 bp for Flt3-L and 460 bp for Tpo. Amplification of the HPRT mRNA is shown as a control. The intensity of ethidium bromide–stained bands was quantified with a CCD camera and Image Quant v1.11 software (Amersham, Pharmacia Biotech).
VEGF 165 stimulates stromal Tpo and Flt-3 ligand mRNAs. Confluent MS-5 cells (7.5 × 105) were incubated for 72 hours in the absence (lane VEGF 0) or the the presence of either 50 ng/mL (lane VEGF 50) or 100 ng/mL (lane VEGF100) VEGF 165. RT-PCRs were performed using 1 μg of total RNA. Expected PCR products are 270 bp for Flt3-L and 460 bp for Tpo. Amplification of the HPRT mRNA is shown as a control. The intensity of ethidium bromide–stained bands was quantified with a CCD camera and Image Quant v1.11 software (Amersham, Pharmacia Biotech).
Different Neuropilin-1 Ligands Are Expressed in Stromal Cells and in Immature Hematopoietic Cells
In vitro, neuropilin-1 is also able to bind secreted semaphorins related to semaphorin III, ie, semaphorins V and E. Neuropilin-1 is also able to bind semaphorin IV, but with a lower affinity.25
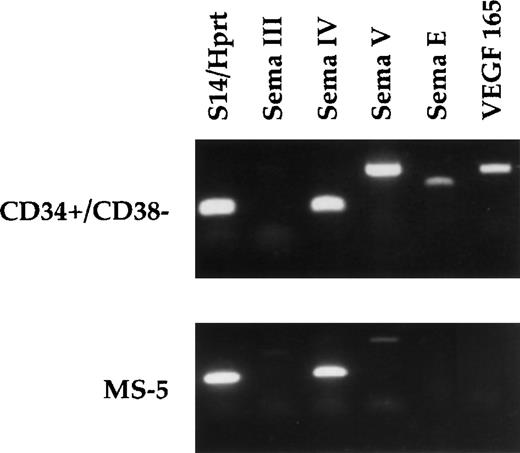
We investigated whether stromal cells and immature hematopoietic cells expressed known ligands for neuropilin-1 using RT-PCR experiments. As shown in Fig 8, high expression of semaphorin IV and weak expression of semaphorin III and semaphorin V were detected in MS-5 cells. Neither VEGF 165 nor semaphorin E expression was detected. Expression profile in murine marrow-derived adherent cells was similar (data not shown). Then, we analyzed expression of neuropilin-1 ligands in CD34+/CD38− hematopoietic progenitors. These cells express high levels of semaphorins IV, V , E, and VEGF 165. No semaphorin III expression could be detected.
Differential expression of secreted semaphorins and VEGF 165. RT-PCRs were performed using total RNA from human CD34+/CD38− cells and from murine MS-5 cells (see Materials and Methods). Expected PCR products are 130 bp for human semaphorin IV, 430 bp for human semaphorin III, 410 bp for human semaphorin V, 255 bp for human and murine semaphorin E, 260 bp for VEGF 165, 260 bp for murine semaphorin IV, 720 bp for murine semaphorin V, and 530 bp for murine semaphorin III. Controls consist of amplification with either S14 primers (human samples) or HPRT primers (murine samples).
Differential expression of secreted semaphorins and VEGF 165. RT-PCRs were performed using total RNA from human CD34+/CD38− cells and from murine MS-5 cells (see Materials and Methods). Expected PCR products are 130 bp for human semaphorin IV, 430 bp for human semaphorin III, 410 bp for human semaphorin V, 255 bp for human and murine semaphorin E, 260 bp for VEGF 165, 260 bp for murine semaphorin IV, 720 bp for murine semaphorin V, and 530 bp for murine semaphorin III. Controls consist of amplification with either S14 primers (human samples) or HPRT primers (murine samples).
Therefore, we conclude that although neuropilin-1 ligands are expressed in both stromal cells and hematopoietic cells, their expression profiles are clearly different.
DISCUSSION
Bone marrow stroma is made of several cell types and an extracellular matrix, which together form a suitable microenvironment for growth and differentiation of hematopoietic stem cells. So far, the precise mechanisms by which stromal cells influence the fate of hematopoietic cells have not been elucidated. To overcome the complexity of stromal cells, primary marrow-derived cells have been substituted by established cell lines that exhibit similar supportive functions. One of the most studied, the murine MS-5 cell line, allows expansion of human primitive CD34+/CD38− cells in the absence of exogenous growth factors. The hematopoietic promoting activity of MS-5 cells cannot be related to expression of the major hematopoietic cytokines.6 Furthermore, addition of hydrocortisone dramatically decreases the ability of MS-5 cells to support human hematopoiesis.7 This inhibition cannot be reversed by addition of exogenous growth factors. This suggests that hydrocortisone may either downregulate an activity which promotes differentiation of CD34+/CD38− cells or may upregulate an inhibitory activity.
The action of hydrocortisone is mediated by the glucocorticoid receptor, a transcription factor that regulates transcription through DNA-binding–dependent and –independent mechanisms.26Howewer, examples have been reported that suggest a role for the glucocorticoid receptor in posttranscriptional regulation.27,28 Direct binding of the glucocorticoid receptor to RNA, especially transfer RNAs,29 has been reported. This RNA binding activity may reflect regulation of gene expression through posttranscriptional mechanisms, such as alterations in translational efficiency. Therefore, it is likely that the effects of hydrocortisone on MS-5 are due to both transcriptional and to posttranscriptional events.
A number of approaches have been used to clone genes with differential expression. These include differential cDNA library construction,30 differential hybridization,31,32 and differential display RT.33 Identification of a candidate gene using these methods relies on its differential mRNA levels, and genes that are posttranscriptionally regulated are not taken into account. Gene trapping is an efficient way to target active genes and allows monitoring of the expression of the endogeneous gene by a LacZ reporter gene. This method has been used successfully to clone retinoid acid induced genes in embryonal carcinoma P19 cells34 or in embryonic stem (ES) cells.35 We chose the gene trap strategy because it may allow cloning of transcriptionally and posttranscriptionally regulated genes. In addition, the vector we used, pGT1.8TM, allows the screening of stable clones for insertional mutations in genes encoding secreted and membrane-spanning proteins. This particular gene trap relies on capturing the amino-terminal signal sequence of a gene to produce an active β-galactosidase fusion protein. In our screen, 4% of the clones selected for G418 resistance displayed β-galactosidase activity, a percentage similar to that obtained in experiments performed in ES cells.16
In the 15D3 clone, insertion has occurred within the neuropilin-1 gene. Sequence of the 15D3 fusion transcript showed an insertion of bacterial DNA between the En-2 sequences and the neuropilin-1 sequences. This event may be due to multiple insertions. In this case, splicing may have occurred within vector tandems. Insertion of multiple and rearranged copies of the vector was confirmed by Southern blot experiments (data not shown). However, because only 1 band was detected in Northern blot experiments, only 1 fusion transcript is produced in 15D3 cells.
In 15D3 cells, β-galactosidase activity was downregulated in the presence of hydrocortisone. To ensure that this modulation reflected neuropilin-1 activity, we studied VEGF 165 binding to MS-5 cells in the presence of hydrocortisone. We showed that it was downregulated 2-fold in the presence of hydrocortisone. Regulation of neuropilin-1 by glucocorticoids may be a posttranscriptional event, since we observed no difference in mRNA levels (data not shown).
Neuropilin-1 is expressed in adult endothelial cells, in some tumor-derived cells, and in a variety of tissues including placenta, heart, lung, liver, skeletal muscle, kidney, and pancreas.18 During embryonic development, neuropilin-1 is expressed in particular neuronal circuits, in the cardiovascular system, and in limb buds. Neuropilin-1 is a receptor for the axonal repellent semaphorin III. When semaphorin III binds to the receptor on the growing tips of neurons, its repels the cells, keeping them from getting off-track. Recently, neuropilin-1 has been described as a new receptor for the VEGF 165 isoform and may stimulate blood vessel growth. The role of neuropilin-1 in angiogenesis seems to be different from its role in axonal guidance. In developing blood vessels, neuropilin-1 seems to work in concert with KDR to stimulate vessel growth. Cells in which both receptors are expressed are 4-fold more effective at binding VEGF 165 and 3-fold more effective at migrating than cells carrying KDR alone.18
In our study, semaphorin III-AP and VEGF 165 could bind to MS-5 cells, both a similar kd of approximately 120 pmol/L. This value is comparable to the estimated kd for the interaction of semaphorin III with neuropilin-1 (325 to 1,500 pmol/L) and for interaction of VEGF 165 with neuropilin-1 (333 pmol/L). We have performed binding experiments in the presence of neuropilin-1 blocking antibodies (provided by Drs Z. He and M. Tessier-Lavigne). Although these antibodies block the ability of semaphorin III to repel axons and to induce collapse of their growth cones,8 they were unable to block semaphorin III binding in our assays. They were actually raised against a portion of the neuropilin-1 ectodomain that is dispensable for semaphorin III binding.36 37 Therefore, the antibodies we used are expected to block biological activity but not semaphorin III binding (data not shown). Semaphorin III-AP binding to MS-5 cells is displaced by VEGF 165 while VEGF 165 binding is competed by V55 and semaphorin III-AP. When 125I-VEGF was cross-linked to MS-5 cells, a 165-kD complex was detected and recognized by anti–neuropilin-1 antibodies. These results indicated that both VEGF 165 and semaphorin III bound to MS-5 cells through the neuropilin-1 receptor.
To elucidate the role of neuropilin-1 on stromal cells, we first investigated whether genes encoding secreted semaphorins or VEGF 165 are expressed in stromal cells. In MS-5 cells, as in bone marrow–derived adherent cells, we detected no VEGF 165 mRNA expression, a high expression of semaphorin IV, and a weak expression of semaphorins III and V. These results indicate that neuropilin-1 and secreted semaphorins may act as mediators of a cross-talk between stromal cells to organize or maintain the stromal structure of the bone marrow.
Another possibility is that neuropilin-1 may bind ligands produced by primitive hematopoietic cells. Therefore, we looked for neuropilin-1 ligands in primitive hematopoietic cells and we found that semaphorin IV, V, E, and VEGF 165 mRNAs were expressed in CD34+/38− progenitors. These results suggest that semaphorins and/or VEGF 165 secreted by hematopoietic cells bind to neuropilin-1 on stromal cells. We showed that although VEGF 165 was unable to stimulate the growth of MS-5 cells, it was able to increase both Tpo and Flt3-L mRNA expression. Interestingly, these 2 cytokines have been shown to regulate early hematopoiesis by increasing numbers of primitive hematopoietic progenitors in vitro.38 ,39
It was shown previously that stromal cultures are a source of early acting cytokines, especially Tpo, SCF, and Flt3-L,40 and that the ability of MS-5 cells to enhance the hematopoietic potential of ES cells is, in part, due to their secretion of Tpo.41These data and ours suggest that interaction between stromal cells and hematopoietic cells through neuropilin-1 may induce cytokine production by the former and proliferation of the latter.
ACKNOWLEDGMENT
We are very grateful to Drs Marc Tessier-Lavigne and Zhigang He for the gift of the semaphorin III-AP secreting cell line and anti–neuropilin-1 antibodies, and for their advice and encouragement; to Dr William Skarnes for the gift of pGT1.8TM and for his advice; to Drs Alain Chédotal, Isabelle Godin, and Patrick Mayeux for helpful discussions; and to Prof André Baruchel and Dr Vincent Mignotte for their encouragement.
Supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), the Centre National de la Recherche Scientifique, the Fondation de France, and the Ligue Nationale contre le Cancer. R.T. was supported by the INSERM. N.O. was supported by the Ligue Nationale contre le Cancer.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Valérie Lemarchandel, PhD, INSERM U474, Hopital Henri Mondor, 94010 Créteil, France; e-mail: valerie@im3.inserm.fr.









This feature is available to Subscribers Only
Sign In or Create an Account Close Modal