Abstract
A Toledo strain cytomegalovirus (CMV) containing the gene for green fluorescent protein (GFP) under the control of elongation factor-1 promoter was used to study infection of human marrow stromal cells. Two stromal cell lines were used: HS-5, which secretes copious amounts of known cytokines and interleukins; and HS-27a, which does not secrete these activities. CMV growth and spread was monitored by counting green plaques and quantitating GFP intensity. Initial studies indicated that, whereas HS-5 and 27a have similar susceptibilities to infection, as evidenced by the same number of GFP+ cells at day 2, HS-5 appears more resistant to growth and spread of CMV. Furthermore, conditioned media from HS-5 (HS-5 CM) inhibited CMV plaque formation in HS-27a, suggesting that factors secreted by HS-5 are responsible for limiting CMV growth. Neutralizing antibodies against interleukin-1 (IL-1) and IL-1β completely blocked the ability of HS-5 CM to limit viral growth, suggesting that IL-1, which is known to be present in HS-5 CM, is responsible for this effect. When exogenous IL-1β was added to CMV-infected HS-27a, both the number of plaques and the intensity of GFP was significantly reduced in IL-1–treated HS-27a compared with untreated HS-27a (the number of plaques by day 18 was 20 ± 3 v 151 ± 12/well, respectively; GFP intensity was 535 ± 165 v 6,516 ± 652/well, respectively, in 4 separate experiments). At day 21, when IL-1β–treated, CMV-infected cultures were passaged and then cultured in the absence of IL-1β, CMV growth progressed with the kinetics of the original untreated culture, indicating that the IL-1β effect is reversible. Because HS-27a expresses the type I IL-1 receptor, we speculate that the antiviral effects are mediated through IL-1–induced changes in cellular gene expression. DNA chip analysis of mRNA from IL-1β–treated and nontreated HS-27a cells has identified some candidate molecules.
A SIGNIFICANT PROPORTION of marrow transplant recipients has a history of cytomegalovirus (CMV) infection, as evidenced by positive serology, antigenemia testing, or viral cultures.1-3 A proportion of these patients will develop myelosuppression attributable to CMV.4-6 Currently, both in vitro studies and clinical case reports suggest that CMV-mediated myelosuppression may result, at least in some cases, from altered function of CMV-infected marrow stromal cells.2,7-10However, it is unclear why only a subset of patients at risk will develop this complication. These observations may be explained in part by recent data suggesting that genetic differences in CMV strains as well as the immune competency of the host may influence this outcome.11 12 How these variables affect cellular mechanisms that control the persistence, latency, and subsequent reactivation of CMV is still unknown.
To begin to address this issue, we have examined the infection of two stromal cell lines by human CMV (HCMV). The human stromal cell lines used included HS-5, which secretes copious amounts of known cytokines and interleukins such as interleukin-1 (IL-1), granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-6, granulocyte colony-stimulating factor (G-CSF), IL-8, and leukemia inhibitory factor (LIF), such that conditioned media from HS-5 (HS-5 CM) is capable of stimulating and supporting the proliferation of CD34+/CD38− hematopoietic progenitor cells.13 The second stromal line, HS-27a, does not secrete copious amounts of growth factors or support extensive progenitor proliferation, but does support contact-dependent, long-term maintenance of immature progenitor cells.13,14 Both lines were generated from a single long-term marrow culture (LTMC) derived from a normal CMV-seronegative donor. The cell lines, as characterized, represent two of the several different functional cell types that comprise an LTMC.13
To facilitate the study of HCMV infection in these cell lines, we used a recombinant virus, HV5.111, that expresses green fluorescence protein (GFP) under the control of the elongation factor-1α promoter. Initial studies indicated that there was a difference in growth of HCMV in HS-5 and HS-27a cells, with HS-5 cells appearing more resistant to CMV. We report here that media from HS-5 cells inhibited plaque formation in HS-27a cells in a reversible manner. The activity in HS-5 CM responsible for this effect was determined to be IL-1α and IL-1β.
MATERIALS AND METHODS
Marrow Stromal Cell Lines
All experiments were conducted using immortalized human marrow stromal cell lines, HS-5 and HS-27a.13 The cells were grown in media (RPMI 1640 medium) supplemented with L-glutamine (0.4 mg/mL), sodium pyruvate (1 mmol/L), penicillin (100 U/mL), streptomycin sulfate (100 μg/mL), and 10% fetal calf serum (FCS) at 37°C under 95% air-5% CO2. After reaching confluency in T-75 flasks, cells were trypsinized, transferred to 48-well plates, and allowed to reach 80% to 100% confluency before the experiments were initiated.
Conditioned Media (HS-5 CM)
HS-5 cells were grown to confluency and then fed with supplemented media. The conditioned media was harvested after 5 days, clarified by centrifugation, and then concentrated five times using Centriprep 10 concentrator (Amicon, Beverly, MA). The concentrated media were diluted with 4 vol of RPMI supplemented with 5% FCS before use. Media from mock cultures were concentrated, diluted, and used as control CM.
Virus
HV5.111 was constructed using pQ91, which consists of the BamHI (position 197042 of the AD169 sequence)15 to Sal I (position 200171) fragment of HCMV (AD169) containing the US9 and US10 orfs cloned into pUC21. Into pQ91 at the Apa I site between US9 and US10, shown to be transcriptionally silent,16 a cassette was inserted consisting of the GFP gene (EGFP; Clontech, Palo Alto, CA) expressed by the elongation factor 1α promoter (EF-1)17 and the Escherichia coliguanosine-hypoxanthine phosphoribosyltransferase gene (gpt) under the control of the mouse phosphoglycerate kinase (PGK) promoter to create pQ111. pQ111 was digested with BamHI and Sal I to release the vector before it was used to transfect HF to generate recombinant virus as described.17 The infected cells were harvested when a greater than 90% cytopathogenic effect was evident, sonicated, and centrifuged at 1,200 rpm for 5 minutes. The supernatant was titered using HFF and frozen in aliquots at −70°C.
Detection and Quantitation of GFP-CMV Growth in HS-27a Cells
To quantitate production of GFP-CMV, HS-27a cells were grown to confluency in 48-well plates in the supplemented media and then exposed to the recombinant HS5.111 (GFP-CMV) at 0.2 to 1 MOI for 4 hours. After washing three times, the cultures were fed with fresh media and incubated for various times at 37°C, 5% CO2, with fresh media added every other day. GFP-CMV growth was measured as fluorescence intensity of GFP using a fluorescent plate reader (CytoFluor II; PerSeptive Biosystems, Framingham, MA) with 485 nm excitation and 530 nm emission lights. Fluorescence intensity of the wells with uninfected cells was subtracted as background fluorescence. GFP-containing cells were also visualized using an inverted phase/fluorescence microscope with a direct camera attachment (Diaphot-TMD; Nikon, Melville, NY).
Experimental culture conditions.
To assess the role of HS-5 CM, interferon-γ (IFN-γ), IL-6, and IL-1β on the production of GFP, HS-27a cells were grown in 12-well plates at 80% to 100% confluency and exposed to GFP-CMV at an MOI of 0 to 100 in the presence or absence of 20% of 5× HS-5 CM, 0.1 to 30 ng/mL IL-1β, 0.1 to 30 ng/mL IL-6, or 2 ng/mL IFN-γ. Complete media with additives was replaced every other day. In some experiments, the HS-27a cells were exposed to factors before the addition of GFP-CMV.
For experiments involving the neutralization of HS-5 CM, which contains both IL-1α and IL-1β, antibodies with neutralizing activity against both IL-1β and IL-1α (R&D Systems, Minneapolis, MN) were used. HS-5 CM was preincubated with the neutralizing antibodies (1 μg/well each) at 37°C for greater than 30 minutes before use in cultures of HS-27a cells. The cells were cultured in control CM or HS-5 CM with or without the antibodies for 2 days and then exposed to GFP-CMV. Viral growth was determined as measured by fluorescence intensity of GFP after 15 days of culture.
Cell Proliferation Assay
To study the effect of IL-1β on cell proliferation, HS-27a cells were cultured in 48-well plates in the presence or absence of 2 ng/mL of IL-1β. At various time points, the plates were gently inverted, blotted onto paper towels to remove residual media, and frozen at −70°C until assayed. Cell numbers were estimated using a green fluorescent dye, CyQuant GR (Molecular Probes, Eugene, OR), to label all cells, and the CytoFluor plate reader was used to quantitate the fluorescence intensity.
Chip Analysis
Confluent cultures of stromal cells were incubated for 4 days with or without 2 ng/mL IL-1β or 5% HS-5 CM. After washing three times with phosphate-buffered saline (PBS), total RNA was isolated using RNAgents Total RNA Isolation Systems (Promega, Madison, WI), followed by poly A+ RNA isolation using Poly A Tract mRNA Isolation Systems (Promega) according to the manufacturer’s instructions. To amplify and label the RNA, it was first converted to double-stranded cDNA using an oligo dT primer that has a T7 RNA polymerase site on the 5′ end [5′-GGCCAGTGAATTGTAATACGACTCACTATAGGGAGGCGG(T24)-3′]. The cDNA was then used directly in an in vitro transcription reaction in the presence of biotinylated nucleotides Bio-11-UTP and Bio-11-CTP (Enzo, Farmingdale, NY). To improve hybridization kinetics, the labeled antisense RNA was fragmented by incubating at 94°C for 35 minutes in 30 mmol/L MgOAc, 100 mmol/L KOAc. Hybridization to Genechips (Affymetrix, San Jose, CA) was performed at 40°C overnight in a mix that included 10 μg fragmented RNA, 6× SSPE, 0.005% Triton-X100, and 100 μg/mL herring sperm DNA in a total volume of 200 μL. Chips were washed, stained with phycoerythrin-streptavidin, and read using an Affymetrix GeneChip scanner and accompanying gene expression software. The software includes algorithms that determine whether a gene is absent or present and whether the expression level of a gene in an experimental sample is significantly increased or decreased relative to a baseline sample.
RESULTS
GFP-CMV Growth in HS-27a Marrow Stromal Cell Lines
The HCMV recombinant strain of Toledo containing the GFP gene regulated by the EF-1 HCMV promoter was used to infect marrow stromal cells. Cells were exposed to GFP-CMV at an MOI of 1 (as determined on human fibroblasts) and cultured for 12 days. The extent of infection was easily visualized in growing cultures by phase contrast fluorescence microscopy as shown in Fig 1. Infection was also quantitated using the CytoFluor fluorescent plate reader. Comparisons of GFP intensity at day 12 past infection indicated that HS-5 cells (fluorescence intensity of 1798) were relatively resistant to CMV compared with HS-27a cells (fluorescence intensity of 5214). This difference was also readily apparent by microscopy (Fig1).
Expression of GFP by HS-27a cells (A and B) and HS-5 cells (C and D) after 12 days of culture in control media. The image is of viable cells examined under a phase/fluorescent inverted microscope (original magnification × 100), with a direct camera attachment. The same field is photographed under illumination with a 450 to 490 nm light source on the left and a combination of 450 to 490 nm plus incandescent light on the right to simultaneously visualize both GFP-CMV–infected and noninfected cells in confluent cultures.
Expression of GFP by HS-27a cells (A and B) and HS-5 cells (C and D) after 12 days of culture in control media. The image is of viable cells examined under a phase/fluorescent inverted microscope (original magnification × 100), with a direct camera attachment. The same field is photographed under illumination with a 450 to 490 nm light source on the left and a combination of 450 to 490 nm plus incandescent light on the right to simultaneously visualize both GFP-CMV–infected and noninfected cells in confluent cultures.
Effect of HS-5 Conditioned Media (HS-5CM) on GFP-CMV Infection of HS-27a Cells
HS-5 CM is known to contain high levels of various cytokines and interleukins.13 We hypothesized that activities present in HS-5 CM may inhibit viral growth. To test this hypothesis, HS-5 CM was added to cultures of CMV-infected HS-27a cells. Figure 2 shows the effect of HS-5 CM on GFP-CMV growth in HS-27a cells. Viral growth was determined both by fluorescence intensity of GFP and as plaque-forming units (PFU). In both assays, HS-5 CM shows a strong inhibition of GFP-CMV growth in HS-27a cells, indicating that a secreted factor in HS-5 CM may influence viral growth.
Effect of HS-5 conditioned media (HS-5 CM) on CMV infection of HS-27a stromal cells measured as fluorescence intensity of GFP or as PFU. HS-27a cells were exposed to recombinant GFP-CMV at 0.2 MOI and cultured in control media (Control) or HS-5 CM for 13 days. Fluorescence intensity was determined using a CytoFluor plate reader; PFU were detected using an inverted fluorescence microscope. Data represent the means ± SE of four determinations in one of two representative experiments.
Effect of HS-5 conditioned media (HS-5 CM) on CMV infection of HS-27a stromal cells measured as fluorescence intensity of GFP or as PFU. HS-27a cells were exposed to recombinant GFP-CMV at 0.2 MOI and cultured in control media (Control) or HS-5 CM for 13 days. Fluorescence intensity was determined using a CytoFluor plate reader; PFU were detected using an inverted fluorescence microscope. Data represent the means ± SE of four determinations in one of two representative experiments.
Gene Expression in HS-5 Cells: The Role of IL-1β
Previous studies using oligonucleotide array chips were used to screen simultaneously for differential expression in HS-5 and HS-27a cells of 250 human genes known to play a role in hematopoiesis and immunology. Based on these results and previous data indicating that HS-5, but not HS-27a, secretes large amounts of IL-1α, IL-1β, and IL-6,13 we hypothesized that one of these activities present in HS-5 CM may contribute to the HS-5 CM-mediated inhibition of CMV growth in HS-27a. This analysis also indicated that HS-27a cells express the type I IL-1 receptor that can bind both IL-1α and IL-1β.18
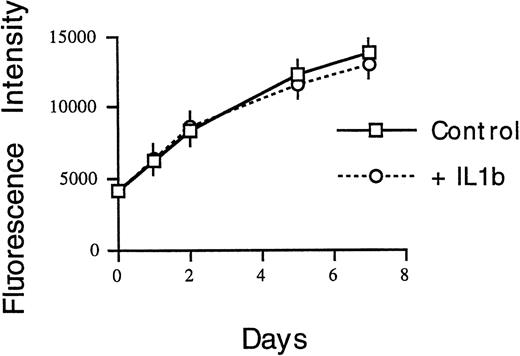
To test this hypothesis, recombinant IL-1β or IL-6 was added to cultures of HS-27a at the time of virus exposure. Data shown in Fig 3 indicate that IL-1β, but not IL-6, inhibits GFP-CMV. The inhibitory effect of recombinant IL-1β could be blocked with the addition of neutralizing anti–IL-1β antibodies (Fig 4); the inhibitory effect of HS-5 CM was blocked after the addition of both the anti–IL-1α and anti–IL-1β antibodies. Both were required because HS-5 CM contains IL-1α and IL-1β and both bind the type I receptor expressed on HS27a.
Effect of recombinant human IL-1β or IL-6 on CMV growth in HS-27a stromal cells as measured by fluorescence intensity of GFP. The cells were infected with recombinant GFP-CMV at 1 MOI and cultured in media containing 0 to 30 ng/mL of IL-1β or IL-6 for 10 days. Values represent means ± SE of four determinations in one of two representative experiments.
Effect of recombinant human IL-1β or IL-6 on CMV growth in HS-27a stromal cells as measured by fluorescence intensity of GFP. The cells were infected with recombinant GFP-CMV at 1 MOI and cultured in media containing 0 to 30 ng/mL of IL-1β or IL-6 for 10 days. Values represent means ± SE of four determinations in one of two representative experiments.
Effect of neutralizing antibodies against IL-1β and IL-1 on the ability of IL-1β and HS-5 conditioned medium (HS-5 CM) to inhibit GFP-CMV. The cells were cultured in the presence or absence of IL-1β (left panel) and HS-5 CM (right panel) with or without antibodies for 2 days before infection with GFP-CMV. They were then exposed to GFP-CMV at 0.2 MOI and cultured in the above-described conditions. Fluorescence intensity of GFP was detected at day 15. Data represent the means ± SE of four replicate cultures in one of two representative experiments.
Effect of neutralizing antibodies against IL-1β and IL-1 on the ability of IL-1β and HS-5 conditioned medium (HS-5 CM) to inhibit GFP-CMV. The cells were cultured in the presence or absence of IL-1β (left panel) and HS-5 CM (right panel) with or without antibodies for 2 days before infection with GFP-CMV. They were then exposed to GFP-CMV at 0.2 MOI and cultured in the above-described conditions. Fluorescence intensity of GFP was detected at day 15. Data represent the means ± SE of four replicate cultures in one of two representative experiments.
IL-1β Does Not Prevent Initial GFP-CMV Infection
HS-27a cells were grown in the presence or absence of 2 ng/mL IL-1β for 24 hours before exposure to GFP-CMV. Data shown in Fig 5A indicate that the amounts of GFP detected on days 1 and 2 were comparable regardless of the presence or absence of IL-1β. Inverted fluorescent microscopic evaluation indicated a comparable number of single green cells detectable under both conditions. However, as shown in Fig 5B, subsequent GFP-CMV growth from days 8 to 13 was severely suppressed in the presence of IL-1β. Importantly, the exposure of HS-27a to IL-1β had no effect on cell growth kinetics as measured by quantitation of cell number over time using CyQuant GR dye intensity detected by a fluorescent plate reader (Fig 6).
Effect of IL-1β on initial infection and growth of GFP-CMV in HS-27a stromal cells. Cells were cultured in the presence or absence of 2 ng/mL of IL-1β for 1 day and then infected with GFP-CMV at an MOI of 0 to 100. The cultures were continued in the presence or absence of IL-1β for 13 days. Fluorescence intensity of GFP was measured using a fluorocytometer as described. (A) shows that comparable levels of GFP for control and IL-1β–treated groups, regardless of MOI, were detected 1 and 2 days after infection. (B) shows a significant difference in GFP intensity between control and IL-1β–treated cultures after day 10.
Effect of IL-1β on initial infection and growth of GFP-CMV in HS-27a stromal cells. Cells were cultured in the presence or absence of 2 ng/mL of IL-1β for 1 day and then infected with GFP-CMV at an MOI of 0 to 100. The cultures were continued in the presence or absence of IL-1β for 13 days. Fluorescence intensity of GFP was measured using a fluorocytometer as described. (A) shows that comparable levels of GFP for control and IL-1β–treated groups, regardless of MOI, were detected 1 and 2 days after infection. (B) shows a significant difference in GFP intensity between control and IL-1β–treated cultures after day 10.
An estimate of cell number over time is shown for HS-27a cells cultured with or without IL-1β. Viable cell numbers are estimated by fluorescence intensity of CyQuant GR dye measured by a fluorescence plate reader.
An estimate of cell number over time is shown for HS-27a cells cultured with or without IL-1β. Viable cell numbers are estimated by fluorescence intensity of CyQuant GR dye measured by a fluorescence plate reader.
GFP Fluorescence Intensity Correlates With CMV Titer
The use of GFP-CMV for these studies greatly simplified the estimate of differences among our experimental groups. Counting green plaques under an inverted fluorescent microscope is much easier than standard technique and permitted us to follow the same cultures over time. Quantitating GFP with a plate reader also provided unequivocal data. However, these methods assume that GFP intensity reflects viral activity and, critically, that the absence of GFP means the absence of virus. To test this assumption, HS-27a cells were cultured in the presence or absence of 2 ng/mL of IL-1β for 18 days, harvested, and sonicated for virus titration. Standard plaque-forming units of CMV obtained from HS-27a cells cultured in control media versus 2 ng/mL of IL-1β were significantly different (500 ± 100 and 51 ± 6, respectively).
IL-1β Effect on the GFP-CMV Growth Is Reversible
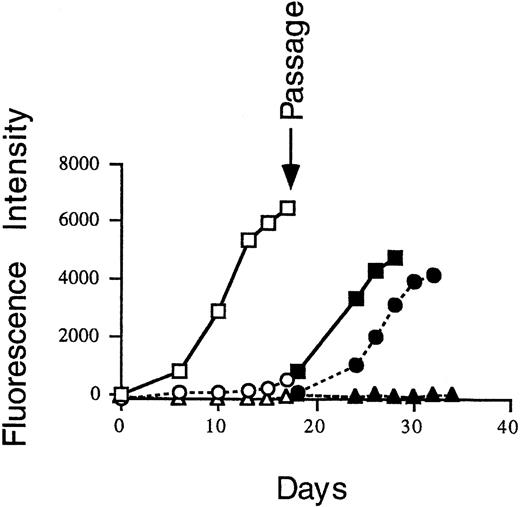
To determine whether the effect of IL-1β is reversible, cell cultures infected with GFP-CMV in the presence of control media, IL-1β, or IFN-γ were passaged into fresh media. As shown in Fig 7, GFP-CMV from IL-1β–treated cells can regrow in the control media. In contrast, GFP-CMV could not revive after IFN-γ treatment. These data suggest that the mechanisms of GFP-CMV suppression by IL-1β and IFN-γ are different and that the IL-1β effect is reversible once IL-1β is removed from the system.
HS-27a cells were exposed at day 0 to GFP-CMV at 0.2 MOI and then incubated in control media (□), 2 ng/mL IL-1β (○), or 2 ng/mL IFN-γ (▵). At day 17, cell cultures were passaged into fresh medium (solid symbols). The amount of GFP-CMV as measured by fluorescence intensity was determined on the days indicated using a CytoFluor plate reader, as described.
HS-27a cells were exposed at day 0 to GFP-CMV at 0.2 MOI and then incubated in control media (□), 2 ng/mL IL-1β (○), or 2 ng/mL IFN-γ (▵). At day 17, cell cultures were passaged into fresh medium (solid symbols). The amount of GFP-CMV as measured by fluorescence intensity was determined on the days indicated using a CytoFluor plate reader, as described.
HS-5 CM and IL-1β Have Similar Effects on HS-27a Gene Expression
Data suggesting that the IL-1β inhibition of GFP-CMV was reversible was interpreted to indicate that the IL-1β was not acting directly on the virus but rather that it altered the HS-27a cells in a way that resulted in inhibition of CMV replication. To identify changes in HS-27a cells after treatment with IL-1β, mRNA was harvested from HS-27a cells before and after treatment with IL-1β or HS-5 CM. Data shown in Fig 8 indicate that both IL-1β and HS-5 CM upregulate the expression of IL-1β, IL-6, GM-CSF, and several chemokines. Which, if any, of these is responsible for the viral protective effect is unknown. However, the data do reinforce our hypothesis that the active factor in HS-5 CM is IL-1β, because both elicited comparable changes in HS-27a gene expression.
Transcriptional profiles of HS-27a cells in response to HS-5 CM or IL-1β. RNA isolated from untreated HS-27a cells or cells treated with 1× HS-5 CM or 2 ng/mL IL-1β for 4 days was hybridized to an oligonucleotide array chip that quantitatively monitors the expression of 250 genes of interest in hematopoiesis and immunology. Genes showing a detectable expression level are plotted. Dots represent expression level in the untreated cells, and a line is drawn to the expression level after cell treatment. Dots without lines represent genes whose expression level did not change in response to cell treatment. Lines in bold indicate genes whose expression levels significantly increased (lines to the right of the dots) or decreased (lines to the left of the dots) as determined by an algorithm that makes a call based on several statistical criteria.35 In addition to the genes plotted, MIP-1, CD44, SAA, LMP2, and LMP7B were only detected after IL-1β treatment and N-CADHERIN and ICAM-1 were detected only after treatment with HS-5 CM.
Transcriptional profiles of HS-27a cells in response to HS-5 CM or IL-1β. RNA isolated from untreated HS-27a cells or cells treated with 1× HS-5 CM or 2 ng/mL IL-1β for 4 days was hybridized to an oligonucleotide array chip that quantitatively monitors the expression of 250 genes of interest in hematopoiesis and immunology. Genes showing a detectable expression level are plotted. Dots represent expression level in the untreated cells, and a line is drawn to the expression level after cell treatment. Dots without lines represent genes whose expression level did not change in response to cell treatment. Lines in bold indicate genes whose expression levels significantly increased (lines to the right of the dots) or decreased (lines to the left of the dots) as determined by an algorithm that makes a call based on several statistical criteria.35 In addition to the genes plotted, MIP-1, CD44, SAA, LMP2, and LMP7B were only detected after IL-1β treatment and N-CADHERIN and ICAM-1 were detected only after treatment with HS-5 CM.
DISCUSSION
HCMV is a herpes virus demonstrated to cause pathogenic effects in many different tissues.19,20 Even within the hematopoietic system, CMV-associated neutropenia has been attributed to several different mechanisms, including direct infection of myeloid progenitors, infection of critical monocyte-derived accessory cells, or infection of various components of the marrow stroma.2,7,21-25 Given the plethora of available data, it is reasonable to conclude that various strains of CMV will infect different cellular targets, with the outcome dependent on the immune competency of the host. The significant degree to which CMV can impact morbidity and mortality in immune-compromised patients is well established.4 Consequently, the increase in number of immune-compromised patients, either from acquired immunodeficiency syndrome (AIDS) or organ and marrow transplantation, has emphasized the need to understand the HCMV life cycle so that infection or reactivation can be prevented.26
In this report, we provide data indicating that HCMV infection of marrow stromal cells can be limited by IL-1β. Unlike IFN-γ, which also limits HCMV infection of stroma,27 the effect of IL-1β is reversible, so that removal of IL-1β results in GFP-CMV growth kinetics that are comparable to that of the original infection. The prompt recovery of detectable GFP-CMV after the removal of IL-1β suggests that IL-1β has rendered the HS-27a cells temporarily nonpermissive for viral replication or spread. Given that our previous studies have shown that HS-27a cells do express type I IL-1 receptors, it is reasonable to assume that the consequences of IL-1β signal transduction are responsible for the anti-CMV effect. This raises the question as to how IL-1β alters gene expression in HS-27a cells. To begin to address this issue, we have analyzed gene expression using oligonucleotide arrays to simultaneously assess differential expression of 250 human genes in HS-27a cells before and after exposure to HS-5 CM and IL-1β. As expected and previously reported, IL-1β increases the production of IL-6, GM-CSF, and IL-1β by stromal cells.28In addition, several chemokines, including MCP-1, IL-8, MGSA, and ENA-78, are also significantly upregulated. Whether any of these contribute to the inhibitory effect on HCMV remains to be determined.
The potential in vivo relevance of these observations remains speculative. However, given that steroids have been shown to inhibit IL-1 production by monocytes29-31 and that steroid treatment has also been associated with CMV disease,32-34it is reasonable to speculate that reduced IL-1 production by monocytes in vivo may increase the likelihood of CMV disease in steroid-treated patients. CMV disease is also increased in patients treated with either radiation or chemotherapy, both of which are know to cause monocytopenia. Taken together, these observations suggest that the increase incidence and severity of CMV disease seen in patients after chemotherapy, radiation therapy, or treatment with steroids may be related to the loss of IL-1 production by the monocyte population. This hypothesis remains to be formally tested.
ACKNOWLEDGMENT
The authors thank Ludmilla Golubev for maintaining the HS-5 and HS-27a cell lines, Ken Griffiths for the visual presentation of the chip data, and Harriet Childs and Bonnie Larson for preparing the manuscript.
Supported in part by Grants No. CA18221, DK34431, DK51417, and HL36444 awarded by the National Institutes of Health, Department of Health and Human Services (Bethesda, MD).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Beverly Torok-Storb, PhD, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: btorokst@fhcrc.org.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal