To the Editor:
In their recent report in Blood, Neves et al1demonstrated the close proximity of BCR and ABL in hematopoietic cells in late S-phase of the cell cycle and provided a basis for a model to explain the t(9;22) chromosomal abnormality. We have studied the relative positioning of BCR and ABL in cells that happened to be accumulating in late S- and G2M-phase, and observed a similar proximity of the 2 genes. In our evaluation, the juxtaposition appears to occur at the time of the replication of the second of the 2 BCR loci.
The cells analyzed were from a 49-year-old man who had just begun reinduction for a relapsed acute leukemia. The patient was initially diagnosed morphologically as having acute myelogenous leukemia a year earlier at an outside hospital, but on transfer to the University of Chicago was found to have circulating blasts (white blood cell [WBC] count, 4.9 × 109/L, 8% blasts) with a common precursor B-cell phenotype (CD19+, CD10+, TdT+, CD34+, sIg−, CD13−, and CD33−, MyPx−). Fluorescence in situ hybridization (FISH) analysis with probes forMBCR and ABL (Oncor, Gaithersburg, MD) was performed on a peripheral blood buffy coat smear (2% blasts) to determine if the lymphoblasts were BCR/ABL+, and if so whether other cell types were also positive.2 Inadvertently, the specimen for FISH was obtained ∼15 hours after the onset of therapy with cyclophosphamide, daunorubicin, vincristine, and prednisone.
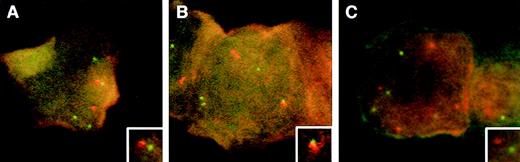
The FISH results, when correlated with the morphology of the previously Wright-stained cells, showed no consistent BCR/ABL fusion signals in the blasts or other cell types. This was supported by cytogenetic and molecular analyses of an involved bone marrow, which showed that the patient had a normal karyotype, and no BCR/ABLfusion transcripts for p190 or p210 by reverse transcriptase-polymerase chain reaction (RT-PCR) analysis. The FISH results did show, however, that most of the blasts (16 of 27 evaluated) appeared to be in late S- or G2M-phase, with many cells showing 3 or 4 copies of both ABL and BCR (Fig 1A through C). This cell-cycle distribution presumably was an effect of the recently administered vincristine.3The analysis also showed that the BCR and ABL signals tended to approach one another when there were 6 or 7 total signals (consistent with late S-phase) (Fig 1A), to merge at a time when the second BCR seemed to be replicating (Fig 1B, and inset), and to separate at G2M (Fig 1C). The distance between the 2 closest BCR and ABL signals was shortest (approaching 1 signal diameter length) when there were 6 or 7 total signals, and this was statistically different from the shortest separation in cells with 4/5 or 8 total signals (P < .0001 and P < .001, respectively).
FISH analysis with a probe set for MBCR andABL, in blasts from a patient with recently treated acute lymphoblastic leukemia shown to be Philadelphia-chromosome–negative and BCR/ABL-negative. The digoxigenin-labeled probe forBCR is detected with anti-dig rhodamine (red), and the biotinylated probe to ABL is detected with fluoreceinated avidin (green). The 3 illustrations represent cells in late S- (A and B) and G2M-phase (C) of the cell cycle. In (A), there are 4 green and 3 red signals with close proximity of BCR/ABL(inset). In (B), there are 4 green and 3 red signals, including a doubling BCR (red) signal which is juxtaposed to ABL(inset). The cell in (C) is at G2M and shows 4 green and 4 red signals with no juxtaposition of BCR and ABL.
FISH analysis with a probe set for MBCR andABL, in blasts from a patient with recently treated acute lymphoblastic leukemia shown to be Philadelphia-chromosome–negative and BCR/ABL-negative. The digoxigenin-labeled probe forBCR is detected with anti-dig rhodamine (red), and the biotinylated probe to ABL is detected with fluoreceinated avidin (green). The 3 illustrations represent cells in late S- (A and B) and G2M-phase (C) of the cell cycle. In (A), there are 4 green and 3 red signals with close proximity of BCR/ABL(inset). In (B), there are 4 green and 3 red signals, including a doubling BCR (red) signal which is juxtaposed to ABL(inset). The cell in (C) is at G2M and shows 4 green and 4 red signals with no juxtaposition of BCR and ABL.
Our chance evaluation of cells from a patient who had already begun therapy permitted us to observe BCR and ABL positioning in the otherwise infrequent cells of late S and G2M. Although the small number of cells studied provide somewhat limited data, our finding supports the results of Neves et al, showing close juxtaposition of the 2 genes in late S-phase of primitive hematopoietic cells. Our finding further advances their model by suggesting that the actual juxtaposition of the 2 genes may occur around the time of the replication of BCR. Our illustrations clearly show an approach, convergence, and separation of the 2 genes when there is full tetrapolid complement of ABL signals, and when BCR copy number goes from 3 to 4. Although the close proximity of BCRand ABL was identified in late S-phase cells in the work of Neves et al, the replication status or copy number of the BCRand ABL genes is not apparent from their data. In fact, it is curious that in their Fig 1, the late S-phase cell depicted seems to have only a diploid complement (2 signals each) of BCR andABL.
Our finding of BCR and ABL closest juxtaposition in late S-phase at a time near an apparent BCR replication would support the possibility of the BCR/ABL fusion developing during a replication error in BCR. Recombination may more likely occur during DNA replication, and this may be the case with regard to the development of BCR/ABL and t(9;22).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal