We have recently reported that cyclophilin B (CyPB), a secreted cyclosporine-binding protein, could bind to T lymphocytes through interactions with two types of binding sites. The first ones, referred to as type I, involve interactions with the conserved domain of CyPB and promote the endocytosis of surface-bound ligand, while the second type of binding sites, termed type II, are represented by glycosaminoglycans (GAG). Here, we further investigated the interactions of CyPB with blood cell populations. In addition to lymphocytes, CyPB was found to interact mainly with platelets. The binding is specific, with a dissociation constant (kd) of 9 ± 3 nmol/L and the number of sites estimated at 960 ± 60 per cell. Platelet glycosaminoglycans are not required for the interactions, but the binding is dramatically reduced by active cyclosporine derivatives. We then analyzed the biologic effects of CyPB and found a significant increase in platelet adhesion to collagen. Concurrently, CyPB initiates a transmembranous influx of Ca2+ and induces the phosphorylation of the P-20 light chains of myosin. Taken together, the present results demonstrate for the first time that extracellular CyPB specifically interacts with platelets through a functional receptor related to the lymphocyte type I binding sites and might act by regulating the activity of a receptor-operated membrane Ca2+ channel.
CYCLOPHILINS are known to be the main binding proteins for the immunosuppressive drug cyclosporine A (CsA).1,2 The first characterized isoform was cyclophilin A (CyPA), an abundant cytosolic protein that is considered to be the major target for CsA into the cell.3,4 Cyclophilin B (CyPB)4-6 and cyclophilin C (CyPC)7 are two other isoforms structurally related to CyPA, but their mRNA encodes a signal sequence thought to mediate translocation into the endoplasmic reticulum. Both the cyclophilins and the structurally unrelated FK506-binding proteins (FKBP) exhibit peptidyl-prolyl cis-transisomerase activity (PPIase)8-10 and inhibit the phosphatase activity of calcineurin in the presence of their respective ligand.11,12 The latter property is thought to be relevant to the immunosuppressive activity of both drugs. Indeed, the inhibition of calcineurin activity has been shown to be a crucial step that effectively blocks the early T-cell activation cascade and constitutes the basis of the prevention of graft rejection.13
The presence of a released form of CyPB in human milk and plasma6,14 has led us to investigate the properties of this protein. We first characterized specific surface binding sites on T lymphocytes,15 mainly associated with the helper/inducer T-cell subset.16 Most recently, we provided evidence that interactions of CyPB with sulfated glycosaminoglycans (GAG) may occur on the T-cell surface. In addition, we identified a second type of CyPB binding sites, referred as type I sites versus type II for GAG interactions.17 The binding of CyPB to type I and type II sites requires interactions with two distinct areas of the protein, the catalytic/CsA-binding domain and the N-terminal extremity of CyPB, respectively. Moreover, we demonstrated that the type I binding sites are involved in an endocytosis process of the protein, which supports the hypothesis that they may correspond to a functional receptor of CyPB.17 The presence of surface binding sites on T lymphocytes was consistent with the hypothesis that secreted CyPB may interact with specific membrane receptors. However, the presence of CyPB binding sites on the other human blood cells has not yet been investigated. In the present report, we analyzed the distribution of CyPB binding sites in blood cell populations. In addition to the interactions with T lymphocytes, we found a significant binding of the protein to platelets. CyPB interacts with platelets in a specific manner, with a dissociation constant (kd) value similar to that of T cells. However, CyPB binding to platelets did not involve interactions with GAG, while it was strongly reduced in the presence of active cyclosporine derivatives. Platelets are largely represented in human blood and their activation is critically important in blood coagulation and inflammatory events.18 Here, we demonstrated that incubation of platelets together with CyPB enhances adhesion to collagen, but is ineffective in terms of degranulation or aggregation. In addition, CyPB binding initiates an influx of extracellular Ca2+ and some kinase activation, which demonstrates that the platelet receptor is coupled to a transduction pathway. The present results suggest that CyPB interacts with platelets through a functional receptor related to the lymphocyte type I binding sites and is probably involved in the regulation of a receptor-operated membrane channel.
MATERIALS AND METHODS
Materials.
Human citrated venous blood samples from healthy donors were obtained from the local blood transfusion center (Etablissement de Transfusion Sanguine, Lille, France). Recombinant human CyPA and CyPB were produced and purified as previously described.3,6 Recombinant human CyPC7 and cyclosporine derivatives (CsA, CsG, CsH)19 were a generous gift from Novartis (Basel, Switzerland). Peptides that corresponded to the N- and C-terminal extensions of CyPB were synthesized as described14 and were provided with the tetrapeptide RGDS by Professor A. Tartar (Institut Pasteur de Lille, France). Collagen mixture (essentially of type I and II) was purified from rat tail.20 [125I]CyPB was prepared as described.15 The specific radioactivity ranged from 4 to 6 × 106 cpm/μg.
Preparation of platelets.
Platelet-rich plasma (PRP) was obtained by centrifugation of whole blood at 200g for 30 minutes. Washed platelets were prepared by filtration on a Sepharose 4-B column (Pharmacia, Uppsala, Sweden) equilibrated in Tyrode’s buffer (NaCl, 137 mmol/L; KCl, 2.7 mmol/L; CaCl2, 2 mmol/L; MgCl2, 1 mmol/L; Na2HPO4, 0.2 mmol/L; NaHCO3, 12 mmol/L; HEPES, 5 mmol/L; 0.1% glucose), pH 7.4, and resuspended in Tyrode’s buffer containing 0.5% bovine serum albumin (BSA). Concentrated platelet suspension was obtained by centrifugation of PRP (1,600g, 10 minutes) and resuspension of the resulting pellet with PRP to a final concentration of 2 × 109/mL.
Surface binding experiments with [125I]CyPB.
Surface binding of [125I]CyPB to blood cell populations was performed by incubating blood samples (1 mL) in the presence of radiolabeled ligand for 1 hour at room temperature. After washing off the plasma, the surface-bound CyPB was counted in the blood cell pellet, to obtain the total binding capacity, and in each isolated cell population, after separation of blood cells on Ficoll separation medium (Nycomed, Oslo, Norway). For platelet binding experiments, PRP was previously incubated in the presence of 1 μmol/L prostaglandin E1 for 30 minutes at 37°C. Platelets (2 × 108 per sample) were then allowed to bind [125I]CyPB at various concentrations. After 1 hour at 22°C, the platelet suspension was filtered over a Whatman glass fiber filter (Maidstone, UK) under mild vacuum and washed twice. Filter-associated (bound ligand) and incubation medium (free ligand) radioactivities were then measured. To analyze interactions with GAG, washed platelets were treated with either 1 U/mL heparinase I, 2 U/mL chondroitinase ABC, or both (Sigma Chemical, St Louis, MO) for 2 hours at room temperature and directly used for binding experiments as described.17 Control untreated platelets were prepared under the same protocol. For all binding experiments with [125I]CyPB, nonspecific interactions were determined in the presence of a 200-fold molar excess of unlabeled ligand and radioactivity was measured using a model 1282 Compugamma LKB-Wallac counter (Gaithersburg, MD).
Platelet function analysis.
Platelet aggregation was typically performed at 37°C for 3 minutes using a turbidimetric method. Aggregation was induced by the addition of fibrinogen (1 mg/mL) or autologous plasma to washed platelet mixture (2 × 108/mL) followed by the addition, 30 seconds later, of CyPB or agonist. To measure platelet degranulation, PRP was incubated with 5-[14C]hydroxytryptamine (5-HT) (0.05 mCi/mL) (ICN Biochemicals, Costa Mesa, CA) for 30 minutes at 37°C. After gel filtration, platelets were incubated with CyPB or agonist and processed as described.21 Released 5-[14C]HT was analyzed using a model LS 6000-TA Beckman counter (Allendale, NJ). For platelet adhesion assays, 96-well microtiter plates were coated with 1 μg/well of collagen in a sodium carbonate buffer, 10 mmol/L, pH 9.6, overnight at 4°C. Nonspecific binding sites were blocked by addition of 2% BSA. Platelets (1 × 107 per well) were incubated in the presence of various concentrations of CyPB and added to collagen-coated wells for a 30-minute incubation at 37°C. After washing, the adherent platelets were quantified using the BCA reagent kit for protein assay (Pierce Chemicals, Rockford, IL).
Calcium measurements.
Platelets were loaded with 3 μmol/L of Fluo 3-acetoxymethylester (Fluo 3-AM) (Molecular Probes, Leiden, Netherlands)22 for 30 minutes at 37°C. After gel filtration to remove extracellular Fluo 3, the final platelet concentration was adjusted to 1 × 106/mL in Tyrode’s buffer. Stimulation was induced by the addition of either various concentrations of CyPB or thrombin at 37°C. Changes in fluorescence were recorded by flow cytofluorimetry using a Becton Dickinson FACScan cytofluorimeter (Mountain View, CA), with excitation and emission wavelengths set on 488 and 515 nm, respectively. This method allowed the analysis of 2,000 fluorescent particles every 30 seconds. The Ca2+-Fluo 3 fluorescence was calibrated with a maximum response induced by the addition of ionomycin to the suspension. The levels of cytosolic Ca2+were calculated for each fluorescence mean value, with a kd of 400 nmol/L for Fluo 3.22
Measurement of inositol phosphate formation.
Concentrated platelet suspensions were labeled withmyo-[3H]inositol (50 μCi/ml) (ICN Biochemicals) for 3 hours at 37°C. Following washing and resuspension in amyo-inositol–free buffer, platelets were stimulated with either CyPB or thrombin and processed as described.23
Protein phosphorylation analysis.
Platelets (2 × 108 per sample) were incubated in the presence of either CyPB or thrombin at 37°C. At various times, platelets were transferred into Tyrode’s buffer containing 10 mmol/L EDTA, 1 mmol/L o-vanadate and 100 mmol/L NaF, to inhibit protein phosphatases and rapidly washed by centrifugation (2,500g, 30 seconds). Proteins from platelet lysates were separated on a 12% sodium dodecyl sulfate-polyacrylamide gel electropheresis (SDS/PAGE) and transblotted onto nitrocellulose paper.24 After blocking in Tris-buffered saline (TBS), pH 8.2, which contained 3% gelatin, blots were incubated with mouse monoclonal antibodies to phosphoserine or phosphotyrosine residues (Sigma) in TBS-gelatin 0.5% for 2 hours and exposed to horseradish peroxidase–labeled antimouse IgG antibodies (1/2,500) (BioSys, Compiègne, France) for another 2-hour incubation. Development was performed with o-phenylene diamine kit (Sigma). Because platelet controls from different individuals showed varying degrees of protein phosphorylation, the effects of CyPB were evaluated in terms of variations of phosphorylation intensity using Quantiscan software (Biosoft, Cambridge, UK).
Statistical analysis.
Results are expressed as mean values ± SEM for at least three independently performed experiments conducted with separate donors. Statistical significance between the different values was analyzed by Student’s t-test for unpaired data with a threshold ofP < .05.
RESULTS
Characterization of CyPB binding sites on human blood cells.
On the assumption that only T lymphocytes were involved in the interactions with CyPB, the total binding capacity of [125I]CyPB was estimated at 50 fmol/mL in whole blood. Indeed, we previously reported that approximately 40% of the peripheral blood lymphocyte population showed significant binding of CyPB, with a capacity ranging from 30,000 to 120,000 sites per cell.15-17 Surprisingly, the surface-bound ligand was found to be 415 ± 80 fmol/mL, much higher than the calculated value. The distribution of [125I]CyPB was then analyzed after separation of the blood cell populations (Table1). Ten percent to 15% of the total binding capacity of [125I]CyPB was found associated with the lymphocyte fraction, which corresponds to the expected value we had calculated. No significant amounts of radioactivity were measurable in the monocyte population. A weak but significantly measurable proportion of CyPB was associated with granulocytes. This value might reflect either a poor expression of CyPB binding sites on the membrane of this whole cell population, or a restricted CyPB binding on a specific subpopulation of polymorphonuclear cells. More than 80% of the bound protein was found associated with the platelet fraction. Owing to the large amount of platelets in human blood, this value implies that the number of surface-bound CyPB would range from 500 to 1,200 on platelets. Finally, the radioactivity found associated with the erythrocyte population is probably due to [125I]CyPB binding to contaminating platelets or lymphocytes; otherwise it would correspond to less than one binding site per erythrocyte, according to the number of red blood cells.
Surface Binding of CyPB to Human Blood Cell Populations
| . | Surface-Bound CyPB (fmol/mL) . | % of Cellular Binding . |
|---|---|---|
| Whole blood cells | 415 ± 80 | 100 |
| Lymphocytes | 51 ± 10 | 12 ± 3 |
| Monocytes | NS | — |
| Granulocytes | 1 ± 0.5 | <1 |
| Platelets | 353 ± 65 | 86 ± 4 |
| Erythrocytes | 5 ± 4 | 1 ± 1 |
| . | Surface-Bound CyPB (fmol/mL) . | % of Cellular Binding . |
|---|---|---|
| Whole blood cells | 415 ± 80 | 100 |
| Lymphocytes | 51 ± 10 | 12 ± 3 |
| Monocytes | NS | — |
| Granulocytes | 1 ± 0.5 | <1 |
| Platelets | 353 ± 65 | 86 ± 4 |
| Erythrocytes | 5 ± 4 | 1 ± 1 |
Abbreviation: NS, not significant (below the limit of detection).
Surface binding of CyPB to platelets.
The binding parameters of CyPB were determined by incubating platelets with increasing concentrations of [125I]CyPB. Figure1 illustrates one representative experiment performed with washed platelets. The binding was specific since a 200-fold molar excess of unlabeled ligand inhibited [125I]CyPB binding by 60% to 80%. After subtraction of nonspecific interactions, the binding was found to be concentration-dependent and saturable (Fig 1A). Scatchard analysis resulted in a linear plot compatible with a single affinity binding site. The apparent kd was 9 ± 3 nmol/L and the number of binding sites was estimated at 960 ± 60 per platelet (Fig 1B). Similar data were obtained when Ca2+ and Mg2+ were omitted from the incubation medium, which indicates that the presence of the divalent cations is not required for CyPB binding to platelets. In additional experiments, platelets were incubated in the presence of 50 nmol/L [125I]CyPB for 1 hour at 22°C, then extensively washed to remove unbound ligand and resuspended in the same buffer containing a 10-fold molar excess of unlabeled CyPB. A rapid removal of surface bound radiolabeled ligand was observed, which indicates that the binding of CyPB to platelets is reversible (data not shown). To ensure that CyPB binding may occur under physiologic conditions, PRP was adjusted to 2 × 108 platelets/mL with citrated buffer and directly used for binding experiments (n = 3). In this case, the kd value and number of sites were estimated at 10 ± 3 nmol/L and 900 ± 120 per platelet, which indicates that the presence of plasma does not significantly modify the binding parameters. In addition, endogenous plasma CyPB levels were measured by enzyme-linked immunosorbent assay (ELISA)14 and found to be less than 5 nmol/L, which is too low to account for a large part in the binding site occupancy.
Surface binding of [125I]CyPB to platelets. Dose-dependence and saturation of CyPB binding were studied by incubating platelets with the indicated concentrations of [125I]CyPB for 1 hour at 22°C. The specific binding (•) was obtained after subtraction of nonspecific (▴) from total counts (▪). Points represent the mean values of triplicates from a representative experiment (A). (B) Scatchard plot of the binding data.
Surface binding of [125I]CyPB to platelets. Dose-dependence and saturation of CyPB binding were studied by incubating platelets with the indicated concentrations of [125I]CyPB for 1 hour at 22°C. The specific binding (•) was obtained after subtraction of nonspecific (▴) from total counts (▪). Points represent the mean values of triplicates from a representative experiment (A). (B) Scatchard plot of the binding data.
Specificity of CyPB binding to platelets.
The specificity of CyPB binding to platelets was first analyzed by competitive experiments. For these studies, platelets were incubated with 50 nmol/L of [125I]CyPB in the presence of various concentrations of unlabeled CyPA, CyPB or CyPC (Fig2A). As expected, [125I]CyPB binding was inhibited by greater than 80% from a 200-fold molar excess of unlabeled CyPB. The concentration of CyPB required for half-maximal inhibition (IC50) of the total [125I]CyPB binding was estimated at 250 nmol/L. CyPA was unable to displace the radio-iodinated ligand from the platelet membrane, which shows that this isoform has no affinity for CyPB binding sites. In contrast, increasing concentrations of CyPC inhibited [125I]CyPB binding to platelets, but to a lesser extent than CyPB. The IC50 was estimated at 2,950 nmol/L, which shows that CyPC has a lower affinity for platelet binding sites. We then examined the involvement of nonconserved regions of CyPB in the ligand binding by using synthetic peptides that copy the most divergent parts of the protein. As previously reported,17 increasing concentrations of the C-terminal peptide were unable to reduce [125I]CyPB binding. Surprisingly, the N-terminal peptide was also inefficient at competing with the ligand for binding to the platelet receptor, although it was previously shown to strongly reduce interactions of CyPB with the lymphocyte type II sites.17 Contrary to CyPA and CyPC, only CyPB possesses a specific RGD motif.4-7 Nevertheless, the involvement of this specific tripeptide in the interactions of CyPB with platelets is unlikely, since the addition of increasing concentrations of the tetrapeptide RGDS, which binds to glycoprotein (GP)IIb/IIIa and inhibits its interaction with RGD-containing ligands such as fibrinogen,25 was also ineffective at reducing [125I]CyPB binding (Fig 2A). We then investigated the role of the CsA-binding/catalytic domain of CyPB in the interactions with the platelet receptor, by using cyclosporine derivatives as competitive inhibitors (Fig 2B). Both CsA and the less active CsG reduced [125I]CyPB binding to the platelet membrane. A significant decrease was only obtained from a 10-fold molar excess of both drugs, which indicates that the inhibition requires that CyPB was maintained in a complexed form. By contrast, CsH, which is unable to interact with CyPB, failed to prevent the ligand binding, which confirms that occupancy of CsA-binding/catalytic domain by active cyclosporine derivatives accounts for the loss of binding activity. On the other hand, treatment of platelets with GAG-degrading enzymes did not significantly modify the binding of [125I]CyPB to platelets. Moreover, incubation of CyPB together with protamine, a polypeptide that inhibits interactions with heparin-like molecules, had no more effect on CyPB binding to platelets, which confirms that CyPB does not interact with platelet GAG (Fig 2B).
Specificity of [125I]CyPB binding to platelets. (A) Competitive binding assays with cyclophilin isoforms and synthetic peptides copying specific sequences of CyPB. Platelets were incubated in the presence of 50 nmol/L [125I]CyPB and increasing concentrations of unlabeled CyPA (▪), CyPB (•), CyPC (⧫), N-terminal peptide of CyPB (○), C-terminal peptide of CyPB (□), or RGDS peptide (⋆). After washing, the amounts of remaining surface-bound [125I]CyPB, expressed as a percentage of the ligand bound in the absence of competitors, are plotted against the molar ratios of [125I]CyPB to the competitors. Data are expressed as mean values from 3 separate experiments conducted with platelets from different donors. (B) Sensitivity of CyPB binding to cyclosporine derivatives, protamine, and GAG-degrading enzymes. Platelets were incubated with of 50 nmol/L [125I]CyPB in the absence (1) or presence of CsA 500 nmol/L (2); CsA 5 μmol/L (3); CsG 500 nmol/L (4); CsG 5 μmol/L (5), CsH 500 nmol/L (6); CsH 5 μmol/L (7); protamine 500 nmol/L (8); or protamine 5 μmol/L (9). In the last cases, platelets were first pretreated with heparinase type I (10), chondroitinase ABC (11), or both (12), and directly used for binding experiments. After washing, the amounts of remaining surface-bound [125I]CyPB were expressed as a percentage of the ligand bound in the absence of any treatment. Data are mean values ± SEM from 3 separate experiments conducted with platelets from different donors.
Specificity of [125I]CyPB binding to platelets. (A) Competitive binding assays with cyclophilin isoforms and synthetic peptides copying specific sequences of CyPB. Platelets were incubated in the presence of 50 nmol/L [125I]CyPB and increasing concentrations of unlabeled CyPA (▪), CyPB (•), CyPC (⧫), N-terminal peptide of CyPB (○), C-terminal peptide of CyPB (□), or RGDS peptide (⋆). After washing, the amounts of remaining surface-bound [125I]CyPB, expressed as a percentage of the ligand bound in the absence of competitors, are plotted against the molar ratios of [125I]CyPB to the competitors. Data are expressed as mean values from 3 separate experiments conducted with platelets from different donors. (B) Sensitivity of CyPB binding to cyclosporine derivatives, protamine, and GAG-degrading enzymes. Platelets were incubated with of 50 nmol/L [125I]CyPB in the absence (1) or presence of CsA 500 nmol/L (2); CsA 5 μmol/L (3); CsG 500 nmol/L (4); CsG 5 μmol/L (5), CsH 500 nmol/L (6); CsH 5 μmol/L (7); protamine 500 nmol/L (8); or protamine 5 μmol/L (9). In the last cases, platelets were first pretreated with heparinase type I (10), chondroitinase ABC (11), or both (12), and directly used for binding experiments. After washing, the amounts of remaining surface-bound [125I]CyPB were expressed as a percentage of the ligand bound in the absence of any treatment. Data are mean values ± SEM from 3 separate experiments conducted with platelets from different donors.
Analysis of CyPB activity on platelet functions.
In an attempt to understand the biological relevance of CyPB binding, we analyzed its effects on platelet functions. The addition of CyPB at different concentrations did not induce any significant aggregate formation and only a poor release of 5-[14C]HT from loaded platelets by comparison with thrombin (Table2). We then examined whether CyPB may potentiate platelet aggregation or secretion after low doses of thrombin. Thrombin at 0.05 U/mL was used because it caused only moderate but significant platelet activation at this concentration. However, the addition of CyPB was found to have no significant enhancing effect on aggregation or 5-HT release (Table 2). Similar conclusions were observed when platelets were challenged in the presence of plasma or activated with adenosine diphosphate (ADP) in place of thrombin (data not shown), which confirms that CyPB is ineffective at inducing any aggregation or degranulation processes and does not act synergistically with low doses of agonist to activate platelets.
Effects of CyPB on Platelet Aggregation and Degranulation
| . | Platelet Aggregation (%) . | 5-HT Release (%) . |
|---|---|---|
| No ligand | 6 ± 5 | 8.4 ± 4.5 |
| CyPB | ||
| 10 nmol/L | 5 ± 4 | 11.1 ± 3.5 |
| 50 nmol/L | 6 ± 4 | 10.0 ± 2.5 |
| 500 nmol/L | 7 ± 3 | 10.1 ± 3.4 |
| Thrombin: 0.05 U/mL | 26 ± 6 | 12 ± 5 |
| +CyPB 50 nmol/L | 28 ± 5 | 16 ± 3.5 |
| +CyPB 500 nmol/L | 25 ± 8 | 14 ± 4 |
| Thrombin: 0.5 U/mL | 84 ± 5 | 89 ± 6 |
| . | Platelet Aggregation (%) . | 5-HT Release (%) . |
|---|---|---|
| No ligand | 6 ± 5 | 8.4 ± 4.5 |
| CyPB | ||
| 10 nmol/L | 5 ± 4 | 11.1 ± 3.5 |
| 50 nmol/L | 6 ± 4 | 10.0 ± 2.5 |
| 500 nmol/L | 7 ± 3 | 10.1 ± 3.4 |
| Thrombin: 0.05 U/mL | 26 ± 6 | 12 ± 5 |
| +CyPB 50 nmol/L | 28 ± 5 | 16 ± 3.5 |
| +CyPB 500 nmol/L | 25 ± 8 | 14 ± 4 |
| Thrombin: 0.5 U/mL | 84 ± 5 | 89 ± 6 |
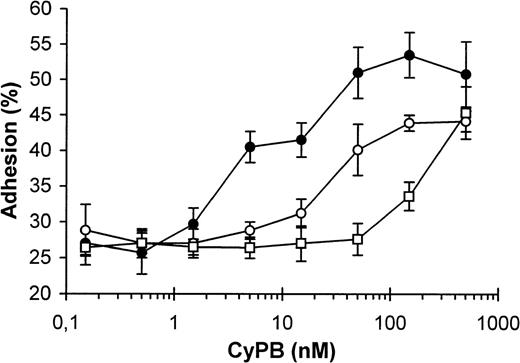
For adhesion analysis, platelets were incubated in the presence of various concentrations of CyPB, and the samples were added to collagen-coated plates. Control values, obtained in the absence of CyPB, were estimated at 20% to 32% of initially added platelets (27% ± 4%). These values were obtained in separate experiments conducted with the platelets from different donors (n = 10) and variations are likely to be due to individual differences in the response to collagen. In the presence of CyPB, a significant increase in platelet adhesion was observed with 5 nmol/L of the protein and the optimal effect was measured at approximately 50 nmol/L. At this concentration, adherent platelets ranged from 43% to 50% (47% ± 2.5%), which reflects an almost twofold increase in platelet adhesion in comparison to control (Fig 3). To determine whether this increasing effect is related to CyPB binding to the platelet receptor, similar experiments were performed in the presence of CsA. In the absence of CyPB, the immunosuppressant had no marked effect on platelet adhesion to collagen. In contrast, the addition of the drug at low and optimal concentrations of CyPB decreased the enhancing effect of the protein. Nevertheless, high concentrations of CsA were necessary to significantly reduce the platelet adhesion to collagen. The basal level in platelet adhesion was only restored in the presence of a 100-fold molar excess of the drug. In contrast, CsA was ineffective in the presence of high concentrations of CyPB, which may be explained by the presence of uncomplexed CyPB at a concentration probably sufficient to obtain an optimal increase in platelet adhesion (Fig 3). Two distinct mechanisms have been distinguished in the promotion of platelet adhesion, one dependent on the presence of divalent cations, and the other cation-independent and reflecting the participation of plasma proteins such as fibronectin or von Willebrand factor. In the last mechanism, adhesive proteins are thought to serve as a bridge between the platelet membrane and the collagen fiber surface.26 To check this hypothesis, collagen-coated plates were pretreated with CyPB and extensively washed before the addition of platelets. In these conditions, a CyPB-mediated increase in platelet adhesion was not observed, which demonstrates that CyPB does not act by forming a link between collagen and the platelet receptor. In contrast, when platelets were pretreated with CyPB and extensively washed to remove unbound ligand, the enhancing effect of the protein on platelet adhesion was preserved, which suggests it may be related to the binding of CyPB to a platelet signalling receptor (Table 3). We then analyzed the role of divalent cations, by incubating platelets in a Ca2+/Mg2+-depleted medium supplemented with 2 mmol/L EGTA. In this case, platelet adhesion was significantly reduced, from 30% to 12%. Nevertheless, no significant increase occurred in the presence of CyPB, which implies that the presence of divalent cations is required for the protein to enhance platelet adhesion. When citrated plasma was used in place of buffer that contained EGTA, platelet adhesion was approximately 20%, which indicates the participation of plasma factors in promoting cation-independent adhesion. Nevertheless, this mechanism was unmodified when CyPB was added to citrated plasma. Finally, adhesion experiments were reproduced with recalcified plasma. To prevent spontaneous aggregation, platelets were pretreated with aspirin (100 μmol/L) and the drug was conserved all along the experiment. In this case, the enhancing effect of CyPB on platelet adhesion to collagen was partially restored, with an almost 1.5-fold increase in adhesion by comparison to control (Table 3). Taken together, these results demonstrate that the action of CyPB is only dependent on the presence of divalent cations and may occur under physiologic conditions.
Enhancing effect of CyPB on platelet adhesion. Washed platelets were incubated in the presence of increasing concentrations of CyPB in the absence (•) or presence of 500 nmol/L (○) or 5 μmol/L (□) of CsA and added to 96-well plates coated with collagen (1 μg/ well) for a 30-minute incubation at 37°C. After washing, adherent platelets were quantified using a BCA protein assay and expressed as percentages of initially added platelets (1 × 107 platelets per well). Results are expressed as mean values ± SEM from quadruplicates and are representative from at least 3 separate experiments conducted with platelets from different donors.
Enhancing effect of CyPB on platelet adhesion. Washed platelets were incubated in the presence of increasing concentrations of CyPB in the absence (•) or presence of 500 nmol/L (○) or 5 μmol/L (□) of CsA and added to 96-well plates coated with collagen (1 μg/ well) for a 30-minute incubation at 37°C. After washing, adherent platelets were quantified using a BCA protein assay and expressed as percentages of initially added platelets (1 × 107 platelets per well). Results are expressed as mean values ± SEM from quadruplicates and are representative from at least 3 separate experiments conducted with platelets from different donors.
Effects of the Modification of Either Incubation Medium or Treatment Procedure on CyPB-Mediated Adhesion of Platelets to Collagen
| Treatment . | Absence of CyPB . | Presence of CyPB (50 nmol/L) . |
|---|---|---|
| Control3-150 | 29 ± 3 | 49 ± 5 |
| Modified incubation medium | ||
| Ca2+/Mg2+-depleted medium | 12 ± 4 | 12 ± 3 |
| Citrated plasma | 19 ± 2 | 20 ± 3 |
| Recalcified plasma | 33 ± 5 | 53 ± 4 |
| Modified incubation procedure: | ||
| Pretreatment of platelets | 28 ± 4 | 48 ± 6 |
| Pretreatment of coated collagen | 29 ± 1 | 28 ± 2 |
| Treatment . | Absence of CyPB . | Presence of CyPB (50 nmol/L) . |
|---|---|---|
| Control3-150 | 29 ± 3 | 49 ± 5 |
| Modified incubation medium | ||
| Ca2+/Mg2+-depleted medium | 12 ± 4 | 12 ± 3 |
| Citrated plasma | 19 ± 2 | 20 ± 3 |
| Recalcified plasma | 33 ± 5 | 53 ± 4 |
| Modified incubation procedure: | ||
| Pretreatment of platelets | 28 ± 4 | 48 ± 6 |
| Pretreatment of coated collagen | 29 ± 1 | 28 ± 2 |
Results are expressed as percentages of initially added platelets (1 × 107 platelets per well) remaining associated to the collagen-coated well.
Control corresponds to direct incubation of platelets with coated collagen in complete Tyrode’s buffer supplemented with 0.5% BSA.
Effects of CyPB on Ca2+ movements and protein phosphorylation.
In the following experiments, we investigated whether the enhancing effect of CyPB on platelet adhesion may be related to the transduction of intracellular signals. In this way, we analyzed possible pathways of CyPB-induced platelet response by measuring the dose- and time-responses of intraplatelet Ca2+ signal generation and protein kinase activation following CyPB addition.
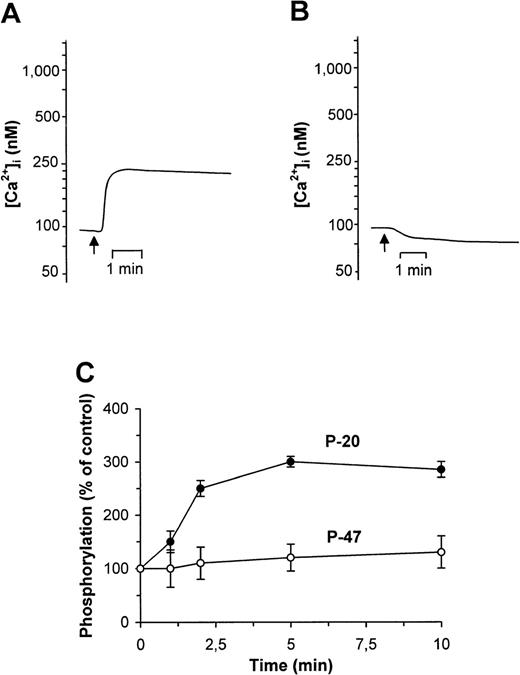
To analyze Ca2+ responses, a series of spectrofluorimetric experiments was performed on platelets loaded with the Ca2+fluorophore Fluo-3. Addition of CyPB (10 to 500 nmol/L) induced an increase in cytosolic free Ca2+ and the concentration of ligand required for a maximum response was estimated at 100 nmol/L. These values are consistent with the CyPB concentrations required for surface binding site occupancy. Stimulation with CyPB resulted in a low and durable Ca2+ flux, with an increase from 90 ± 35 nmol/L to 225 ± 45 nmol/L in the first minute (Fig4A). This elevation in cytosolic free Ca2+ concentration was nevertheless not comparable to that induced by thrombin, which was estimated at 1,220 ± 205 nmol/L. To inhibit extracellular Ca2+ entry, platelets were diluted 1 minute before analysis in a CaCl2-depleted buffer containing 2 mmol/L EGTA. In this case, the effect of CyPB was similar to that observed in the absence of any activator (Fig 4B), which suggests that the elevation in cytosolic free Ca2+initiated by CyPB is likely to be generated by a transmembranous influx of extracellular Ca2+ through a membrane channel. We then examined the influence of CyPB binding on the formation of inositol phosphate (InsPs) derivatives, after platelet labeling withmyo-[3H]inositol. The exposure of platelets to CyPB for times varying from 1 to 10 minutes did not increase the levels of these second messengers (1,260 ± 320 cpm within 5 minutes) in comparison to basal levels (930 ± 240 cpm), while thrombin induced a large and rapid increase in InsPs concentration (14,400 ± 1,080 cpm within 5 minutes). These results further demonstrate that CyPB-induced Ca2+ flux is quite different from that initiated by thrombin and not dependent on the activation of PLC.
Effects of CyPB on Ca2+ flux and phosphorylation of myosin light chains (P-20) and pleckstrin (P-47) in platelets. Ca2+ mobilization, measured in Fluo-3–loaded platelets, was analyzed after the addition of CyPB (100 nmol/L normal (A) or Ca2+-depleted buffer (B). Changes in fluorescence, reflecting changes in cytosolic Ca2+ concentration, were monitored by flow cytofluorimetry. Tracings are representative of 3 distinct experiments conducted with platelets from separate individuals. The arrows indicate addition of the ligand. Platelet response to CyPB (100 nmol/L) was also analyzed in terms of variations of P-20 and P-47 protein phosphorylation (C). Reactions were stopped at the indicated times and the variations in the intensity of serine phosphorylation of P-20 (•) and P-47 (○) were analyzed. Zero time results were obtained without addition of the ligand. Data are calculated as the percentage of intensity at indicated times relative to that at time zero, and expressed as mean values ± SEM from 3 separate experiments conducted with platelets from different donors.
Effects of CyPB on Ca2+ flux and phosphorylation of myosin light chains (P-20) and pleckstrin (P-47) in platelets. Ca2+ mobilization, measured in Fluo-3–loaded platelets, was analyzed after the addition of CyPB (100 nmol/L normal (A) or Ca2+-depleted buffer (B). Changes in fluorescence, reflecting changes in cytosolic Ca2+ concentration, were monitored by flow cytofluorimetry. Tracings are representative of 3 distinct experiments conducted with platelets from separate individuals. The arrows indicate addition of the ligand. Platelet response to CyPB (100 nmol/L) was also analyzed in terms of variations of P-20 and P-47 protein phosphorylation (C). Reactions were stopped at the indicated times and the variations in the intensity of serine phosphorylation of P-20 (•) and P-47 (○) were analyzed. Zero time results were obtained without addition of the ligand. Data are calculated as the percentage of intensity at indicated times relative to that at time zero, and expressed as mean values ± SEM from 3 separate experiments conducted with platelets from different donors.
To analyze the effect of CyPB on protein kinase activation, platelets were incubated in the presence of various concentrations of CyPB and the patterns of phosphorylation were compared with those obtained in the absence of agonist, or in the presence of thrombin (0.5 U/mL) taken as a positive control. CyPB did not induce any significant changes in tyrosine phosphorylation (data not shown). We then compared the profiles of serine phosphorylation of P-47 pleckstrin, substrate for protein kinase C (PKC)27 and P-20 myosin light chains, substrate for myosin light chain kinase (MLCK).28 29 As expected, the addition of thrombin to platelets resulted in the rapid phosphorylation of P-47 and P-20. After CyPB stimulation, an increase in serine phosphorylation was essentially observed for the 20-kD proteins, while P-47 was not significantly modified (Fig 4C). Phosphorylation of P-20 rapidly increased and was maximum within 5 to 10 minutes, which shows that it occurred after the generation of Ca2+ influx. Moreover, the optimal concentration of CyPB was estimated at 100 nmol/L, with a threefold increase in intensity, at a level similar to that obtained with platelets challenged with thrombin (290% ± 10% and 250% ± 15% for CyPB and thrombin, respectively). In contrast, CyPB did not induce any increase in P-20 phosphorylation when similar experiments were reproduced in the absence of extracellular Ca2+. Taken together, these results indicate that the dose- and time-dependent responses to CyPB for increasing P-20 phosphorylation paralleled those observed for elevation of cytosolic free Ca2+ concentration, which suggests that both events are related.
DISCUSSION
Our previous work showed that CyPB specifically binds to the surface of human T cells.15-17 Here, we present new data demonstrating that specific binding sites are also expressed at the surface of human platelets and exhibit similar affinity for CyPB. No binding was observed to erythrocytes and monocytes, and most probably the methods used were not sensitive enough to conclude to the presence or absence of surface CyPB binding sites on granulocytes. We reported that CyPB interacts with two types of binding sites present on the membrane of T lymphocytes.17 The first ones, termed type I binding sites, involve interactions with the CsA-binding domain of CyPB, while the type II sites are mainly represented by GAG present on the T-cell membrane and involve interactions with the N-terminal extension of the protein. The present data demonstrate that the interactions of CyPB with the platelet receptor and the lymphocyte type II binding sites are quite different, which excludes a role for platelet GAG in CyPB binding. Actually, the lymphocyte type II sites were found to mainly correspond to molecules of the heparin/heparan sulfate family.17 Conversely, platelet GAG are almost exclusively represented by chondroitin-4-sulfate,30 which might explain the absence of type II binding sites on platelets. The involvement of the RGD tripeptide in the interactions of a large family of ligands, such as fibrinogen and von Willebrand factor, with platelet membrane receptors has been largely documented.25 Nevertheless, the RGDS peptide was unable to compete with CyPB binding, which rules out the role of integrins of the GPIIb/IIIa family in the binding of CyPB. We then demonstrated that both CsA and CsG, but not CsH, reduced CyPB binding to the platelet membrane. Actually, active drugs overlay the binding domain of CyPB when complexed to the protein and probably lead to a loss of accessibility for the platelet receptor. In addition, CyPC, but not CyPA, was found here to compete with CyPB for binding to platelet receptor. However, the area of the cyclophilin isoforms that interacts with cyclosporine derivatives is strongly conserved,4,6,7 which disagrees with the role of this conserved catalytic domain in receptor recognition. Most probably, divergent regions differentially influence the spatial conformation of these proteins and therefore explain variations in the interactions with specific receptors. Such properties were also observed for the binding of CyPB to lymphocyte type I sites,17 which strongly suggests that both CyPB receptors are related.
The events initiated by CyPB binding to the platelet receptor were unexpected and appeared to differ in important ways to those induced by agonists like thrombin.18 CyPB was found to increase platelet adhesion to collagen, but was unable to promote degranulation or aggregation. The mechanism by which CyPB increases platelet adhesion to collagen is dependent on the presence of extracellular Ca2+ and is accompanied by the elevation of cytosolic free Ca2+ concentration and P-20 phosphorylation. However, it does not require the generation of InsPs, which suggests that stimulation of CyPB membrane sites mediates activation of effectors other than PLC. Moreover, the absence of the PKC-dependent phosphorylation of P-47 demonstrates that there is no generation of diacylglycerol and further confirms the absence of any activation of PLC. In contrast, the effect of CyPB on phosphorylation of P-20 indicates that the increase in cytosolic free Ca2+concentration probably led to the activation of MLCK. Indeed, phosphorylation of the P-light chains of myosin may be induced by direct activation of this kinase by the low elevation of intracellular Ca2+ concentration.18 Similar events are observed when platelets are exposed to cold temperatures. Chilling platelets was reported to promote a Ca2+ entry through the inhibition of the membrane Ca2+/adenosine triphosphatase (ATPase) channel by low temperature and to induce serine/threonine phosphorylation.31,32 These events would be essentially mediated by MLCK in response to the increase in cytosolic Ca2+.33 This is consistent with our hypothesis that CyPB-mediated Ca2+ influx is related to the phosphorylation of P-20 in promoting the activation of MLCK. On the other hand, a CyPB-associated protein, termed calcium-signal–modulating cyclophilin ligand, was already reported to participate in the transmission of Ca2+ influx signals in T cells.34 This protein was postulated to regulate intracellular Ca2+ release or generate a signal responsible for opening plasma membrane channels.34 Such a CyPB-binding protein might be expressed at the surface of platelets and be involved in the control of Ca2+ influx in association with extracellular CyPB. In this way, CyPB might interact with a receptor-operated channel and initiate a transmembraneous influx of Ca2+, leading to the activation of the Ca2+-dependent MLCK. The phosphorylation of P-light chains of myosin and increase in cytosolic free Ca2+ concentration are thought to play a key role in the contractile events associated with platelet shape changes and adhesion.21,28,29 35 It is therefore conceivable to postulate that CyPB-mediated activation of MLCK and Ca2+ entry is related to the enhancing effect of the protein on platelet adhesion.
The addition of CsA significantly reduced the enhanced platelet adhesion. These results indicate that the surface binding and activity of CyPB are related and can be abolished by occupancy of the CsA-binding domain of the protein. This inhibitory effect is likely to be dependent on the concentration of the drug. Most probably, CsA divided between CyPB and other binding sites, eg, platelet proteins and lipids, and large molar excesses of the drug are necessary to form a stable and inactive complexed form of CyPB. Elevated concentrations of CsA are currently measured in blood from transplant recipients, which suggests that the drug could interfere with the biologic activity of CyPB and hemostatic parameters. Many in vivo observations report that CsA increases the risk of thromboembolism in transplant patients, which is mainly related to endothelium damage and abnormalities in platelets and the coagulolytic system. However, data concerning the effect of CsA on hemostasis and platelet functions are often confusing. A prothrombotic effect of the drug was generally reported and attributed to an increased activation of PKC after stimulation by agonists.36,37 In contrast, CsA therapy was reported to increase the levels of antithrombin and protein C, two proteins known to protect against venous thromboembolism.38 Most recently, CsA was demonstrated to have both proaggregatory but anticoagulant effects, which are largely related to platelet reactivity and drug concentration.39 Further studies will therefore be necessary to determine the mechanisms by which CyPB in combination with hemostatic agents could modulate platelet reactivity and coagulation in the presence of CsA.
We reported in a previous work that CyPB levels in plasma from healthy donors were in the range of 5 nmol/L.14 However, we demonstrated here that CyPB-mediated Ca2+ flux generation and increase in platelet adhesion to collagen required higher concentrations. Such concentrations have been measured in the plasma from patients with sepsis, which indicates that CyPB may be secreted as an inflammatory response and exert a cytokine-like activity.40 On the other hand, CyPB was demonstrated to escort and stabilize procollagen chains all along the secretory pathway.41 Thus, CyPB could be secreted together with collagen and accumulate in the subendothelial matrix. As a response to blood vessel offense, platelets adhere to the site of injury and become activated. These events are mainly mediated by the contact of collagen with platelets.18 Therefore, the occurrence of collagen might be associated with the liberation of CyPB from the subendothelial matrix at the site of the injured vessel, which would allow the protein to exert its enhancing effect on platelet adhesion.
In nonexcitable cells such as platelets, extracellular Ca2+entry is thought to be controlled in part by agonists that act directly on plasma membrane Ca2+ channel.42 Studies with permeabilized platelets demonstrated that guanine nucleotide regulatory proteins were involved in the adhesive process to collagen.43 In this way, the platelet CyPB receptor might be associated with such regulatory proteins and control the activity of a membrane channel. Our objective is now to characterize the platelet receptor as a possible Ca2+ channel-associated protein and to ascertain whether it is related to the lymphocyte type I binding sites. This should allow further understanding on the biologic functions of released CyPB.
ACKNOWLEDGMENT
We are grateful to Dr J.J. Huart, Director of the Etablissement de Transfusion Sanguine, Lille, for providing us with blood samples, and to Prof A. Tartar for the synthesis of the peptides used in this work. We also thank Drs J.F. Borel and M. Zurini for generous gifts of cyclosporine derivatives and human recombinant CyPC.
Supported by the Université des Sciences et Technologies de Lille, CNRS (Unité Mixte de Recherche no. 111; Director: Professor A. Verbert) and by a grant from the Conseil Régional du Nord/Pas-de-Calais (contract: “Maladies neurodégénératives et Vieillissement”).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Prof Geneviève Spik, Laboratoire de Chimie Biologique, Unité Mixte du CNRS no. 111, Universitédes Sciences et Technologies de Lille, 8576 Villeneuve d’Ascq Cedex, France; e-mail: genevieve.spik@univ-lille1.fr.

![Fig. 1. Surface binding of [125I]CyPB to platelets. Dose-dependence and saturation of CyPB binding were studied by incubating platelets with the indicated concentrations of [125I]CyPB for 1 hour at 22°C. The specific binding (•) was obtained after subtraction of nonspecific (▴) from total counts (▪). Points represent the mean values of triplicates from a representative experiment (A). (B) Scatchard plot of the binding data.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/3/10.1182_blood.v94.3.976.415k19_976_983/5/m_blod41519001x.jpeg?Expires=1769135406&Signature=IMHinDSMLoGg3mczrXWti11YfnJFG4lYVupS~PHxAhK97cAIJb5qjV2lLV~b7xTAPRKGqfbdqUWqn6KuQXaVfhoJHOPSY6CCqoIB02BhnXd8o9Y7aE9Z7l6Jrg3n3-ubtXyamdRxAT9d83I6jtAN0gYZdv5eZBwugeGz8gjErm7UPZ3IxWxcL~jCZO9b~b5F1rY84U~I56PuXbtCmYpwKpQLP5uCeMsKYJcpEd0aTUJND3vzH6npudXQmZsQemuLdrxiSwWmIced1AAz22WpwetnyVWT29MMyMx70Ja1GVoCdjXudq8yWn45YwuZ5MRxZpldTPd7cvVSYDaIGrGu4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Specificity of [125I]CyPB binding to platelets. (A) Competitive binding assays with cyclophilin isoforms and synthetic peptides copying specific sequences of CyPB. Platelets were incubated in the presence of 50 nmol/L [125I]CyPB and increasing concentrations of unlabeled CyPA (▪), CyPB (•), CyPC (⧫), N-terminal peptide of CyPB (○), C-terminal peptide of CyPB (□), or RGDS peptide (⋆). After washing, the amounts of remaining surface-bound [125I]CyPB, expressed as a percentage of the ligand bound in the absence of competitors, are plotted against the molar ratios of [125I]CyPB to the competitors. Data are expressed as mean values from 3 separate experiments conducted with platelets from different donors. (B) Sensitivity of CyPB binding to cyclosporine derivatives, protamine, and GAG-degrading enzymes. Platelets were incubated with of 50 nmol/L [125I]CyPB in the absence (1) or presence of CsA 500 nmol/L (2); CsA 5 μmol/L (3); CsG 500 nmol/L (4); CsG 5 μmol/L (5), CsH 500 nmol/L (6); CsH 5 μmol/L (7); protamine 500 nmol/L (8); or protamine 5 μmol/L (9). In the last cases, platelets were first pretreated with heparinase type I (10), chondroitinase ABC (11), or both (12), and directly used for binding experiments. After washing, the amounts of remaining surface-bound [125I]CyPB were expressed as a percentage of the ligand bound in the absence of any treatment. Data are mean values ± SEM from 3 separate experiments conducted with platelets from different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/3/10.1182_blood.v94.3.976.415k19_976_983/5/m_blod41519002x.jpeg?Expires=1769135406&Signature=kRxeczJGxHDQxZcLPLEgEMEYzzvunK5PfHfmClWbNTUin0PLmQlbQ0fzdg2-IKSw6afQuIXqx379zxOG6EK4MlaLyT2NxQGl~zU7hB~skgXp00JpsJy8KdxExdJtkGUbiK70vKOQZ4hxsxhDSIBD2-dUgbyfeO4RaHloGt36L3a1ywr9J~4DdvKvPZGRD5GVPrh715KUHNpZEkp7gVFhfO-~L0z1ViFyYDiQZ0KVMvznKYdTEknyM0pJGRW2kcYCb6We7yKxUONLOweOTtwF6mSAPtd-PAiaa9aYAjbBWyjU2ifTsoCPtsMW0e0kNheq7rkpM86~Mnc5umMvMNxyCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal