Abstract
Plasminogen activator inhibitor-1 (PAI-1) is present in the platelet -granule and is released on activation. However, there is some debate as to whether the megakaryocyte and platelet synthesize PAI-1, take it up from plasma, or both. We examined the expression of PAI-1 in differentiating megakaryocytic progenitor cells (UT-7) and in CD34+/CD41− cells from cord blood. UT-7 cells differentiated with thrombopoietin (TPO) resembled megakaryocytes (UT-7/TPO) with respect to morphology, ploidy, and the expression of glycoprotein IIb-IIIa. PAI-1 messenger RNA (mRNA) expression was upregulated and PAI-1 protein synthesized in the UT-7/TPO cells accumulated in the cytoplasm without being released spontaneously. In contrast, erythropoietin (EPO)-stimulated UT-7 cells (UT-7/EPO) did not express PAI-1 mRNA after stimulation with TPO because they do not have endogenous c-Mpl. After cotransfection with human wild-typec-mpl, the cells (UT-7/EPO-MPL) responded to phorbol 12-myristate 13-acetate (PMA), tumor necrosis factor- (TNF-), and interleukin-1β (IL-1β) with enhanced PAI-1 mRNA expression within 24 to 48 hours. However, induction of PAI-1 mRNA in UT-7/EPO-MPL cells by TPO required at least 14-days stimulation. UT-7/EPO cells expressing c-Mpl changed their morphology and the other characteristics similar to the UT-7/TPO cells. TPO also differentiated human cord blood CD34+/CD41− cells to CD34−/CD41+ cells, generated morphologically mature megakaryocytes, and induced the expression of PAI-1 mRNA. These results suggest that both PAI-1 mRNA and de novo PAI-1 protein synthesis is induced after differentiation of immature progenitor cells into megakaryocytes by TPO.
PLATELETS MAY AFFECT clot lysis by a variety of mechanisms. Platelets contain factor XIII, which, on activation, cross-links fibrin monomer and α2-plasmin inhibitor (α2-PI), thereby rendering it more resistant to digestion by plasmin.1,2 Activated platelets also secrete plasminogen activator inhibitor-1 (PAI-1) and α2-PI, which inhibit plasmin formation and activity, respectively.3,4 PAI-1 is a member of serine protease inhibitor super family, and is the major physiological inhibitor of fibrinolysis. Platelets are the main reservoir of PAI-1, with approximately 85% of circulating PAI-1 contained within platelet α-granules.5 It has been reported that platelet PAI-1 exists predominantly in a latent or inactive form,6,7 suggesting that its effect on fibrinolysis may be limited. However, Fay et al reported that PAI-1–deficient platelets inhibited tissue-type plasminogen activator (t-PA)–mediated clot lysis to a substantially lesser extent than normal platelets.8 Positive PAI-1 immunostaining of human platelets and megakaryocytes has also been shown.9However, PAI-1 messenger RNA (mRNA) was neither detected by Northern blot analysis of human platelet RNA, nor amplified from reverse-transcribed human platelet RNA.10 Therefore we questioned whether a relationship may exist between thrombopoietin (TPO)-induced megakaryocytic differentiation from progenitor cells and PAI-1 synthesis.
TPO, the recently isolated and cloned ligand for the cytokine receptor Mpl,11-15 is a hematopoietic growth factor that regulates platelet production. TPO stimulates the proliferation of megakaryocyte progenitor cells, promotes megakaryocyte terminal differentiation, and is essential for the production and maintenance of normal levels of thrombopoiesis.16-19 In the past, the study of megakaryocytic differentiation was limited because of the rarity of megakaryocytes in normal bone marrow, the poorly defined cell population, and inadequate assay methods. The UT-7 cell line was established from bone marrow cells of a patient with acute megakaryoblastic leukemia.20 In particular, recently isolated UT-7/TPO cells have an absolute dependence on TPO and show mature megakaryocytic features.21 In contrast, the UT-7/erythropoietin (EPO) cell line shows erythroid development without TPO dependency due to a lack of endogenous c-Mpl expression.22 Therefore, comparison of these cell lines will be useful for evaluating the PAI-1 response during megakaryocyte development induced by TPO.
In the present study, we used these UT-7 cell lines and their transfectants with or without transfection with c-mplcomplementary DNA (cDNA) as a model system and show expression of PAI-1 mRNA and de novo synthesis of PAI-1 protein with TPO-dependent differentiation. We also confirm these results using CD34+progenitor cells isolated from human cord blood.
MATERIALS AND METHODS
Hematopoietic growth factors and reagents.
Recombinant human TPO was provided by the Kirin Brewery Co, Ltd (Gumma, Japan). Recombinant human EPO was a gift from the Life Science Research Institute of Snow Brand Milk Company (Tochigi, Japan). Recombinant human granulocyte-macrophage colony stimulating factor (GM-CSF) was provided by Sumitomo Pharmaceutical Company (Osaka, Japan). Human cDNA clones for full-length c-mpl P (wild type) andc-mpl K (truncated) were kindly provided by Dr M. Okada (Eisai, Tsukuba, Japan) and Dr S. Gisselbrecht (INSERM, Paris, France), respectively.
Cell culture.
The original UT-7 cell line (UT-7/OR) was established from bone marrow cells obtained from a patient with acute megakaryocytic leukemia20 and maintained in liquid culture with Iscove’s modified Dulbecco’s medium (IMDM; GIBCO Laboratories, Grand Island, NY) containing 10% fetal calf serum (FCS; Hyclone Laboratories, Logan, UT) and 1 ng/mL of GM-CSF. UT-7/GM was isolated after long-term culture of UT-7 cells and maintained as described for the UT-7 cells.23 The UT-7/EPO cell line, which is a subclone of UT-7, was maintained continuously in IMDM containing 10% FCS and 1 U/mL of EPO.22 The UT-7/TPO cell line was maintained in IMDM containing 10% FCS and 10 ng/mL of TPO.21 Primary human umbilical cord-derived endothelial cells (HUVEC) were harvested from human umbilical cord veins treated with 0.1% collagenase as described elsewhere,24 and grown on fibronectin-precoated culture plate in Medium-199 (GIBCO Laboratories), containing 15% FCS, 2 mmol/L of glutamine, 15 mmol/L of HEPES, 100 μg/mL of heparin, and 60 μg/mL of endothelial cell growth supplement (Equitech-Bio Inc, Ingram, TX).
Reverse-transcriptase polymerase chain reactions (RT-PCR) and Southern blotting analysis.
Total RNA was isolated from cells according to the methods of Chomczynski and Sacchi.25 RT-PCR was performed using oligonucleotide primers as follows. The PAI-1 forward 5′-GAACAAGGATGAGATCAGCACC-3′ (nucleotides 402-423) and reverse 5′-ACTATGACAGCTGTGGATGAGG-3′ (nucleotides 1151-1172) primers; GP-Ibα forward 5-AAGCTGGAGAAGCTCAGTCTGG-3′ (nucleotides 535-556) and reverse 5′-CTCCTTAGTGGATTCTTGTGTTGG-3′ (nucleotides 1072-1095); PF-4 forward 5′-GCTGAAGCTGAAGAAGATGGG-3′ (nucleotides 98-108) and reverse 5′-TAGCAAATGCACACACGTAGG-3′ (nucleotides 324-344); urokinase-type plasminogen activator (u-PA) forward 5′-GATCTGATGCTCTTCAGCTGG-3′ (nucleotides 389-409) and reverse 5′-CTGCTCCGGATAGAGATAGTCG-3′ (nucleotides 1038-1059); Protease-activated receptor 1 (PAR-1) forward 5′-TGTCTGTGTCAGCAGCATAAGC-3′ (nucleotides 1292-1313) and reverse 5′-CTTGGAATAACACCGTCATCTCG-3′ (nucleotides 1688-1710); PAR-3 forward 5′-GGTAACATGTGGACTGGTGTGG-3′ (nucleotides 777-798) and reverse 5′-AATGGAGCTCCTTGCACTATGC-3′ (nucleotides 1334-1355);c-mpl P forward 5′-CAAGGCTTCTTCTACCACAGC-3′ (nucleotides 934-954) and reverse 5′-TCAGTCTCCTGTAGTGTGCAGG-3′ (nucleotides 1552-1573); glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward 5′-CCCATGGCAAATTCCATGGCA-3′ (nucleotides 215-235) and reverse 5′-GGTGGACCTGACCTGCCGTCTAGA-3′ (nucleotides 759-782). Each first-strand cDNA was synthesized by RT using a commercial kit (Roche Molecular System, Branchburg, NJ). Total cellular RNA (1 μg) was RT using oligo-dT16 primers, and amplified by PCR (94°C for 60 seconds, primer annealing at 56°C for 60 seconds, extension at 72°C for 60 seconds) for 30 cycles in a Program Temp Control System PC-700 (Astec, Tokyo, Japan), and a final incubation at 72°C for 10 minutes. Amplification products were separated on 1.5% agarose gels stained with ethidium bromide and photographed. The RT-PCR products were transferred to nylon membranes and incubated with PAI-1 and c-mpl P cDNA labeled with32P-αCTP by the random-priming method. After an overnight incubation at 65°C in 5× SSPE (150 mmol/mL NaCl, 10 mmol/mL sodium phosphate, pH 7.4, containing 1 mmol/mL EDTA) with 5× Denhardt’s solution, 20 μg/mL of nonhomologous salmon sperm DNA, and 0.5% (wt/vol) sodium dodecyl sulfate (SDS), the blots were washed three times with 2× SSC (150 mmol/mL NaCl, 15 mmol/mL sodium citrate, pH 7.0), 0.5× SSC, 0.1× SSC, plus 0.1% SDS for 15 minutes each. The membranes were autoradiographed using Kodak XAR-5 film (Eastman Kodak Co, Rochester, NY) with an intensifying screen at −80°C.
Preparation of c-mpl transfectant.
A c-Mpl expression vector was generated by ligation of full-lengthc-mpl cDNA into the pRc cytomegalovirus (CMV) mammalian expression vector. Electroporation was used for stable transfection of the plasmid into UT-7/EPO cells, as described previously.26In brief, 20 μg of pRcCMV containing c-mpl cDNA was introduced into 1 × 107 UT-7/EPO cells resuspended in 0.25 mL of RPMI 1640 medium containing 10% fetal bovine solution (FBS) by electropulse at 250V, 960 μFD. Transfected cells were seeded at 3 to 5 × 107 cells/mL in IMDM medium containing 10% FBS and 1 U/mL of EPO and neomycin- (Life Technologies Inc, Grand Island, NY) resistant clones were selected.
Metabolic labeling and immunoprecipitation.
UT-7/OR, UT-7/TPO cells, and HUVEC (1 × 107 cells/mL) were metabolically labeled for 15 minutes with 250 μCi/mL35S-methionine (EXPRE35S35S; Du Pont Co, Wilmington, DE) in 2.5 mL of methionine-free medium, as previously described.27 The cells were further incubated in serum-free medium supplemented with unlabeled methionine, and were harvested at various time intervals. Culture media was separated from cell pellets by centrifugation, and stored at −80°C immediately. The cells were washed with phosphate-buffered saline (PBS) and lysed on ice in lysis buffer composed of 20 mmol/L Tris-HCl (pH 7.4), 135 mmol/L NaCl, 20% glycerol, 1% NP-40, 1 mmol/L phenylmethylsulfonylfluoride (PMSF), 15 μg/mL aprotinin, and 2 mmol/L sodium orthovanadate. The culture media and cell lysates were precleared with protein A-Sepharose (Pharmacia Biotech), and incubated at 4°C for 1 hour with shaking with mouse antihuman PAI-1 monoclonal antibodies (MoAb) (JTI-3 and JTI-4) and nonimmune mouse immunoglobulin G (IgG) (Cappel ICN Pharmaceuticals Inc, Aurora, OH) bound to protein A-Sepharose beads.28 The immunoprecipitates were washed to remove unbound proteins and the bound proteins were eluted from the Sepharose by heating at 100°C for 5 minutes and then subjected to electrophoresis on 10% SDS-polyacrylamide gels.29 The gels were dried and exposed to radiograph film for autoradiography at −80°C.
Separation of human CD34-positive cells and megakaryocytic colony formation.
Human cord blood was obtained with informed consent from women who underwent normal vaginal delivery, and approximately 50 mL of cord blood was collected. To deplete adherent cells, cord blood cells were incubated with silica beads (KAC-2, Japan Antibody Institute, Gumma, Japan) at 37°C for 30 minutes. The cells were separated as the interface mononuclear FH cells by centrifugation (400g, 30 minutes at 25°C) over Ficoll Hypaque (FH; 1.077 g/cm3; Pharmacia Biotech, Uppsala, Sweden). The nonadherent mononuclear cells were adjusted to 5 × 107 cells/mL in PBS without Ca2+ and Mg2+, and CD34+ progenitor cells were isolated using a magnetic cell sorting system (Dynal CD34 progenitor cell selection system; Dynal, Oslo, Norway) according to the manufacturer’s instructions. CD34+-enriched cells were plated at 1 × 106 cells/mL in megakaryocyte culture medium containing 20% heparinized plasma (from the human cord blood) in 24-well tissue culture plates (Costar Corp, Cambridge, MA) as described previously.30-32 They were cultured at 37°C with 5% CO2 for 12 days.
Flow cytometry.
Cell-surface antigens were detected by immunofluorescence using fluorescein isothiocyanate (FITC; Becton Dickinson, Mountain View, CA) conjugated mouse antihuman CD34 and phycoerythrin (PE)-conjugated antihuman CD41 MoAb. In brief, UT-7 cells or isolated cord blood CD34+ progenitor cells were incubated for 30 minutes at 4°C with the appropriately diluted antibodies. After washing, cell-bound fluorescence on 5,000 to 10,000 cells/sample was determined with a flow cytometer (FACScan; Becton Dickinson).
RESULTS
Expression of GPIb-α, PF-4, u-PA, and PAI-1 in UT-7 megakaryocytic cell lines.
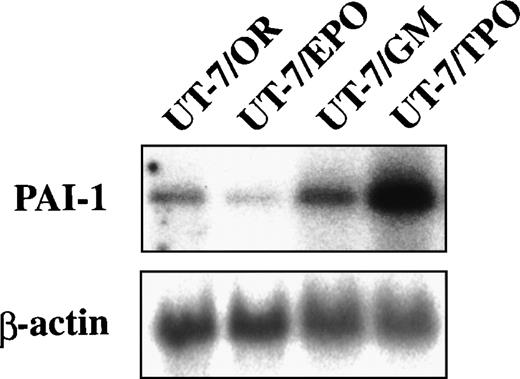
We examined the expression of GPIb-α, PF-4, and u-PA in the UT-7 cell lines by means of RT-PCR. The GPIb-α transcript was detected in the original UT-7 cell lines and in UT-7/TPO cells, but was undetectable in UT-7/EPO or UT-7/GM cells. PF-4, a protein specific to megakaryocytes and platelets, was detected solely in UT-7/TPO cells, and was entirely absent in UT-7/OR, UT-7/EPO, or UT-7/GM cells. In contrast, the u-PA mRNA was scarcely detectable UT-7/TPO cells as well as in platelets (data not shown). These results and previous studies21suggest that the UT-7/TPO cells have mature megakaryocyte characteristics with respect to morphology, ploidy, and the expression of megakaryocyte-specific proteins. Thus, we examined the effect of differentiation into megakaryocytes on PAI-1 expression using these cell lines. As shown in Fig 1, the PAI-1 expression level was detected in UT-7/OR and UT-7/GM cells, with abundant expression in the UT-7/TPO cells, but the transcript was scarcely detected in UT-7/EPO cells.
Expression of PAI-1 mRNAs in UT-7 cell lines. Total cellular RNAs were extracted from UT-7 cell lines (UT-7/OR, UT-7/EPO, UT-7/GM, and UT-7/TPO) and expressions of PAI-1 mRNA were evaluated by RT-PCR (see Materials and Methods). The RT-PCR products were resolved by agarose gel electrophoresis, and the bands were transferred to a membrane and hybridized to 32P-labeled PAI-1 or β-actin cDNA probes.
Expression of PAI-1 mRNAs in UT-7 cell lines. Total cellular RNAs were extracted from UT-7 cell lines (UT-7/OR, UT-7/EPO, UT-7/GM, and UT-7/TPO) and expressions of PAI-1 mRNA were evaluated by RT-PCR (see Materials and Methods). The RT-PCR products were resolved by agarose gel electrophoresis, and the bands were transferred to a membrane and hybridized to 32P-labeled PAI-1 or β-actin cDNA probes.
Pulse-chase analysis of PAI-1 protein in UT-7/TPO cells.
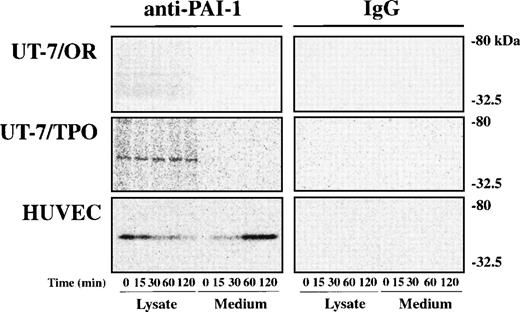
We evaluated the synthesis of PAI-1 and its secretion from UT-7/OR, UT-7/TPO cells, and HUVECs by pulse-chase analysis. The cells were pulse-labeled with [35S]-methionine for 15 minutes and were chased with cell lysate and culture medium for various periods. As shown in Fig 2, PAI-1 protein was not synthesized in UT-7/OR cells. In contrast, the protein produced for 15 minutes was apparent in the UT-7/TPO cell lysate and did not appear in the culture medium. Although UT-7/TPO cells express protease-activated receptor-1 (PAR-1) and PAR-3, a recently identified thrombin receptor of platelets and megakaryocytes,33addition of thrombin to the pulse-labeled cells had no effect on the release of PAI-1 protein (data not shown).
Pulse-chase analysis of PAI-1 in UT-7/OR, UT-7/TPO, and HUVEC. UT-7/OR, UT-7/TPO, and HUVEC were incubated for 60 minutes in methionine-free medium and then pulse labeled with35S-methionine for 15 minutes, followed by a chase with excess of unlabeled methionine for the indicated period. Cells and media were harvested at appropriate intervals, and PAI-1 was immunoprecipitated from each sample with mouse antihuman PAI-1 MoAb (JTI-3 and JTI-4) or normal mouse IgG. Subsequently, they were subjected to SDS-polyacrylamide gel electrophoresis (10% separating gels) and autoradiography.
Pulse-chase analysis of PAI-1 in UT-7/OR, UT-7/TPO, and HUVEC. UT-7/OR, UT-7/TPO, and HUVEC were incubated for 60 minutes in methionine-free medium and then pulse labeled with35S-methionine for 15 minutes, followed by a chase with excess of unlabeled methionine for the indicated period. Cells and media were harvested at appropriate intervals, and PAI-1 was immunoprecipitated from each sample with mouse antihuman PAI-1 MoAb (JTI-3 and JTI-4) or normal mouse IgG. Subsequently, they were subjected to SDS-polyacrylamide gel electrophoresis (10% separating gels) and autoradiography.
The effect of TPO and other ligands on PAI-1 mRNA expression.
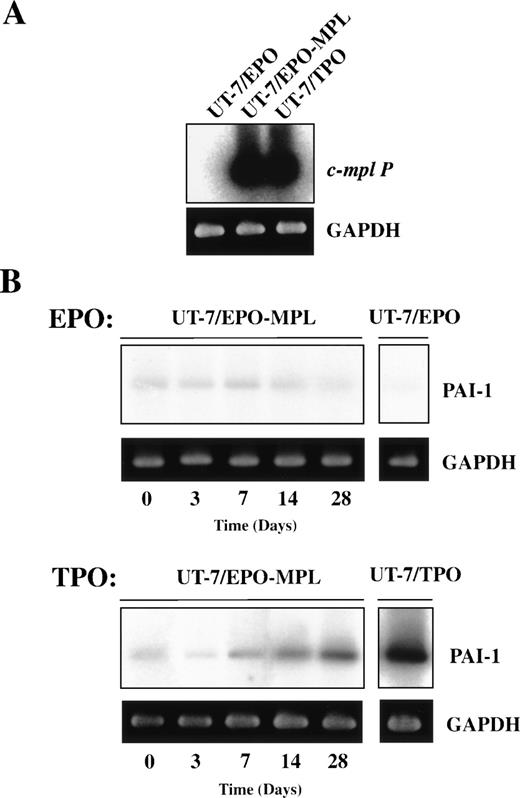
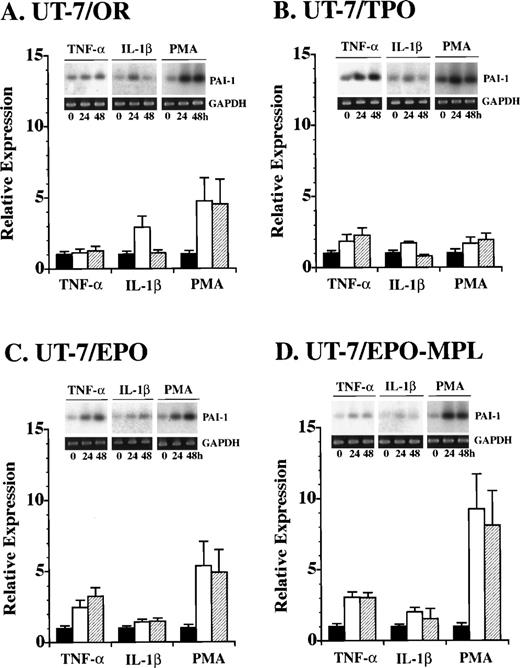
Because UT-7/EPO cells are committed to the erythroid lineage,22 and do not express endogenous c-Mpl, we introduced full-length c-mpl cDNA into the UT-7/EPO cells (Fig3A). We selected neomycin-resistant clones expressing high levels of c-Mpl on the surface of the cells as determined by flow cytometry with a polyclonal antibody against the extracellular domain of c-Mpl. These cells were designated as UT-7/EPO-MPL cells. Using UT-7/OR, UT-7/TPO, UT-7/EPO, and UT-7/EPO-MPL cells, we examined the effect of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, phorbol 12-myristate 13-acetate (PMA), and TPO on the expression of PAI-1 mRNA. TNF-α and IL-1β induced the expression of PAI-1 mRNA within 48 hours in all cell lines (Fig 4). Incubation with 10 nmol/mL PMA resulted in a more-rapid increase in the PAI-1 mRNA expression. Although the expressions of GP-Ibα and PF-4 mRNA were also upregulated by PMA for 24 hours, UT-7/EPO-MPL cells did not obviously change their morphology, except for mild adhesion to the plastic dishes and extension of pseudopadia (data not shown). In contrast, TPO did not increase PAI-1 mRNA expression until day 7 (Fig3B). The effect of TPO on the growth of UT-7/EPO-MPL cells was similar to that of EPO when incubated for several days. However, over a long-term culture of 14 to 28 days, the TPO-stimulated UT-7/EPO-MPL cells gradually enlarged and became nearly identical with UT-7/TPO cells. In parallel with these morphological changes, PAI-1 transcript was increased and visible by RT-PCR on day 14.
Effect of TPO on the expression of PAI-1 mRNA in UT-7/EPO transfected with c-Mpl (UT-7/EPO-MPL). (A) Total cellular RNAs extracted from UT-7/EPO, UT-7/EPO transfected with c-Mpl (UT-7/EPO-MPL), and UT-7/TPO were RT and amplified by PCR. The RT-PCR products were resolved by agarose gel electrophoresis, and the bands were transferred to a membrane and hybridized to32P-labeled c-mpl P cDNA probes. (B) UT-7/EPO-MPL cells were cultured in the presence of 1 U/mL EPO or 10 ng/mL TPO. The cells were harvested at the indicated time points (0 to 28 days) and subjected to RT-PCR analysis for PAI-1 and GAPDH mRNA expression. PAI-1 RT-PCR products were analyzed on 2% agarose gels and transferred to membranes and hybridized to 32P-labeled PAI-1 cDNA probes.
Effect of TPO on the expression of PAI-1 mRNA in UT-7/EPO transfected with c-Mpl (UT-7/EPO-MPL). (A) Total cellular RNAs extracted from UT-7/EPO, UT-7/EPO transfected with c-Mpl (UT-7/EPO-MPL), and UT-7/TPO were RT and amplified by PCR. The RT-PCR products were resolved by agarose gel electrophoresis, and the bands were transferred to a membrane and hybridized to32P-labeled c-mpl P cDNA probes. (B) UT-7/EPO-MPL cells were cultured in the presence of 1 U/mL EPO or 10 ng/mL TPO. The cells were harvested at the indicated time points (0 to 28 days) and subjected to RT-PCR analysis for PAI-1 and GAPDH mRNA expression. PAI-1 RT-PCR products were analyzed on 2% agarose gels and transferred to membranes and hybridized to 32P-labeled PAI-1 cDNA probes.
Effect of TNF-, IL-1β, and PMA on the expression of PAI-1 mRNA in UT-7 cell lines. At the initiation of (A) UT-7/OR, (B) UT-7/TPO, (C) UT-7/EPO, and (D) UT-7/EPO-MPL culture, any one of 10 ng/mL TNF-, 10 ng/mL IL-1β, and 10 nmole/mL PMA was added to the culture. The cells were harvested at the indicated times (0, 24, and 48 hours) and examined by RT-PCR followed by Southern blot analysis. Each value (mean ± SD, n = 3) shows the ratio of PAI-1 expression after 24 and 48 hours of culture versus the respective control (0 hour) expression.
Effect of TNF-, IL-1β, and PMA on the expression of PAI-1 mRNA in UT-7 cell lines. At the initiation of (A) UT-7/OR, (B) UT-7/TPO, (C) UT-7/EPO, and (D) UT-7/EPO-MPL culture, any one of 10 ng/mL TNF-, 10 ng/mL IL-1β, and 10 nmole/mL PMA was added to the culture. The cells were harvested at the indicated times (0, 24, and 48 hours) and examined by RT-PCR followed by Southern blot analysis. Each value (mean ± SD, n = 3) shows the ratio of PAI-1 expression after 24 and 48 hours of culture versus the respective control (0 hour) expression.
Expression of PAI-1 during short-term liquid culture of CD34+ cord blood cells.
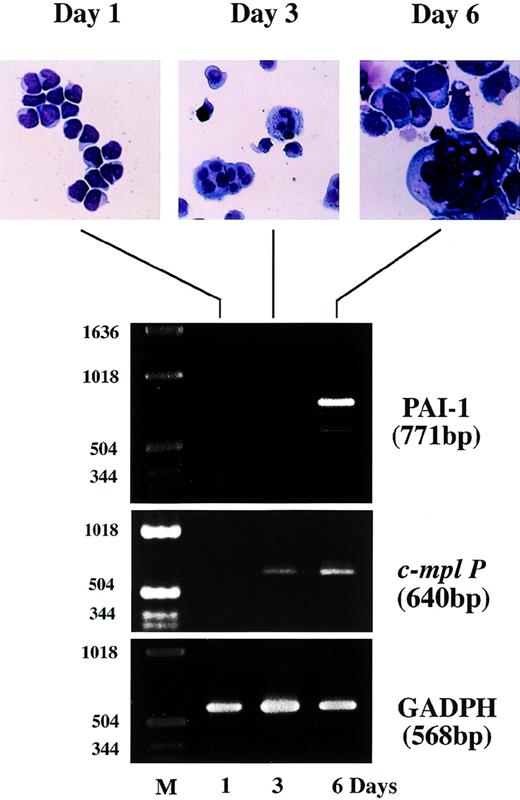
To verify the parallel increase of PAI-1 mRNA expression with apparent morphological change to a more megakaryocytic-like cell, we examined the expression of c-mpl P and PAI-1 mRNA transcripts in the presence of TPO in a short-term liquid culture of cord blood. CD34+ cells were isolated from normal human cord blood using a magnetic cell sorting system and cultured for 6 to 12 days in a megakaryocyte culture medium as previously described.31,32 34 As shown in the upper panel of Fig 5, cells with morphologic features of immature megakaryocytes, including basophilic cytoplasm and budding, began to appear after 3 days of culture. Mature megakaryocytes-like features with polyploid nuclei were observed after 6 days. Total cellular RNA was isolated on days 1, 3, and 6, and the expression of c-mpl P and PAI-1 mRNA was examined by semiquantitative RT-PCR analysis. On day 1, CD34+ cells expressed neither c-mpl P nor PAI-1 mRNA transcript just after isolation, whereas GAPDH mRNA, which was used as an internal control, was readily detectable (Fig 5, lower panel, day 1). The expression of c-mpl P was observed after 3 days of culture, which is apparently preceded by polyploidization. In contrast, PAI-1 mRNA transcript was not present on day 3, but was apparent only after 6 days of culture in parallel with the appearance of mature megakaryocytes. This chronological order of PAI-1 expression is consistent with the findings obtained with the UT-7 cell lines.
DISCUSSION
Platelets can modify fibrinolysis through several mechanisms. Platelet arterial thrombi are more resistant to lysis by plasminogen activators than platelet-poor thrombi.35 Several studies suggest that PAI-1, which inhibits fibrinolysis by binding irreversibly to the active site of t-PA and u-PA, is a major determinant of the resistance of platelet-rich clots to lysis by t-PA.36,37 Activation of platelets results in release of PAI-1 from the α-granules. In this study, we have investigated the mechanism of production of PAI-1 during the process of megakaryocyte differentiation. Among the UT-7 cell lines, the UT-7/OR cells have some properties of megakaryocytes, including polyploidy and positive staining of platelet peroxidase.20 In contrast, UT-7/EPO cells have progressed further in erythroid development than the parent UT-7 cells,22 and UT-7/GM cells are of the erythroid-megakaryocytic bipotential lineage, because they have the capacity to differentiate into erythroid or megakaryocytic lineages by treatment with EPO and TPO, respectively.23Morphologically, UT-7/TPO cells have mature megakaryocytic characteristics, such as a developed demarcation membrane in the cytoplasm and a multinucleated appearance with a high level of DNA content.21 In addition, UT-7/TPO cells contain high levels of GP-Ibα, PF4 and PAI-1 mRNA (Fig 1). Therefore, we initially hypothesized that PAI-1 expression might be closely related to megakaryocyte differentiation induced by TPO. In contrast, u-PA mRNA transcript was decreased in the UT-7/TPO cells and not detected in platelets. Because immature cells and many cell lines originated from cancer cells are apt to have both PAI-1 and u-PA mRNAs,38 39 this result may also support the differentiation of UT-7, developed from megakaryoblastic leukemia, into a more megakaryocytic cell line, UT-7/TPO.
PAI-1 protein was synthesized de novo in UT-7/TPO cells as shown by the [35S]-methionine–labeled pulse-chase analysis (Fig 2). Interestingly, unlike HUVECs and other cell lines,40UT-7/TPO cells did not secrete PAI-1 immediately after synthesis (Fig2). This may represent a storage pool of PAI-1, although whether or not it is eventually secreted awaits further experimentation. Furthermore, PAI-1 was not secreted after thrombin stimulation, although UT-7/TPO cells express both PAR-1 and PAR-3 protease receptors capable of binding thrombin (data not shown). This indicates that the signal transduction system for these receptors probably differ between megakaryocytes and platelets.
To clarify the mechanism of TPO-dependent induction, we compared the induction of PAI-1 by other agents such as PMA, IL-1β, and TNF-α. Analyses of the mechanism involved in megakaryocytic differentiation and the expression of megakaryocytic genes were performed with PMA-induced human megakaryocytic cell line models.41Hill40 and Konkle42 showed that PMA-induced PAI-1 mRNA expression and the accumulation of PAI-1 protein in Dami cells and CHRF-288 cell lines, respectively. However, because PMA is a chemical agent and not a physiological regulator, its action on megakaryoblastic cell lines may not always mimic normal megakaryocytopoiesis. Because c-Mpl specifically regulates megakaryocytopoiesis and thrombopoiesis through activation by its ligand TPO,18 we forced c-Mpl expression in UT-7/EPO cells, which do not express endogenous c-Mpl, and studied the effect of TPO stimulation on the transfected cells (UT-7/EPO-MPL). UT-7/EPO-MPL cells not only depended on EPO for growth and survival, but they also acquired the ability to proliferate and differentiate in the presence of TPO. PAI-1 mRNA expression was induced by PMA in UT-7/EPO-MPL cells within 12 to 48 hours (Fig 4). In addition, the proinflammatory cytokines, IL-1β and TNF-α, which are known to enhance PAI-1 production through increased transcription rate, also induced PAI-1 mRNA in UT-7/EPO-MPL for 48 hours without inducing apparent morphological changes. Furthermore, the comparisons of PAI-1 mRNA expression between UT-7/OR and UT-7/TPO or UT-7/EPO and UT-7/EPO-MPL show that upregulations of PAI-1 production by these cytokines are independent of c-Mpl presentation. In contrast, PAI-1 mRNA transcripts were detectable in UT-7/EPO-MPL cells only after 14 days stimulation with TPO, a time when these cells changed morphology to mature megakaryocytes-like feature (Fig 3). Our data suggest that megakaryocytes response to TPO through c-Mpl is essential for their maturation, differentiation, and constitutive expression of PAI-1 mRNA. However, because PAI-1 message is not detected in platelets but the protein is,9,10 it is also possible that mRNA turnover might contribute in part to the regulation of PAI-1 message level.43 Recently, PU.1/Spi-1, an Ets-related transcription factor, was found to be selectively induced by TPO and it increased the transcription activity of megakaryocyte-related gene promoters, whereas PMA did not.44 Because the PAI-1 gene promoter contains several Ets-binding core sequences GGAA (GGAA −621, −460, −396, and −61) and a predicted GATA-binding site (GATA −426),45 these elements could be responsible for the TPO action.
In normal cord blood CD34−/CD41+ colonies induced by TPO, c-mpl mRNA expression was induced before the detection of PAI-1 mRNA transcript and PAI-1 mRNA expression peaked at 6 days (Fig 5), and reached a plateau on day 12 (data not shown). Although PAI-1 mRNA appeared faster in these colonies than in the UT-7/EPO-MPL cells, a difference in the maturation stage between these two groups may be a contributing factor. Thus, the expression of PAI-1 was first shown at the transcriptional level both in leukemic and normal megakaryocytes.
Expression of PAI-1 and c-Mpl mRNA during short-term culture of normal human cord blood CD34+ progenitor cells with TPO. The CD34+ cells were isolated from human cord blood using antihuman CD34 MoAb bound to magnetic microspheres and cultured in the presence of 10 ng/mL TPO. The cells were harvested at the indicated time points and subjected to morphologic examination on Wright-Giemsa staining cytospin slides (upper panel) and RT-PCR analysis for PAI-1, c-mpl P and GAPDH mRNA expression (lower panel). Amplified products were analyzed on 2% agarose gels followed by ethidium bromide staining. M, molecular size marker.
Expression of PAI-1 and c-Mpl mRNA during short-term culture of normal human cord blood CD34+ progenitor cells with TPO. The CD34+ cells were isolated from human cord blood using antihuman CD34 MoAb bound to magnetic microspheres and cultured in the presence of 10 ng/mL TPO. The cells were harvested at the indicated time points and subjected to morphologic examination on Wright-Giemsa staining cytospin slides (upper panel) and RT-PCR analysis for PAI-1, c-mpl P and GAPDH mRNA expression (lower panel). Amplified products were analyzed on 2% agarose gels followed by ethidium bromide staining. M, molecular size marker.
In summary, we report here that PAI-1 mRNA is expressed in megakaryocytes and is accompanied by de novo PAI-1 protein synthesis. This endogenous PAI-1 synthesis may be closely related to TPO-dependent megakaryocyte maturation.
ACKNOWLEDGMENT
We thank Dr Hiroshi Tomizuka for helpful discussion regarding the flow cytometry analyses of cultured cells.
Supported in part by a Grant-in-Aid for Scientific Research, No. 09470234 from the Ministry of Education, Science, Sports, and Culture of Japan, by a grant from Nippon Foundation, and by a grant from Jichi Medical School Young Investigator Award.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal