Abstract
Within multi-subunit Ig receptors, the FcR γ-chain immunoreceptor tyrosine-based activation motif (ITAM) plays a crucial role in enabling antigen presentation. This process involves antigen-capture and targeting to specific degradation and major histocompatibility complex (MHC) class II loading compartments. Antigenic epitopes are then presented by MHC class II molecules to specific T cells. The high-affinity receptor for IgG, hFcγRIa, is exclusively expressed on myeloid lineage cells and depends on the FcR γ-chain for surface expression, efficient ligand binding, and most phagocytic effector functions. However, we show in this report, using the IIA1.6 cell model, that hFcγRIa can potentiate MHC class II antigen presentation, independently of a functional FcR γ-chain ITAM. Immunoelectron microscopic analyses documented hFcγRIa -chain/rabbit IgG-Ovalbumin complexes to be internalized and to migrate via sorting endosomes to MHC class II-containing late endosomes. Radical deletion of the hFcγRIa -chain cytoplasmic tail did not affect internalization of rabbit IgG-Ovalbumin complexes. Importantly, however, this resulted in diversion of receptor-ligand complexes to the recycling pathway and decreased antigen presentation. These results show the hFcγRIa cytoplasmic tail to contain autonomous targeting information for intracellular trafficking of receptor-antigen complexes, although deficient in canonical tyrosine- or dileucine-targeting motifs. This is the first documentation of autonomous targeting by a member of the multichain FcR family that may critically impact the immunoregulatory role proposed for hFcγRIa (CD64).
RECEPTORS FOR THE Fc region of antibodies (FcR) play a coordinating role in immunity. They are expressed on various types of cells and mediate functions ranging from endocytosis, phagocytosis, antibody-dependent cell-mediated cytotoxicity (ADCC), and cytokine production, to facilitation of antigen presentation. Antigen presentation represents a process in which antigens are captured, targeted to appropriate compartments, and processed before binding to major histocompatibility complex (MHC) molecules. MHC class II distribution is dependent on the species origin, cell type, and maturation state of the cell, and peptide loading can occur in both early and late endocytic compartments (reviewed in Watts1and Geuze2). In general, MHC class II molecules are found along the endocytic pathway,3 accumulating in late endosomal and lysosomal compartments, called MIICs (MHC class II enriched compartments).4 Some cell types contain MHC class II molecules in specialized class II vesicles (CIIVs), which are distinct from conventional endocytic compartments.5 After loading of MHC class II molecules with antigenic epitopes, they are transported to the cell surface, where they serve to stimulate antigen-specific CD4+ T cells.1
Leukocyte FcR for IgG (FcγR) comprise a multigene family, divided in 3 classes (FcγRI, II, and III) based on differences in receptor structure, cell distribution, and affinity for IgG.6 FcγR molecules can potently enhance antigen presentation, and the type of FcγR involved has been shown a crucial determinant for the types of epitopes presented by the antigen-presenting cell.7 The human high-affinity receptor for IgG, hFcγRIa (CD64), is constitutively expressed on antigen-presenting cells such as monocytes, macrophages, and dendritic cells, and hFcγRIa-targeted antigens are presented very efficiently both in vitro8 and in vivo.9
Most FcR exist as multi-subunit receptor complexes with unique ligand binding α-chains, and promiscuous accessory (γ-, ζ-, or β-) signaling chains. The hFcγRIa ligand binding α-chain depends on the γ-chain for stable surface expression in vivo,10 optimal ligand binding capacity,11 and effector-functions such as phagocytosis, cytokine production, and ADCC (Van Vugt et al10 and own unpublished data). An immunoreceptor tyrosine-based activation motif (ITAM) within the FcR γ-chain was found to be crucial for initiation of these FcγRIa-triggered functions. Similar data were generated for FcγRIIIa (CD16),12 FcγRIIa (CD32),13 and FcαRI (CD89).14 In all these FcR complexes, the integrity of the γ-chain ITAM proved indispensable for antigen presentation.12-14 In the present report, we assessed the role of FcR γ-chain in hFcγRIa-mediated antigen presentation. Using IIA1.6 cell transfectants, we show that the hFcγRIa α-chain potentiates MHC class II antigen presentation, independently of a functional FcR γ-chain. Furthermore, the hFcγRIa cytoplasmic domain was found to contain autonomous targeting information for intracellular trafficking of receptor-antigen complexes.
MATERIALS AND METHODS
cDNAs.
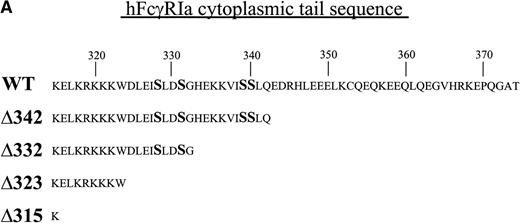
FcγRIa wild-type (WT),10 FcγRIa tail truncated mutant (Δ), and point mutant cDNAs were cloned into the pRC-CMV vector. FcγRIa Δ342, Δ332, Δ323, and Δ315 represent mutant molecules with progressively truncated cytoplasmic tails. FcγRIa Ser (328, 331, 339, 340) represents a mutant molecule in which serine (S) residues were mutated to alanines. Truncations of the FcγRIa cytoplasmic tail were obtained by insertion of stop codons, and point mutations were generated by two-step overlap extension polymerase chain reaction (PCR). Integrity of cDNAs was confirmed by sequence analysis. The wild-type murine FcR γ-chain cDNA15 and mutant γY65F,Y76F cDNA, representing a molecule in which both tyrosines within the ITAM were changed to phenylalanines, were cloned into the pNUT expression vector, allowing selection of transfectants with methotrexate (MTX).16
Cells.
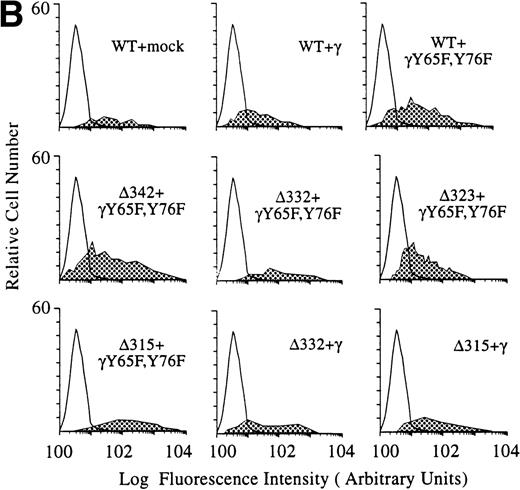
The FcγR and FcR γ-chain negative murine IIA1.6 cell line,17,18 the ovalbumin (OVA)-specific T-cell hybridoma 3DO-54.8,19 and the interleukin-2 (IL-2)–dependent CTLL-16 cell line were cultured as described.13 IIA1.6 cells were transfected by electroporation performed with Bio-Rad (Richmond, CA) equipment set at 250 V, 960 μF.10 Cotransfectants of FcγRIa (WT), (Δ), or point mutants with mock vector, wild-type γ-chain, or γY65F,Y76F were cultured in the presence of 5 μmol/L MTX (Pharmachemie, Haarlem, The Netherlands) to ensure high levels of hFcγRIa expression.17
Immunofluorescence and reverse transcription-PCR (RT-PCR).
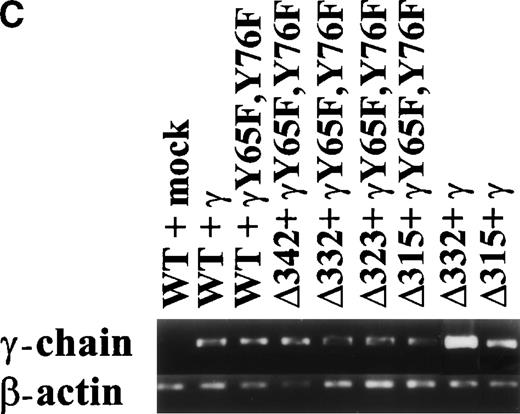
hFcγRIa expression levels of the various cotransfectants were checked using fluorescein isothiocyanate (FITC)-labeled CD64 specific monoclonal antibodies (MoAbs) 22 and 32.2 (Medarex, Annandale, NJ). Cells were incubated with immunofluorescence buffer (1% bovine serum albumin [BSA]/0.1% azide, pH 7.4) alone or with MoAbs for 30 minutes at 4°C, followed by washing twice. Cells were analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Murine FcR γ-chain wild-type and γY65F,Y76F expression was checked by RT-PCR on transfected cells using specific primers as described.17cDNA quality was confirmed by amplification of a 345-bp β-actin band, using specific primers.17
Internalization of hFcγRIa-IgG-OVA complexes.
Rabbit IgG-OVA complexes were generated by incubation of 40 μg/mL chicken egg albumin (Sigma, St Louis, MO) with 20 μg/mL specific rabbit IgG antiserum (Sigma) for 20 minutes at 37°C.13125I-labeled donkey-antirabbit F(ab′)2fragments (DαR; 0.1 μCi; Amersham, Roosendaal, The Netherlands) were then added at a final concentration of 50 ng/mL to the rabbit IgG-OVA complexes (200 ng/mL). IIA1.6 cells (5 × 106) expressing either FcγRIa WT/γ-chain, FcγRIa WT/mock vector, or FcγRIa Δ315/γY65F,Y76F were incubated with 100 ng/mL125I-IgG-OVA for 15 minutes on ice. After washing three times with ice-cold phosphate-buffered saline (PBS) supplemented with 1% BSA (PBA), the temperature was shifted to 37°C for different periods of time. Samples were washed three times with either PBA (control for total binding) or PBA-HCl at pH 2.5 (to remove surface-bound radiolabeled complexes). Samples were centrifuged for 3 minutes (850g), and supernatants were removed by aspiration, before analysis of pellets by gamma spectometry. The percentage of internalization of 125I-labeled complexes at each point was calculated from the ratio of acid-resistant binding to total (acid-resistant + acid-released) binding.
Modulation of FcγRI expression.
Transfected IIA1.6 cells (2.5 × 105) were seeded in 100 μL of RPMI 1640 medium with 10% fetal calf serum (FCS) in microtiter wells and either 0, 10, 100, or 1,000 ng/mL rabbit IgG-OVA complexes were added. Samples were incubated at 37°C for 16 hours, washed, and stained with CD64 specific MoAb 32.2 followed by phycoerythrin (PE)-labeled F(ab′)2 fragments of goat antimouse IgG1 antiserum (Southern Biotechnology, Birmingham, AL). The percentage of modulation was determined as the reduction in receptor expression relative to untreated transfectants.20
Antigen presentation assays.
Immune complexes were generated as described above. Transfected cells (1 × 105) were cultured with different concentrations of immune complexes for 6 hours at 37°C, followed by washing twice. Cells were subsequently incubated with 2 × 104 OVA-specific T cells for 16 hours at 37°C. The presence of IL-2 released by T cells in the supernatants was determined by using IL-2–dependent CTLL-16 cells exactly as described.13 The capacity of OVA-specific T cells to produce IL-2 was checked for each transfectant by addition of a large excess of OVA (100 μg/mL). In control experiments, IIA1.6 transfectants were cultured with immune complexes in the absence of T cells, and 3H-thymidine was never found incorporated in CTLL-16 cells. This confirmed IL-2 to be produced exclusively by T cells under these experimental conditions. In select experiments, antigen-presenting IIA1.6 transfectants were preincubated for 3 hours at 37°C with 10 μg/mL cycloheximide (Sigma). Cycloheximide, furthermore, remained present during incubation with immune complexes or OVA alone, until incubation with T cells. In other experiments, IIA1.6 transfectants were preincubated with the protein kinase inhibitor H7 (75 μmol/L; Biomol, Plymouth Meeting, PA) for 30 minutes at 37°C and, furthermore, during incubation with immune complexes (or OVA alone) until incubation with T cells.
Preparation of OVAgold conjugates and cationic HRP (cHRP).
OVA was coupled to 5-nm gold particles as described.21Briefly, 30 μg/mL OVA was added to 200 mL gold sol for 15 minutes at pH 5.3 and then ultracentrifuged to obtain a condensed pellet of 5-nm OVAgold particles. This was added to cells at a final concentration of OD 0.5. cHRP (type IV; Sigma) was prepared according to Rennke et al.22
Immunoelectron microscopy (IEM).
Cells were fixed and prepared for ultrathin cryosectioning and immunolabeling as described.23 Briefly, cells were fixed in 2% paraformaldehyde plus 0.2% glutaraldehyde in phosphate buffer. After washing with PBS and PBS/glycine, cell pellets were embedded in 10% gelatin, cut in small blocks, and infiltrated with 2.3 mol/L sucrose for 4 hours at 4°C. Finally, the blocks were mounted and frozen in liquid nitrogen. For better visualization of membranes, ultrathin cryosections were picked up with a mixture of 2.3 mol/L sucrose and 2% methyl cellulose. In the immunolabeling, MHC class II MoAb M5/11424 and transferrin (TfR) MoAb H68.4 (Zymed, San Francisco, CA) were visualized with 10-nm protein A gold particles in single labelings. Ultrathin sections were embedded in a mixture of 2% methyl cellulose and 0.4% uranyl.
Ovagold and cHRP internalization.
Tubes containing cells (5 × 106) were placed on ice for 15 minutes and incubated with rabbit IgG anti-OVA for 15 minutes at 4°C, washed, and incubated with OVAgold particles (5 nm) for 15 minutes at 4°C. Cells were washed and resuspended in RPMI 1640 medium with 10% FCS and incubated at 37°C for 60 minutes, before fixation. For cHRP internalization, 5 × 106 cells were washed in RPMI 1640 medium without FCS and incubated with the endocytosis marker cHRP (1 mg/mL) for 60 minutes at 37°C. Subsequently, cells were washed three times and fixed as described above.
Quantative analysis of internalized OVAgold and cHRP.
Ultrathin cryosections of hFcγRIa WT/γY65F,Y76F, Δ315/γY65F,Y76F, WT/γ-chain, and Δ315/γ-chain cells, incubated with OVAgold as described above, were analyzed by electron microscopy. Three × 20 or 4 × 20 cell profiles were randomly selected. Gold particles were counted in intracellular compartments, including early endosomes, late endosomal multivesicular bodies, lysosomes, and small coated and noncoated vesicles and tubules. The distribution of cHRP after 1 hour of continuous uptake at 37°C was evaluated on ultrathin sections of both transfectants, which were immunolabeled with anti-HRP polyclonal antibody (Sigma) and 10-nm protein A gold particles.
Statistics.
Statistical analyses were performed with a paired Student’st-test. Significance was accepted at the P < .05 level.
RESULTS
hFcγRIa transfectants.
To dissect the role of individual subunits of the hFcγRIa-complex in antigen presentation, we generated a panel of wild-type (WT) and truncated (Δ) FcγRIa-constructs (Fig1A). Because FcR γ-chain has been shown to be important for stable surface expression of hFcγRIa under physiological conditions,10 α-chain constructs were cotransfected in IIA1.6 cells with either wild-type or mutant FcR γ-chain (γY65F,Y76F). In all multi-subunit Fc receptor complexes analyzed to date, the integrity of the γ-chain ITAM proved indispensable for antigen presentation. To uncouple signaling directed through the hFcγRIa α-chain from that via the γ-chain, we cotransfected a γY65F,Y76F mutant in which both tyrosines were mutated to phenylalanines. These mutations ablated antigen presentation via other multi-subunit FcR (Amigorena et al7,12 and own unpublished observations). To study hFcγRIa WT α-chain’s ability to trigger antigen presentation in the absence of (stabilizing) γ-chain, a hFcγRIa WT/mock vector transfectant was established. As previously described,10 cotransfection with the mock vector enabled MTX-selection–driven (unstable) hFcγRIa expression. hFcγRIa expression levels were checked weekly using CD64-specific MoAb 22 and remained high during the course of the experiments described here (Fig 1B). All transfectants were, in addition, equally capable of binding rabbit IgG-OVA complexes, indicating that intracellular deletions and γ chain mutations do not affect extracellular binding of immune complexes (data not shown). The presence of wild-type or mutant γ-chain molecules in the transfectants was verified by RT-PCR (Fig 1C). cDNA quality was checked by amplifying β-actin (Fig 1C).
Schematic illustration of hFcγRIa mutants and expression levels of hFcγRIa and FcR γ-chain in IIA1.6 transfectants. (A) Schematic representation of the cytoplasmic tails of wild-type (WT) and truncated (▵) hFcγRIa -chains. Numbers correspond to amino acid residues counted from the initiating methionine of hFcγRIa. (B) hFcγRIa expression levels in IIA1.6 transfectants. Cells were incubated with immunofluorescence buffer alone (open profiles) or buffer with FITC-labeled CD64-specific MoAb (shaded profiles). Fluorescence was recorded as arbitrary units on a logarithmic scale and plotted against relative cell number. (C) FcR γ-chain expression in IIA1.6 transfectants. Expression was checked by RT-PCR using specific primers. β-Actin RT-PCR served as a control.
Schematic illustration of hFcγRIa mutants and expression levels of hFcγRIa and FcR γ-chain in IIA1.6 transfectants. (A) Schematic representation of the cytoplasmic tails of wild-type (WT) and truncated (▵) hFcγRIa -chains. Numbers correspond to amino acid residues counted from the initiating methionine of hFcγRIa. (B) hFcγRIa expression levels in IIA1.6 transfectants. Cells were incubated with immunofluorescence buffer alone (open profiles) or buffer with FITC-labeled CD64-specific MoAb (shaded profiles). Fluorescence was recorded as arbitrary units on a logarithmic scale and plotted against relative cell number. (C) FcR γ-chain expression in IIA1.6 transfectants. Expression was checked by RT-PCR using specific primers. β-Actin RT-PCR served as a control.
hFcγRIa internalization and modulation is independent of FcR γ-chain.
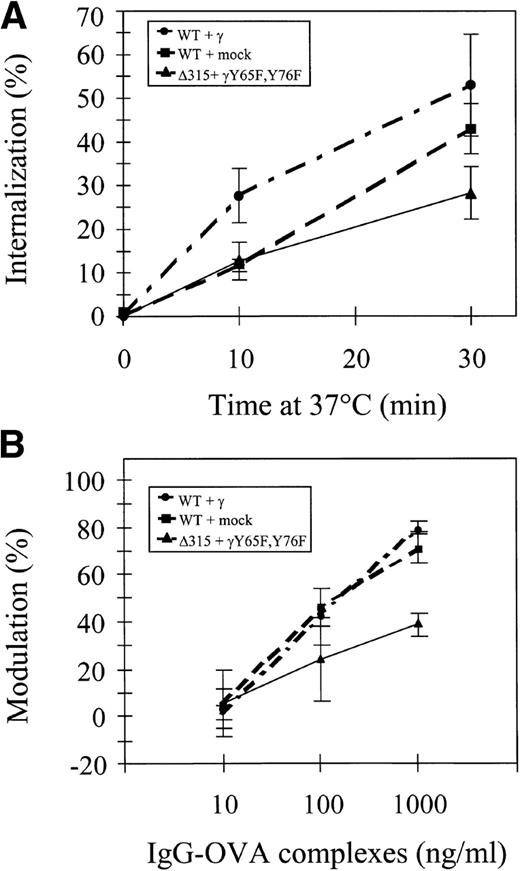
We first analyzed the capacity of different hFcγRIa receptor complexes to mediate ligand internalization by measuring uptake of125I-labeled rabbit IgG-OVA complexes. hFcγRIa/γ-chain, hFcγRIa/mock vector, and tail-deleted hFcγRIa Δ315/γY65F,Y76F-transfected IIA1.6 cells were incubated at 4°C with 125I-labeled immune complexes, washed, and subsequently incubated at 37°C for different periods of time. The internalized fraction was determined after acid elution of cell surface-bound immune complexes (Fig 2A). We found 25% to 50% of labeled immune complexes to be internalized after 30 minutes. No significant differences were detected between the three types of IIA1.6 transfectants. hFcγRIa-mediated internalization of immune complexes, thus, seems independent of coexpression of functional γ-chain.
hFcγRIa internalization and modulation. (A) Internalization of 125I-labeled rabbit IgG-ovalbumin complexes in IIA1.6 cells expressing hFcγRIa WT/γ-chain, hFcγRIa WT/mock vector, or hFcγRIa ▵315/γY65F,Y76F.125I-labeled complexes were prebound at 4°C and internalization at 37°C was determined as described in Materials and Methods. No detectable binding/internalization was observed in nontransfected IIA1.6 cells (n = 3). (B) Modulation of hFcγRIa expression by rabbit IgG-ovalbumin complexes. Transfectants were incubated overnight with complexes as detailed in Materials and Methods. Receptor expression was determined using CD64-specific MoAb 32.2 and PE-labeled goat antimouse IgG1. The percentage of modulation was calculated as defined in Materials and Methods (n = 3). Error bars indicate standard errors of the mean.
hFcγRIa internalization and modulation. (A) Internalization of 125I-labeled rabbit IgG-ovalbumin complexes in IIA1.6 cells expressing hFcγRIa WT/γ-chain, hFcγRIa WT/mock vector, or hFcγRIa ▵315/γY65F,Y76F.125I-labeled complexes were prebound at 4°C and internalization at 37°C was determined as described in Materials and Methods. No detectable binding/internalization was observed in nontransfected IIA1.6 cells (n = 3). (B) Modulation of hFcγRIa expression by rabbit IgG-ovalbumin complexes. Transfectants were incubated overnight with complexes as detailed in Materials and Methods. Receptor expression was determined using CD64-specific MoAb 32.2 and PE-labeled goat antimouse IgG1. The percentage of modulation was calculated as defined in Materials and Methods (n = 3). Error bars indicate standard errors of the mean.
We next assessed immune complex-induced hFcγRIa modulation in IIA1.6 cells. hFcγRIa WT/γ-chain WT, hFcγRIa/mock vector, and hFcγRIa Δ315/γY65F,Y76F transfectants were incubated overnight with rabbit IgG-OVA complexes. After this incubation, receptor expression was determined by flow cytometry with CD64-specific MoAb 32.2. hFcγRIa surface expression in all three transfectants was significantly decreased (P < .05) upon incubation with 100 and 1,000 ng/mL rabbit IgG-OVA complexes. Receptor modulation by 1,000 ng/mL IgG-OVA complexes in hFcγRIa Δ315/γY65F,Y76F-transfected cells was, furthermore, found to be significantly lower compared with the other two cell lines (Fig 2B, n = 3).
The hFcγRIa ligand binding chain potentiates antigen presentation.
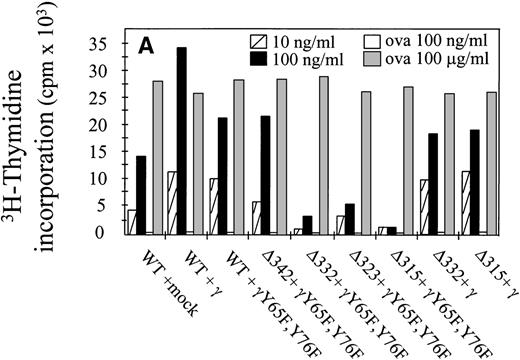
The capacity of hFcγRIa α-chain to mediate antigen internalization (Fig 2A) triggered the question of whether this could, in addition, lead to enhanced antigen presentation. To assay the role of α-chain, we used a panel of hFcγRIa transfectants (Fig 1B). hFcγRIa WT/mock vector, hFcγRIa WT/γ-chain, and hFcγRIa WT/γY65F,Y76F IIA1.6 transfectants were incubated with rabbit IgG-OVA complexes, washed, and incubated with OVA-specific T cells. Antigen presentation was assessed by measuring T-cell–secreted IL-2 using CTLL proliferation assays. No CTLL proliferation was observed when transfectants were incubated in control medium without immune complexes (data not shown) or with 100 ng/mL OVA alone (Fig 3A). However, hFcγRIa WT α-chain transfectants incubated with either 10 or 100 ng/mL immune complexes consistently presented OVA to T cells, independently of the presence of a functional γ-chain (Fig 3). Furthermore, we found the hFcγRIa WT/γ-chain–transfected cells to be significantly more efficient in antigen presentation, compared with the hFcγRIa WT/mock or hFcγRIa WT/γY65F,Y76F-transfected cells (Fig 3).
Antigen presentation by hFcγRIa-transfected IIA1.6 cells. (A) Cells were incubated with rabbit IgG-ovalbumin complexes (10 or 100 ng/mL) or OVA alone (100 ng/mL or 100 μg/mL) for 6 hours, washed, and incubated with ovalbumin-specific T cells for 16 hours at 37°C. IL-2 released by T cells was determined by CTLL proliferation assays. Data represent means of duplicate determinations in 1 representative experiment of 8. (B) Antigen presentation observed in 8 individual experiments was compared by calculating antigen presentation indices (API). API were defined by dividing cpm of incorporated3H-thymidine obtained in the presence of complexes by background cpm (obtained in the absence of complexes). Error bars represent standard errors of the mean.
Antigen presentation by hFcγRIa-transfected IIA1.6 cells. (A) Cells were incubated with rabbit IgG-ovalbumin complexes (10 or 100 ng/mL) or OVA alone (100 ng/mL or 100 μg/mL) for 6 hours, washed, and incubated with ovalbumin-specific T cells for 16 hours at 37°C. IL-2 released by T cells was determined by CTLL proliferation assays. Data represent means of duplicate determinations in 1 representative experiment of 8. (B) Antigen presentation observed in 8 individual experiments was compared by calculating antigen presentation indices (API). API were defined by dividing cpm of incorporated3H-thymidine obtained in the presence of complexes by background cpm (obtained in the absence of complexes). Error bars represent standard errors of the mean.
We next sought to define the part of the hFcγRIa α-chain responsible for facilitation of antigen presentation. The cytoplasmic tail of hFcγRIa does not contain any established tyrosine-based7,12 or dileucine-based25 26targeting motifs. To start dissecting the cytoplasmic determinants involved in antigen presentation, we made a series of sequential truncations (Fig 1A and B). We observed a decrease in antigen presentation by the hFcγRIa Δ332/γY65F,Y76F transfectant, the hFcγRIa Δ323/γY65F,Y76F transfectant, and the hFcγRIa Δ315/γY65F,Y76F transfectant (Fig 3A). The capacity of these latter transfectants to present OVA to T cells was assessed by adding a large excess of OVA (100 μg/mL) to assay fluid phase-mediated antigen presentation (Fig 3A). Coexpression of a functional FcR γ-chain restored the capacity to present immune-complexed OVA by these transfectants comparable to the observed capacity of the hFcγRIa WT/γY65F,Y76F and hFcγRIa Δ342/γY65F,Y76F transfectants (Fig3).
hFcγRIa-mediated antigen presentation requires newly synthesized MHC class II molecules and protein serine kinase activity.
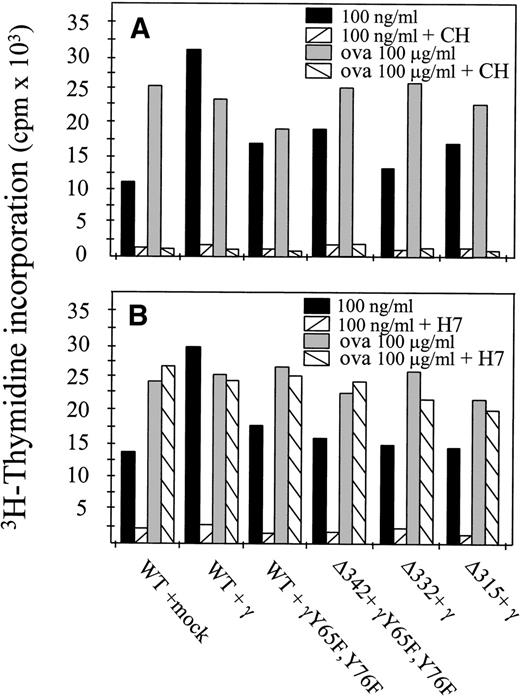
It is well accepted now that at least two different pathways for MHC class II-mediated antigen presentation exist. The first one involves newly synthesized MHC class II molecules en route to the cell surface that are loaded with antigen-derived peptides. The second pathway involves a fraction of cell surface MHC class II molecules that are internalized and recycled back to the cell surface.27To determine whether newly synthesized MHC class II molecules were involved in hFcγRIa α-chain–mediated antigen presentation, we performed experiments in the presence of cycloheximide, a potent protein synthesis blocker.27 hFcγRIa-transfected cells were preincubated with cycloheximide for 3 hours to deplete intracellular pools of newly synthesized class II molecules. Furthermore, cycloheximide was present during the 6-hour antigen presentation assay. Cells were then washed and incubated overnight with OVA-specific T cells. Inhibition of protein synthesis blocked both hFcγRIa-mediated antigen presentation as well as fluid phase-mediated presentation of OVA (Fig 4A). These results supported the idea that hFcγRIa-mediated antigen presentation involves newly synthesized MHC class II molecules.
hFcγRIa triggered antigen presentation depends on newly synthesized proteins and on protein serine kinase activity. (A) Transfectants were incubated in RPMI 1640 medium alone or medium with cycloheximide (CH) and either 100 ng/mL rabbit IgG-OVA complexes or OVA alone. B) Transfectants were incubated in medium alone or medium with H7 and either 100 ng/mL rabbit IgG-ovalbumin complexes or ovalbumin alone. For both (A and B), cells were then washed and incubated with OVA-specific T cells for 16 hours. Levels of IL-2 released by T cells were determined by CTLL proliferation assays. Data represent means of duplicate determinations in a single experiment, repeated at least four times, with essentially identical results.
hFcγRIa triggered antigen presentation depends on newly synthesized proteins and on protein serine kinase activity. (A) Transfectants were incubated in RPMI 1640 medium alone or medium with cycloheximide (CH) and either 100 ng/mL rabbit IgG-OVA complexes or OVA alone. B) Transfectants were incubated in medium alone or medium with H7 and either 100 ng/mL rabbit IgG-ovalbumin complexes or ovalbumin alone. For both (A and B), cells were then washed and incubated with OVA-specific T cells for 16 hours. Levels of IL-2 released by T cells were determined by CTLL proliferation assays. Data represent means of duplicate determinations in a single experiment, repeated at least four times, with essentially identical results.
Because the hFcγRIa α-chain cytoplasmic tail is devoid of tyrosine residues and hFcγRIa-mediated antigen presentation proved independent of the presence of tyrosines within the FcR γ-chain, we assessed the role of serine phosphorylation. Transfected cells were preincubated with the protein kinase inhibitor H7, which potently inhibits serine phosphorylation.28 After washing, cells were incubated overnight with OVA-specific T cells. Inhibition of serine phosphorylation strongly decreased hFcγRIa-mediated antigen presentation, in contrast to fluid phase-mediated antigen presentation (Fig 4B). This showed antigen presentation via hFcγRIa to occur via a process that, in contrast to the fluid-phase internalized OVA, was sensitive to H7. Furthermore, these results suggested serine phosphorylation of either the hFcγRIa α-chain or downstream signaling molecules to be important for hFcγRIa-mediated antigen presentation.
Subcellular localization of FcR/IgG/OVAgold complexes.
Because FcR antibodies failed to work in IEM (own unpublished data), the intracellular trafficking of hFcγRIa WT and hFcγRIa Δ315 α-chains in the presence of γY65F,Y76F was examined by studying ligand internalization. Rabbit IgG (anti-)OVA antibodies were thus bound to hFcγRIa at 4°C, and cells were subsequently incubated with 5-nm OVAgold for 60 minutes at 37°C. After extensive washing, cells were fixed and prepared for IEM. The uptake of OVAgold via fluid phase endocytosis was ruled out by incubating cells with OVAgold in the absence of rabbit IgG, which showed no internalized gold particles (data not shown). Furthermore, conjugation of gold particles to OVA did not disturb the intracellular processing of the protein, because only hFcγRIa WT/γY65F,Y76F cells were able to induce a specific T-cell response after uptake of OVAgold immune complexes (data not shown).
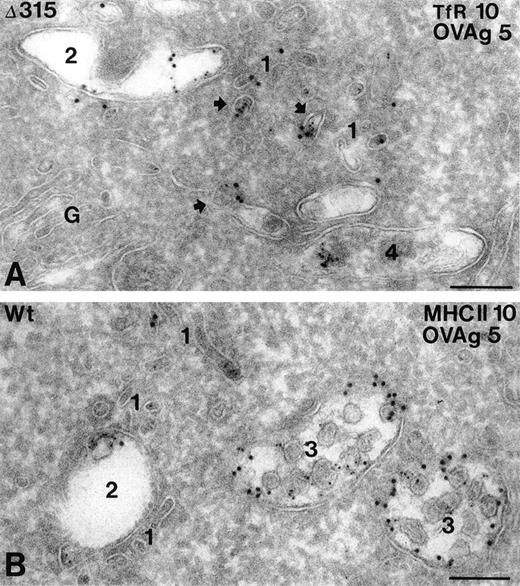
In both hFcγRIa WT/γY65F,Y76F and hFcγRIa Δ315/γY65F,Y76F transfectants, OVAgold was present in several types of compartments, including small coated and noncoated vesicles and tubules (type 1), early endosomes (type 2), late endosomal multivesicular bodies (type 3), and lysosomes (type 4). The type 1 compartment included both incoming and recycling membranes, which were either in the vicinity of the plasma membrane or early endosomes. The majority of these membranes were labeled for TfR, a known recycling surface protein29(Fig 5A). Quantative analysis of OVAgold showed 2.5-fold more OVAgold in the type 1 compartment in hFcγRIa Δ315/γY65F,Y76F cells as compared with the hFcγRIa WT/γY65F,Y76F cells (Table 1). The presence of TfR in this compartment (Fig 5A) strongly indicated that in Δ315/γY65F,Y76F cells a large pool of FcR was diverted into the recycling pathway. Type 3 compartments in Δ315/γY65F,Y76F cells displayed a relatively low percentage of OVAgold compared with hFcγRIa WT/γY65F,Y76F cells. In both cell types, OVAgold accumulated in lysosomes, although the number of gold-containing lysosomes in hFcγRIa WT/γY65F,Y76F transfectants was much higher compared with hFcγRIa Δ315/γY65F,Y76F transfectants (data not shown). MHC class II labeling in hFcγRIa WT/γY65F,Y76F was observed in all types of compartments, although primarly in the late endosomal multivesicular type 3 compartment, in which it colocalized with OVAgold (Fig 5B). To check whether the overall endocytic activity of the transfectants was comparable, an incubation with the endocytic marker cHRP was performed for 60 minutes at 37°C, and ultrathin cryosections were immunolabeled with anti-HRP and 10-nm protein A gold particles. Table 1 shows that, in both types of transfectants, cHRP was transported in a similar fashion, primarly accumulating in type 3 and 4 compartments.
Subcellular localization of internalized hFcγRIa/IgG/OVAgold complexes. Cells expressing hFcγRIa WT/γY65F,Y76F or hFcγRIa ▵315/γY65F,Y76F were incubated with rabbit IgG anti-OVA, followed by 5-nm OVAgold (OVAg), were washed, and were allowed to internalize for 60 minutes at 37°C, before fixation and preparation for IEM. Ultrathin cryosections were immunolabeled with antibodies to TfR (A) or MHC class II (B) and 10-nm protein gold particles. (A) Electron micrograph of a hFcγRIa ▵315/γY65F,Y76F expressing cell, showing that OVAg is present in small vesicles and tubules (1) and in an early endosome (2). Several of the small vesicles (arrows) display colocalization with gold particles for TfR (10 nm). A lysosome (4) shows some clustered OVAg. G, Golgi complex. (B) OVAg in a hFcγRIa WT/γY65F,Y76F transfected cell is present in type 1 vesicles and tubules (1), an early endosome (2), and two multivesicular type 3 compartments (3), the latter containing abundant MHC class II labeling (10 nm). Bars represent 100 nm.
Subcellular localization of internalized hFcγRIa/IgG/OVAgold complexes. Cells expressing hFcγRIa WT/γY65F,Y76F or hFcγRIa ▵315/γY65F,Y76F were incubated with rabbit IgG anti-OVA, followed by 5-nm OVAgold (OVAg), were washed, and were allowed to internalize for 60 minutes at 37°C, before fixation and preparation for IEM. Ultrathin cryosections were immunolabeled with antibodies to TfR (A) or MHC class II (B) and 10-nm protein gold particles. (A) Electron micrograph of a hFcγRIa ▵315/γY65F,Y76F expressing cell, showing that OVAg is present in small vesicles and tubules (1) and in an early endosome (2). Several of the small vesicles (arrows) display colocalization with gold particles for TfR (10 nm). A lysosome (4) shows some clustered OVAg. G, Golgi complex. (B) OVAg in a hFcγRIa WT/γY65F,Y76F transfected cell is present in type 1 vesicles and tubules (1), an early endosome (2), and two multivesicular type 3 compartments (3), the latter containing abundant MHC class II labeling (10 nm). Bars represent 100 nm.
Subcellular Distribution of Internalized OVAg and cHRP



| . | Type 1 . | Type 2 . | Type 3 . | Type 4 . |
|---|---|---|---|---|
| . | . | . | . | . |
| WT/γY65F, Y76F OVAg | 9 ± 3 | 11 ± 4 | 21 ± 5 | 59 ± 13 |
| Δ315/γY65F, Y76F OVAg | 24 ± 2 | 18 ± 1 | 13 ± 1 | 45 ± 2 |
| WT/γY65F, Y76F cHRP | 11 ± 4 | 12 ± 4 | 21 ± 1 | 56 ± 10 |
| Δ315/γY65F, Y76F cHRP | 9 ± 1 | 15 ± 2 | 29 ± 3 | 47 ± 1 |
| WT/γ-chain OVAg | 5 ± 1 | 6 ± 2 | 58 ± 7 | 31 ± 7 |
| Δ315/γ-chain OVAg | 11 ± 6 | 12 ± 6 | 21 ± 2 | 56 ± 11 |
| . | Type 1 . | Type 2 . | Type 3 . | Type 4 . |
|---|---|---|---|---|
| . | . | . | . | . |
| WT/γY65F, Y76F OVAg | 9 ± 3 | 11 ± 4 | 21 ± 5 | 59 ± 13 |
| Δ315/γY65F, Y76F OVAg | 24 ± 2 | 18 ± 1 | 13 ± 1 | 45 ± 2 |
| WT/γY65F, Y76F cHRP | 11 ± 4 | 12 ± 4 | 21 ± 1 | 56 ± 10 |
| Δ315/γY65F, Y76F cHRP | 9 ± 1 | 15 ± 2 | 29 ± 3 | 47 ± 1 |
| WT/γ-chain OVAg | 5 ± 1 | 6 ± 2 | 58 ± 7 | 31 ± 7 |
| Δ315/γ-chain OVAg | 11 ± 6 | 12 ± 6 | 21 ± 2 | 56 ± 11 |
Transfectants were incubated with OVAgold (OVAg) or cHRP as described in Materials and Methods. cHRP was visualized on ultrathin cryosections with an HRP antibody and 10-nm protein A gold particles. Cell profiles were randomly selected, and gold particles were assigned to a compartment when within 20 nm of a membrane. Percentages of gold particles ± SEM, based on three to four analyses of 20 cell profiles, present in each compartment are shown. Four types of compartments were distinguished, ie, incoming and recycling membranes (type 1), early endosomes (type 2), multivesicular late endosomes (type 3), and lysosomes (type 4).
Because both the hFcγRIa and FcR γ-chain are present in the receptor complex under physiological conditions,6 10 we next analyzed the combined contribution of these chains to intracellular targeting of internalized immune complexes. OVAgold disposition was studied in hFcγRIa WT/γ-chain and hFcγRIa Δ315/γ-chain transfectants. Quantitative analysis showed in hFcγRIa WT/γ-chain transfectants a striking 58% of OVAgold particles to accumulate in type 3 compartments and 31% in type 4, whereas only 5% of OVAgold particles were found in type 1 compartments. However, in the hFcγRIa Δ315/γ-chain, 21% of the OVAgold particles were detected in type 3 compartments, 36% in the lysosomal type 4 compartment, and 11% in type 1 incoming and recycling endosomes (Table 1, n = 3). OVAgold distribution in this latter transfectant resembled largely the distribution found in hFcγRIa WT/γY65F,Y76F transfectants (Table 1). These results document the efficiency of targeting of immune complexes to type 3 multivesicular compartments to be cumulative when both hFcγRIa α-chain and FcR γ-chain are present in FcγRI receptor complexes.
DISCUSSION
Most leukocyte receptors for IgG, IgE, and IgA belong to the multichain immune recognition receptor (MIRR) family and are composed of separate ligand binding α-chains and signaling γ-, ζ-, or β-subunits.6 In macrophages, wild-type FcR, bound to monovalent Fab fragments30 or monovalent IgG,31are continuously internalized and recycled back to the cell surface during endocytosis. However, FcR interacting with multivalent ligands internalize and are targeted to later endosomal compartments, resulting in downregulated surface receptor expression.32Internalization and sorting of FcR within endocytic pathways has been shown to be largely dependent on information within the cytoplasmic tails of either the FcR α-chain or the accessory chains. This information consists primarily of short, linear arrays of amino acids that function as internalization and/or sorting signals.33Within FcR, a central role of importance has been documented for the ITAM within the cytoplasmic tail of FcR γ-chain. This motif was shown essential for intracellular trafficking of murine FcγRIIIa,7 hFcγRIIa,13 and hFcαR.14 The only exception to this rule within the FcR family has been reported for FcγRIIb, which contains a dileucine sorting motif.26 34
The analyses presented here document the first example of autonomous targeting by a member of the multichain FcR. Internalization and MHC class II antigen presentation by the high-affinity IgG receptor proved independent of association with the FcR γ-chain (Fig 2). The structural requirements that underly the capacity of the FcγRIa α-chain to internalize upon ligand binding proved to reside in its transmembrane or extracellular region (Fig 2). These findings confirm and extend earlier data in U937 cells31 and COS cell transfectants.35,36 Furthermore, the hFcγRIa α-chain cytoplasmic domain was shown to contain targeting information for MHC class II antigen presentation. Deletion of the majority of the α-chain cytoplasmic tail did not significantly affect internalization rates of IgG complexes (Fig 2), but resulted in accumulation of receptor-ligand complexes in recycling vesicles, whereas wild-type receptor-ligand complexes mainly localized in late endosomal and lysosomal compartments (Fig 5 and Table 1). Data from several laboratories support a model in which recycling of membrane components to the cell surface represents a default pathway of the endocytic system. It has been demonstrated that TfR molecules with cytoplasmic tail mutations/deletions actively recycle to the plasma membrane. This suggested that specific targeting signals are not essential for recycling between endosomes and the cell surface.37Recycling of cytoplasmic tail-deleted hFcγRIa likely explains the lower receptor modulation observed with this mutant (Fig 2B).
It is generally accepted that, upon internalization, antigens have to be degraded to enable stimulation of antigen-specific T cells.38-40 Specific targeting of wild-type hFcγRIa resulted in (>1,000-fold) enhanced antigen presentation of ovalbumin upon complexing with IgG (100 ng/mL), compared with ovalbumine alone (100 ng/mL; Fig 3A). Massive cross-linking of the class I IgG receptor appeared not to be required, because a dimeric ovalbumin-CD64 MoAb construct triggers activation of specific T cells. IIA1.6 cells expressing either hFcγRIa WT/γ-chain or hFcγRIa Δ315/γY65F,Y76F were incubated with H22-OVA, which specifically targets OVA to hFcγRIa. Subsequent incubation with OVA-specific T cells showed potent T-cell activation (Dr Paul Guyre, Dartmouth Medical School, NH, personal communication, May 1998). Electron microscopical analyses showed wild-type hFcγRIa-immune-complexes to be targeted primarily to specific MHC class II-containing multivesicular endocytic and lysosomal compartments (Table 1). These compartments are crucial for proteolytic degradation at low pH and loading of MHC class II molecules.1 In the case of hFcγRIa, only newly synthesized MHC class II molecules were loaded with antigen-derived peptides (Fig 4). Tail-deleted hFcγRIa-immune-complexes were diverted to recycling compartments, and this strongly suggests that lack of efficient targeting to degradation compartments underlies the absence of antigen presentation (Table 1 and Fig 3). Still, hFcγRIa Δ315/γY65F,Y76F cells gold-particles also accumulated in lysosomes (Fig 5 and Table 1). However, these gold particles had a clustered appearance, in contrast to gold particles found in lysosomes of hFcγRIa WT transfectants. The clustered appearance of the gold particles and absence of antigen presentation indicated that the gold was no longer associated with OVA or was only associated with a small part of OVA. In separate studies, others have also shown that mutated type II and III FcR-ligand complexes were targeted to lysosomes without consequent antigen presentation to occur.12 27 A schematic representation of wild-type and tail-deleted hFcγRIa-triggered intracellular trafficking upon internalization is presented in Fig 6.
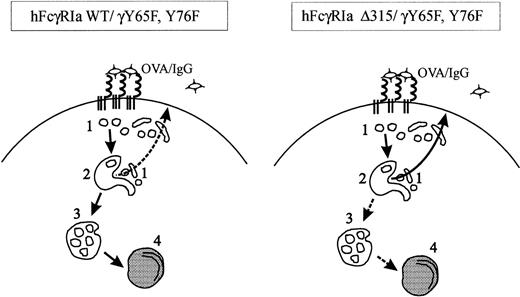
Schematic representation of intracellular trafficking of wild-type and tail-deleted hFcγRIa. In this model, based on our kinetic data and morphologic observations, four types of endocytic compartments are distinguished, ie, small coated and noncoated vesicles and tubules (1), early endosomes (2), multivesicular late endosomes (3), and lysosomes (4). hFcγRIa WT/γY65F,Y76F/IgG-OVA complexes are internalized via type 1 vesicles and transported to the early endosomes. The majority of complexes are sorted to late endosomes and lysosomes for degradation and loading onto MHC class II molecules. Only part of the receptor-ligand complexes recycle back to the cell surface (indicated by the dashed arrow) via type 1 vesicles and tubules. In contrast, the majority of hFcγRIa ▵315/γY65F,Y76F/IgG-OVA complexes is diverted from type 2 early endosomes into the recycling pathway, most likely due to the absence of intrinsic targeting information. Some antigen-receptor complexes are transported further down the endocytic tract to type 3 and 4 compartments (dashed arrows), but this is insufficient for antigen presentation to occur.
Schematic representation of intracellular trafficking of wild-type and tail-deleted hFcγRIa. In this model, based on our kinetic data and morphologic observations, four types of endocytic compartments are distinguished, ie, small coated and noncoated vesicles and tubules (1), early endosomes (2), multivesicular late endosomes (3), and lysosomes (4). hFcγRIa WT/γY65F,Y76F/IgG-OVA complexes are internalized via type 1 vesicles and transported to the early endosomes. The majority of complexes are sorted to late endosomes and lysosomes for degradation and loading onto MHC class II molecules. Only part of the receptor-ligand complexes recycle back to the cell surface (indicated by the dashed arrow) via type 1 vesicles and tubules. In contrast, the majority of hFcγRIa ▵315/γY65F,Y76F/IgG-OVA complexes is diverted from type 2 early endosomes into the recycling pathway, most likely due to the absence of intrinsic targeting information. Some antigen-receptor complexes are transported further down the endocytic tract to type 3 and 4 compartments (dashed arrows), but this is insufficient for antigen presentation to occur.
hFcγRIa does not contain well-known tyrosine-based,12dileucine-based sorting motifs,25,26 or leucine-isoleucine motifs such as found within the Limp II membrane glycoprotein.41 By truncation of the wild-type cytoplasmic domain it was found that residues 315-342 of the tail were important for targeting (Fig 3). However, the exact structural requirements for intracellular targeting of hFcγRIa remain to be characterized in detail. It has been described that intracellular trafficking of furin, polymeric Ig (poly-Ig) receptor, and mannose-6-phosphate receptor is modulated via phosphorylation of casein kinase II sites in their cytoplasmic tails.42-44 These sites contain key serines that can be phosphorylated by casein kinase II. Notably, the hFcγRIa α-chain contains two consensus casein kinase II motifs,45containing serines 331 and 340 (Fig 1). Because serine phosphorylation was shown important for hFcγRIa-mediated antigen presentation (Fig4), a hFcγRIa molecule in which all serines (at positions 328, 331, 339, and 340) were mutated was generated. However, this mutant did not exhibit impaired antigen presentation (data not shown). Notably, this does not exclude involvement of these serines in increasing the affinity of the receptor for cellular components that effect targeting, as has also been shown for the mannose 6-phosphate/insulin growth factor II receptor.46 Whether the cytoplasmic 315-342 segment influences intracellular targeting directly or indirectly via an effect on other sequences (either intracellular, transmembrane, or extracellular) remains unclear.
The hFcγRIa α-chain associates in vivo with the FcR γ-chain,10 and both chains are now considered to contribute to uptake of antigens. The hFcγRIa α-chain is capable of internalizing antigens via endocytosis,35,36 whereas the γ-chain enables FcR-mediated antigen uptake via phagocytosis.10 Our data show that, for efficient targeting of internalized immune complexes to type 3 multivesicular compartments, both the hFcγRIa α-chain and the FcR γ-chain contain targeting signals relevant for optimal antigen processing (Fig 3).
The present study documents that the hFcγRIa α-chain facilitates MHC class II-mediated antigen presentation. This finding may have direct impact on the in vivo role of hFcγRIa, because this receptor is likely occupied continuously with IgG in human serum.6Because hFcγRIa has a restricted cell distribution and the capacity to efficiently internalize and process hFcγRIa-targeted antigens,47 the principal function of this receptor may be immunoregulatory. Such a model fits well with the documented potency of triggering immune responses in vivo upon targeting to hFcγRIa.8,9 47
ACKNOWLEDGMENT
The authors thank Drs Thamar van Dijk and George Posthuma for technical advice and support, Janice Griffith for excellent technical assistance, Dr Peter Peters for helpful discussions, and Dr Aldwin Vriesema for critically reviewing the manuscript.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Jan G.J. van de Winkel, PhD, Department of Immunology, Immunotherapy Laboratory, KC.02-085.2, University Medical Center Utrecht, Lundlaan 6, 3584 EA, Utrecht, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal