LOCAL REMODELING OF chromatin is a key step in the transcriptional activation of genes. Dynamic changes in the nucleosomal packaging of DNA must occur to allow transcriptional proteins contact with the DNA template. The realization that the proteins that regulate the modification of chromatin are themselves disrupted in many leukemic chromosomal rearrangements has generated new excitement in the study of chromatin structure. Recent reports have kindled the hope that pharmacological manipulation of chromatin remodeling might develop into a potent and specific strategy for the treatment of these leukemias. In this review, we will discuss the structure of chromatin and the mechanisms by which cells remodel chromatin, alteration of these pathways in leukemias, and therapeutic approaches.
CHROMATIN: BEADS ON A STRING OR STRING ON BEADS?
The condensation of DNA into an ordered chromatin structure allows the cell to solve the topological problems associated with storing huge molecules of chromosomal DNA within the nucleus. DNA is packaged into chromatin in orderly repeating protein-DNA complexes called nucleosomes.1,2 Each nucleosome consists of approximately 146 bp of double-stranded DNA wrapped 1.8 times around a core of 8 histone molecules (Fig 1). Two molecules each of H2A, H2B, H3, and H4 comprise the histone ramp around which the DNA superhelix winds. Stretches of DNA up to 100 bp separate adjacent nucleosomes. Multiple nuclear proteins bind to this linker region, some of which may be responsible for the ordered wrapping of strings of nucleosomes into higher-order chromatin structures.3 4
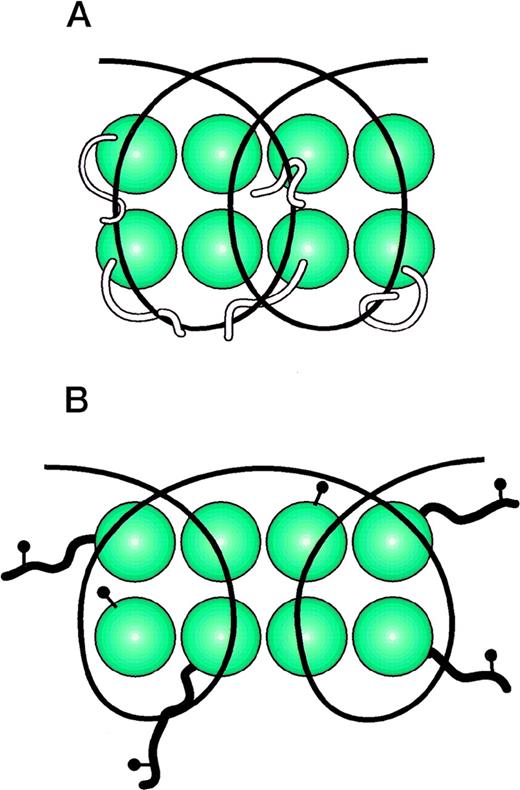
Cartoon of nucleosomal structure. (A) represents the random-coiled tails of the histone octamer intertwined with DNA. (B) represents the nucleosome with histones acetylated (acetyl groups drawn as lollipop structures). The acetylated histone tails do not bind the DNA strands. This allows the DNA to assume a more open configuration that is accessible to the transcriptional machinery.
Cartoon of nucleosomal structure. (A) represents the random-coiled tails of the histone octamer intertwined with DNA. (B) represents the nucleosome with histones acetylated (acetyl groups drawn as lollipop structures). The acetylated histone tails do not bind the DNA strands. This allows the DNA to assume a more open configuration that is accessible to the transcriptional machinery.
Nucleosomal structure has been well characterized by x-ray crystallographic studies, most recently to a resolution of 2.8 Å.1 The histones are arranged as heterodimers of H2A and H2B, H3 and H4; the heterodimers, in turn, form a tetrameric structure known as the octamer core. Each histone heterodimer binds approximately 30 bp of DNA through electrostatic contacts with the phosphate backbone in the minor groove of the DNA. The interaction with histones causes the DNA to become distorted and bend and bulge at several positions: this twisting of the helix results in a deviation of the periodicity of the basepairs as they spiral along the DNA superhelix. The change in periodicity leads to alignment of the DNA minor grooves to form channels, through which pass the random-coil histone tails. The histone tails are thus able to contact the DNA on the exterior of the nucleosomal particle to further stabilize DNA/histone interactions.
Although nucleosomal architecture depends primarily on nonspecific interactions between histones and the phosphate backbone of DNA, the thermodynamic stability of the interaction is influenced by the basepair composition of the DNA. A-T–rich sequences in particular impart the DNA with flexibility that allows it to form the tertiary structures necessary for optimal nucleosomal packing.1 5 In addition, histone leucine residues bound to the minor groove of DNA are able to interact with nearby thymidine residues; arginines form hydrogen bonds with neighboring pyrimidines. These sequence-specific characteristics likely contribute to variations in the number of bases in each nucleosome particle, the length of internucleosomal spacer DNA, and the phasing of adjacent nucleosomes.
NUCLEOSOMAL REMODELING
Both distortion of the periodicity of the superhelix and electrostatic shielding by the positively charged histones act to constrain the access of nonhistone proteins to nucleosomal DNA.6,7Extensive remodeling of chromatin structure, particularly at cis-acting promoter sites necessary for transcriptional initiation, must take place for the DNA template to become accessible to the transcriptional machinery. A complete picture of the way in which DNA separates from nucleosomes during transcription is not yet available, but most likely only partial dissociation from the core histones is necessary.8
Several mechanisms have been identified that contribute to chromatin remodeling. They vary from those that alter histone-DNA interaction to those that physically dissociate the DNA from histones in an ATP-dependent manner to those that destabilize histone-histone tetramerization (see the recent review by Tsukiyama and Wu9for a more detailed discussion). All of these processes likely act simultaneously and in concert to regulate access to the DNA template. Two of the mechanisms are particularly relevant to the discussion of leukemic fusion proteins (see below): histone acetylation and ATPase-mediated DNA-histone dissociation.
The best understood mechanism by which cells regulate chromatin structure is posttranslational modification of histones by acetylation.10-15 Change in electrostatic attraction for DNA and steric hindrance introduced by the hydrophobic acetyl group leads to destabilization of the interaction of histones with DNA.1,11-13,15,16 Lysine residues in the N-terminal extensions of H2B, H3, and H4 that bind to the exterior surface of nucleosomes are particularly accessible to acetylation. Acetylation of residues within the octamer core interferes with formation of the histone heterodimers and tetramers around which the DNA winds. Furthermore, posttranslational modification of histones interferes with interactions with nonhistone, chromatin-associated proteins, such as MeCP2 (which binds to methylated regions of DNA17) and the high-mobility group proteins (HMG,18 19 so named for their properties in electrophoretic fields), which also contribute to higher-order nucleosomal packing. In summary, acetylation of histones disrupts nucleosomes and allows the DNA to become accessible to transcriptional machinery. Removal of the acetyl groups allows the histones to bind more tightly to DNA and to maintain a transcriptionally repressed chromatin architecture. Acetylation is mediated by a series of enzymes with histone acetylase (histone acetyl transferase [HAT]) activity; conversely, acetyl groups are removed by specific histone deacetylase (HDAC) enzymes.
The ability to modulate histone acetylation in a gene-specific fashion presents a challenge for cells. The state of histone acetylation is determined by the balance between competing enzymatic activities of histone acetyltransferases and deacetylases. Fine regulation of the catalytic activities of these enzymes likely involves cooperative subunit interaction and posttranslational modification. However, our limited understanding of the regulation of gene-specific histone acetylation is best captured in the rather simplistic model of local recruitment of acetylases or deacetylases by sequence-specific DNA binding proteins.
The paradigm for local remodeling of chromatin through gene-specific recruitment of HAT activity is the retinoic acid receptor (RAR).20 Because RAR-mediated chromatin remodeling is perturbed in acute promyelocytic leukemia (APL; see below), it is appropriate to consider this model in some depth; similar models have been developed for other transcriptional activators and repressors.21 RAR is a ligand-dependent transcriptional activator.22 Through its zinc (Zn) finger domain, it binds as a heterodimer with a related protein, RXR,23 to a well-defined consensus DNA sequence found in the promoters of retinoic acid-responsive genes.24 In the absence of ligand (retinoic acid [RA]), the RXR/RAR heterodimer binds to DNA and actively represses transcription below the basal level expected from random initiation by the transcriptional machinery.25a It does this through an indirect mechanism. The RXR/RAR heterodimer binds a nuclear corepressor molecule, either N-CoR26,27(Nuclear Receptor Co-Repressor) or SMRT28,29 (Silencing Mediator ofRetinoid and Thyroid Receptors), through specific interaction domains in the ligand-binding region of RAR. N-CoR, the better analyzed of the 2, binds to many sequence-specific DNA-binding transcriptional repressor proteins (Table1). N-CoR (or SMRT) itself binds another intermediary protein, Sin3, which serves as a bridge to HDAC1, a histone deacetylase30-32 (there have been 3 homologous HDACs identified in human cells33). Thus, the end result of the association of unliganded RAR/RXR with N-CoR is to recruit HDAC1 to the local environment of the promoter. By removing acetyl groups from histones and restructuring the chromatin into a repressive configuration, HDAC1 serves as the effector molecule in this pathway. N-CoR, Sin3, and HDAC1 function in many repressive pathways, including transcriptional silencing by the MAD/MAX members of the MYC family,30,31,34,35 ETO36-38 (see below), and other members of the steroid hormone receptor superfamily.26 32
Corepressor Proteins
| Protein . | Homolog . | Binds to . | Deacetylase Activity . |
|---|---|---|---|
| N-CoR (Nuclear Receptor Corepressor) | SMRT | Nuclear receptors, others | No |
| SMRT (Silencing Mediator of Retinoid and Thyroid Receptors) | N-CoR | Nuclear receptors, others | No |
| Sin3 | N-CoR or SMRT DNA-binding proteins | No | |
| HDAC | Sin3 | Yes |
| Protein . | Homolog . | Binds to . | Deacetylase Activity . |
|---|---|---|---|
| N-CoR (Nuclear Receptor Corepressor) | SMRT | Nuclear receptors, others | No |
| SMRT (Silencing Mediator of Retinoid and Thyroid Receptors) | N-CoR | Nuclear receptors, others | No |
| Sin3 | N-CoR or SMRT DNA-binding proteins | No | |
| HDAC | Sin3 | Yes |
Binding of ligand to RAR initiates a conformational change in the C-terminus of RAR, which contains the N-CoR binding site.39-41 An amphipathic helix within RAR (helix 12, which is highly conserved within the steroid receptor superfamily) swings into a position that both locks the ligand into its binding pocket and creates a new hydrophobic domain. N-CoR does not bind to this altered conformation of RAR, and hence the N-CoR/Sin3/HDAC1 complex dissociates itself from liganded RAR. In its place, the newly created hydrophobic domain within RAR binds a large multimolecular complex that enhances transcriptional activation.21,42,43 Several components of this coactivator complex have been identified (Table 2), including the adenovirus E1A-associated protein p300, pCAF (CBP-associated factor44), and a family of homologous 160-kD proteins, including p/CIP (p300/CBP cointegrator-associated protein),43,45 NCoA-146 (nuclear coactivator-1, also known as SRC-142), and NCoA-2 (also known as TIF-246-48). p300, or the homologous cyclic-AMP response-element-binding protein cofactor CBP,21,43,49,50 has been shown to interact through nonoverlapping domains with at least a dozen DNA-binding transcriptional activators, including other members of the steroid hormone receptor family, members of the STAT family, Jun, Fos, AML1, and Myb (see review by Giles et al51). In this regard, p300/CBP serves as a bridge between multiple transcriptional activators. Several members of this complex have HAT activity, including pCAF,44,52 the 160-kD proteins,53 and p300/CBP itself.50 Thus, recruitment of the coactivator complex brings several histone acetylases to the proximity of the promoter to which RAR binds, so that the deacetylated histones can be efficiently acetylated. The coactivator complex serves to reverse the suppressive effects of the N-CoR/Sin3/HDAC1 complex and, by decreasing the affinity of histones for DNA, remodel nucleosomal configuration so that the DNA template can be accessed for transcription. It is as yet unclear which of the proteins in the complex directly interact with histones, which acetylate other proteins in the complex (or the proteins that comprise the basal transcriptional machinery), which stabilize the nascent basal transcriptional machinery or, which, as has been proposed for p300/CBP, function in all 3 capacities.50
Histone Acetylases
| Human Histone Acetylase . | Alternate Name . | Homolog . | Part of Coactivator Complex . |
|---|---|---|---|
| p300 | CBP (CREB-binding protein) | Yes | |
| PCAF (CBP-associated factor) | Yes | ||
| NCoA-1 (nuclear coactivator-1) | SRC-1 | NCoA-2, p/CIP | Yes |
| NCoA-2 | TIF-2, GRIP-1 | NCoA-1, p/CIP | Yes |
| p/CIP (p300/CBP cointegrator-associated protein) | RAC3/AIBI/ACTR | NCoA-1, NCoA-2 | Yes |
| TAFII250 | No | ||
| MOZ | No |
| Human Histone Acetylase . | Alternate Name . | Homolog . | Part of Coactivator Complex . |
|---|---|---|---|
| p300 | CBP (CREB-binding protein) | Yes | |
| PCAF (CBP-associated factor) | Yes | ||
| NCoA-1 (nuclear coactivator-1) | SRC-1 | NCoA-2, p/CIP | Yes |
| NCoA-2 | TIF-2, GRIP-1 | NCoA-1, p/CIP | Yes |
| p/CIP (p300/CBP cointegrator-associated protein) | RAC3/AIBI/ACTR | NCoA-1, NCoA-2 | Yes |
| TAFII250 | No | ||
| MOZ | No |
An interesting twist to this paradigm has recently arisen through studies of DNA methylation, which has been a long-accepted mechanism of transcriptional suppression54,55. A possible contributor to the transcriptional-silencing effect of DNA-methylation may be the MeCP family of proteins, which specifically bind to regions of methylated DNA between adjacent nucleosomes.56 The MeCP2 protein directly binds Sin3/HDAC1.17 Thus, by recruiting a histone deacetylase, MeCP2-binding leads to a change in the state of histone acetylation and in the chromatin structure of methylated DNA. Whether this mechanism directly mediates the transcriptional silencing effects of DNA methylation or whether it serves a synergistic or supporting role is not yet clear. However, it leads to the interesting speculation that DNA-methyltransferase inhibitors such as 5-azacytidine57 might alter transcriptional activity by indirectly remodeling regions of methylated chromatin.
A second mechanism for alteration of chromatin that is less well understood than acetylation of histones involves an ATP-dependent enzymatic activity that directly acts on nucleosomal structure. Based on analogous protein complexes in yeast and Drosophila (known by the acronyms SWI/SNF,58-62 NURF,63 and RSC64,65), it has been proposed that these complexes act as ATP-dependent motors that track along the DNA strands and pull them away from the histone octamer cores.59-62,66 During this shift of histone-DNA contact points, the DNA would presumably become accessible to the transcriptional machinery. However, there is conflicting evidence to suggest that these complexes serve to maintain chromatin in a repressive configuration, perhaps through dissociating other chromatin-associated proteins from the DNA, such as errant TATA-binding protein.67 Reinforcing the concept of interplay between the chromatin remodeling mechanisms is the finding that 2 members of the human p300-associated coactivator complex68 69 have the predicted ATPase activities requisite for a SWI/SNF type of engine.
DISRUPTION OF CHROMATIN REMODELING MECHANISMS IN LEUKEMIA
APL: Abnormal histone deacetylation.
Chromatin remodeling is fundamental to transcription. The models presented above outline the normal control of chromatin remodeling during gene-specific transcription. Disruption of these mechanisms gives rise to transcriptional chaos and leukemic transformation. The best understood example of this is in APL (French-American-British [FAB] M3).
APL holds a unique position in the study of leukemias in that it is the only form of leukemia and the only malignancy described to date that responds to differentiation therapy. First published in Bloodby Huang et al70 from the Shanghai Institute of Hematology, APL blasts undergo terminal differentiation in response to all-trans retinoic acid (ATRA). Differentiation therapy with ATRA has become the mainstay of therapy for this disease.71,72 Although relapses uniformly occur when used by itself, in combination with conventional chemotherapy, ATRA has revolutionized the treatment of APL, generating response rates of close to 90%, with 3-year disease-free survival greater than 75%.73
The explanation for the restriction of the success of ATRA therapy to the M3 subtype of leukemias likely lies in the chromatin alterations induced by the RAR-fusion proteins expressed uniquely in APL cells (Fig2). RARα (there are 3 homologous RAR proteins, called α, β, and γ) is a transcriptional activator that binds to specific DNA sequences and inducibly recruits corepressor or coactivator complexes to regulate transcription of retinoic acid-responsive genes (see above). Ordered expression of retinoic acid-responsive genes is necessary for myeloid development; dominant-negative mutants of RARα inhibit myeloid maturation at the promyelocytic stage.74,75All patients with APL have a chromosomal translocation within the second intron of the RARα locus on chromosome 17q12 to produce a chimeric protein comprised of all but the first 30 AA of RARα.76 The N-terminal fusion partners arePML77,78 on chromosome 15q21,PLZF79 on chromosome 11q23,NPM80 on chromosome 5q31, orNUMA81 on chromosome 11q13. The PML-fusion is the most common: only 8 patients with PLZF-RARα,82 3 with NPM-RARα,80 and 1 with NUMA-RARα81 have been described to date. All of these RARα fusion proteins contain the DNA-binding, heterodimerization, ligand-binding, corepressor-binding, and coactivator-binding motifs of RARα. Like wild-type RARα, they bind to retinoic acid-response elements in DNA and, under appropriate conditions, can activate transcription of RA-target genes.77,80,83 84
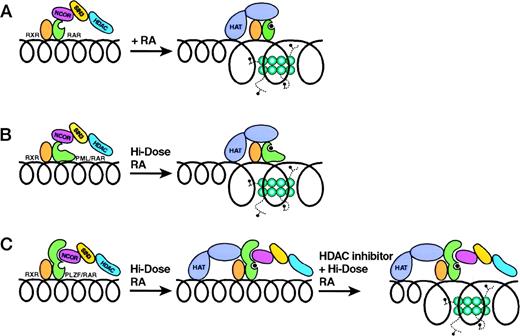
Fusion proteins in acute promyelocytic leukemia. (A) indicates the unliganded interactions of the RXR/RAR heterodimer with an N-CoR/Sin3/HDAC1 complex. Upon binding retinoid acid, the RXR/RAR heterodimer releases the corepressor complex and binds a coactivator complex with histone acetylase activity. (B) indicates the analogous interactions of the RXR/PML-RAR heterodimer with the corepressor complex. Release of the corepressor complex occurs only in the presence of pharmacological levels of retinoic acid. (C) depicts the ligand-independent binding of the corepressor complex to PLZF-RAR. (It has been proposed, but not yet been formally demonstrated, that liganded RXR/PLZF-RAR binds both coactivator and corepressor complexes.) Chromatin remodeling occurs only in the presence of both RA and an HDAC inhibitor.
Fusion proteins in acute promyelocytic leukemia. (A) indicates the unliganded interactions of the RXR/RAR heterodimer with an N-CoR/Sin3/HDAC1 complex. Upon binding retinoid acid, the RXR/RAR heterodimer releases the corepressor complex and binds a coactivator complex with histone acetylase activity. (B) indicates the analogous interactions of the RXR/PML-RAR heterodimer with the corepressor complex. Release of the corepressor complex occurs only in the presence of pharmacological levels of retinoic acid. (C) depicts the ligand-independent binding of the corepressor complex to PLZF-RAR. (It has been proposed, but not yet been formally demonstrated, that liganded RXR/PLZF-RAR binds both coactivator and corepressor complexes.) Chromatin remodeling occurs only in the presence of both RA and an HDAC inhibitor.
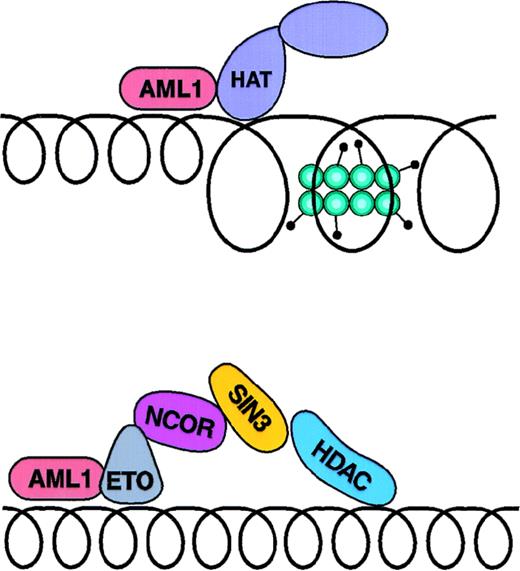
AML-ETO fusion protein. (A) depicts the association of the AML1 transcriptional activator with a coactivator complex; (B) indicates the binding of a corepressor to the AML-ETO fusion protein.
AML-ETO fusion protein. (A) depicts the association of the AML1 transcriptional activator with a coactivator complex; (B) indicates the binding of a corepressor to the AML-ETO fusion protein.
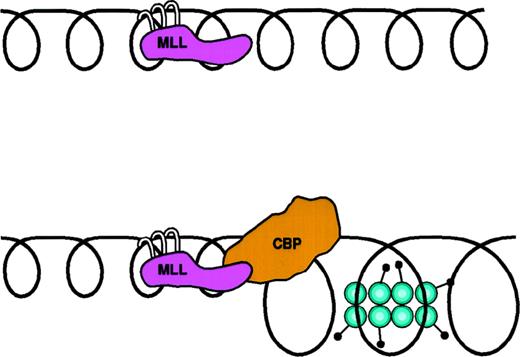
MLL-CBP. One of several models for MLL-CBP function. (A) MLL binds to DNA through interactions between its AT hooks and the minor groove of DNA. (B) MLL-CBP alters chromatin structure at MLL-target sites through the action of the histone acetyltransferase domain of CBP.
MLL-CBP. One of several models for MLL-CBP function. (A) MLL binds to DNA through interactions between its AT hooks and the minor groove of DNA. (B) MLL-CBP alters chromatin structure at MLL-target sites through the action of the histone acetyltransferase domain of CBP.
However, at physiological levels of retinoic acid, PML-RARα represses rather than activates transcription.85-88 This is apparently the consequence of enhanced interaction between PML-RARα and the N-CoR/Sin3/HDAC1 corepressor complex. PML-RARα binds N-CoR at levels of ligand that are otherwise sufficient to release the corepressor complex from wild-type RARα. By maintaining the promoters of RA-responsive genes in a repressive deacetylated configuration, PML-RARα suppresses transcription and produces the identical phenotype to that experimentally produced by a dominant-negative inhibitor of RARα.74 Only at pharmacological levels of ligand does PML-RARα release N-CoR, recruit a coactivator complex, and allow histone acetylation and chromatin remodeling to proceed. Thus, for PML-RARα–expressing APL cells, pharmacological levels of RA are needed to induce differentiation.
If the end result of PML-RARα binding to the corepressor complex is active repression of transcription through a HDAC1-dependent pathway, then one would predict that the APL phenotype might be overcome by inhibitors of HDAC. This prediction has been validated by the finding that RA and inhibitors of HDAC1 (see below) synergize to induce differentiation of the APL cell line, NB4, or U937 cells engineered to express PML-RARα.85-87 The molecular mechanism underlying the strong interaction of PML-RARα with N-CoR is not yet known: there is apparently no direct binding between PML and N-CoR. Fusion with PML presumably inhibits the ligand-induced conformational changes necessary for release of the N-CoR complex. A similar mechanism may also be at play with the NPM-RARα t(5;17) fusion, which is also retinoic acid-responsive.89
The t(11;17)(q23;q12) chromosomal translocation of APL fuses the same sequences of RARα to the N-terminus of PLZF.79 PLZF is itself a DNA-binding transcription factor, capable of binding N-CoR via the 120 AA N-terminal POZ motif90, 91 (conserved betweenpoxvirus and zinc finger proteins) retained in the PLZF-RARα fusion.92-94 As a result, PLZF-RARα interacts with N-CoR through 2 binding sites: a ligand-dependent site in the RARα domain and a ligand-independent site in the PLZF N-terminus.85-88,93 When retinoic acid binds to the ligand-binding domain of PLZF-RARα, the RARα domain of the fusion protein loses its attraction for N-CoR, but the PLZF ligand-independent domain continues to bind N-CoR. As a result, even in the presence of pharmacological levels of retinoic acid, N-CoR/Sin3/HDAC1 remains tethered to RA-responsive promoters, permanently suppressing transcription and blocking differentiation. This model explains the lack of response of t(11;17) patients to ATRA differentiation therapy95 and may explain in part the observation that t(11;17) blasts show morphologic features indicating less differentiation than classical APL cells.82 Based on this model, one would predict that inhibitors of HDAC might partially overcome the suppressive effects of PLZF-RARα. This is indeed the case: several groups have recently demonstrated that inhibitors of HDAC1 synergize with retinoic acid to induce differentiation of otherwise nonresponsive PLZF-RARα cells.85-88
AML1-ETO: Exchanging a coactivator for a corepressor.
The AML1-ETO oncoprotein has recently been shown to alter gene expression through an analogous mechanism of errant recruitment of an N-CoR repressor complex. AML1-ETO is derived from the fusion of the AML1 gene on chromosome 21q22 with the ETO (also called MTG8) locus on chromosome 8q22 (see review by Hiebert et al96). Accounting for over 10% of acute myeloid leukemias (AML), t(8;21) is seen exclusively in FAB M2. Patients with t(8;21) have a better response rate to chemotherapy and a higher remission rate than M2 patients with normal karyotype.97 98
AML1 is a sequence-specific DNA binding protein that complexes with core binding factor β (CBFβ) to activate transcription of target genes.99 Many of the AML1-dependent genes have been implicated in myeloid maturation, including interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), myeloperoxidase, and neutrophil elastase.96 Unlike the RARα model discussed in detail above, AML1/CBFβ binds no ligand; regulation of AML1 activity occurs as a result of transcriptional and posttranslational modulation of AML1 (as yet, these mechanisms have been poorly defined). Recently, it has been shown that the C-terminus of AML1 interacts with a p300-containing coactivator complex,100 suggesting that one of the mechanisms by which AML1 activates transcription is through local histone acetylation and nucleosomal remodeling.
AML1-ETO, on the other hand, inhibits transcription of AML1-responsive genes.99,101AML1-ETO knock-in mice102have similar embryonic-lethal phenotype as aml1 knock-out mice,25 which show absent fetal liver hematopoiesis. The AML1-ETO protein contains the N-terminal 177 AA of AML1, including therunt homology domain that mediates sequence-specific DNA binding,99 fused to near full-length ETO protein.103 ETO was originally identified through its involvement in the t(8;21) translocation. Expressed in CD34 cells and in the brain,104 ETO shares regions of homology with theDrosophila Nervy protein.103 It contains 2 putative C-terminal Zn-finger domains, but has not yet been shown to directly interact with DNA. Little is known about its normal function. It acts as a transforming oncogene when overexpressed in NIH3T3 cells.105 In tests of its ability to modulate transcription, ETO acts as a repressor, although it is not yet clear how this relates to the function of wild-type ETO.36
Recently, our group demonstrated that ETO’s ability to repress transcription is mediated through its interaction with N-CoR and recruitment of an N-CoR/Sin3/HDAC1 complex.36 These results have been confirmed by 2 other laboratories.37,38 Because the N-CoR interaction domain maps to the Zn-finger regions of ETO, a region that is retained in the AML1-ETO fusion, the same domain likely mediates AML1-ETO’s repressive effects. AML1-ETO mutants that lack this domain lose their ability to recruit N-CoR and lose their ability to repress transcription and inhibit differentiation.36 38The model that has developed is the following (Fig 3): AML1-ETO binds to AML1 consensus sequences in DNA. Unlike wild-type AML1, the fusion protein does not initiate p300/CBP-directed histone acetylation and transcriptional activation. Rather, the opposite occurs: through its ETO domain, AML1-ETO recruits N-CoR/Sin3/HDAC1. By permanently tethering this repressive complex to AML1-responsive promoters, AML1-ETO actively suppresses transcription by maintaining the histones in a deacetylated conformation and making DNA inaccessible to the transcriptional apparatus. Inhibition of the expression of AML1-responsive genes leads to a block in myeloid development and leukemic transformation of the maturing hematopoietic progenitors. One might predict that HDAC inhibitors could overcome the effects of AML1-ETO and lead to reversal of the leukemic phenotype; preliminary data suggests that this strategy of chromatin-remodeling therapy holds promise for the treatment of t(8;21) acute myeloid leukemia (see below).
MLL fusions: A SET of alterations.
The MLL locus is involved in a greater assortment of chromosomal rearrangements in leukemias than any other gene (see recent reviews by Downing and Look106 and Cimino et al107). Translocations or inversions of this gene on chromosome 11q23 are associated with a variety of FAB subtypes and some lymphoid malignancies as well (thus the name, MixedLineage Leukemia). Because of its involvement in the t(4;11) pediatric B-cell acute lymphoblastic leukemia (ALL),108MLL is also calledALL1. MLL rearrangement is found in both de novo leukemias and chemotherapy-associated (most often topoisomerase-inhibitor) secondary leukemias. All of the 11q23 leukemias are aggressive, respond poorly to chemotherapy, and have a poor prognosis. More than 40 translocation sites have been identified for MLL, and nearly 20 fusion partners have been cloned. Partial tandem duplication of MLL with an abnormalMLL/MLL fusion occurs in AML patients with the (+11) karyotype109 and is sometimes seen in leukemias with normal karyotype. Possibly relating to a fragile site in the MLLlocus, all of the rearrangements cluster within exons 5-11.110
The MLL gene111-114 is more than 100 kb long, encodes an 11.7-kb transcript with 23 exons,110 and gives rise to a protein of 3972 AA. Clues to the function of MLL have come from the identification of domains that share homology with other known proteins. The most significant match is seen in 4 domains that are conserved with the Drosophila protein Trithorax (TRX), giving rise to the alternative name for MLL as the human trithorax gene (HRX or HTRX).111-114 InDrosophila, TRX positively regulates homeotic gene expression,115-118 which in turn directs development of embryonic structures. In mammalian hematopoiesis, MLL is thought to regulate expression of homeobox (HOX) genes,119which serve a similar critical role as transcriptional activators of developmentally expressed gene families. It follows that dysregulation of HOX gene expression would have global consequences for hematopoietic cells and give rise to aggressive malignant transformation. As expected, murine mll −/− knockouts are embryonic lethal.119Mll +/− animals have numerous developmental skeletal abnormalities, as well as abnormal hematopoiesis.119
MLL has a series of N-terminal domains known as AT-hooks and a Zn-finger motif in its C-terminus.111-115 As with other AT-hook containing proteins, it is thought that the AT-hooks allow MLL to bind to the minor groove of DNA (Fig 4A). Although it has not been shown to recognize specific DNA sequence motifs, TRX does localize to discrete areas of polytene chromosomes inDrosophila.120 Several of these sites have been identified as regulatory regions of target genes, supporting the hypothesis that TRX and MLL associate physically with cis-acting elements of target genes to facilitate their expression during appropriate stages of development. Unlike TRX, MLL has an N-terminal region that shares homology with the regulatory region, but not the catalytic domain, of DNA methyltransferases112; the significance of this is not yet clear, although one could speculate that this region might participate in chromatin structure recognition. Perhaps key to their function, MLL and TRX share a highly conserved C-terminal 150 AA protein interaction domain, known as the SET domain.120 This region is also found in a number of other proteins that have transcriptional regulatory roles. Through its SET domain, MLL has been shown to bind INI1 and SNR1, 2 proteins that are homologous to members of the SWI/SNF complex.120 SWI/SNF complexes alter chromatin structure in an ATP-dependent fashion (see above). To date, coprecipitation experiments have failed to identify MLL or TRX as part of a stable SWI/SNF-like complex, indicating that the interaction may be a transient one.120
It is reasonable to speculate that MLL serves as a chromatin-binding protein that serves a regulatory function necessary for expression of target genes. Through its SET domain, MLL recruits an SWI/SNF-like ATPase-engine that changes the nucleosomal structure to maintain an open chromatin conformation. Supporting this hypothesis is the observation that initial transcription of downstream targets of MLL occurs normally in mll-null mice; however, in the absence of MLL, transcription of MLL-target genes cannot be maintained.119
It is difficult to propose a single model that would suggest a common mechanism for all of the MLL fusion proteins, including the partial-tandem duplication of MLL itself (Table3). In all of the MLL fusions (with the exception being the partial-tandem duplication), the N-terminal AT hooks and methyltransferase homology domains are retained, but the C-terminal Zn-finger and SET domains are lost.121 One simple model is that loss of the ability to recruit the SWI/SNF complex disrupts MLL function as a regulator of gene expression. In the tandem MLL duplication, in which the SET domain is preserved, one might postulate that the altered MLL conformation, and the abnormal distance of the SET domain from the N-terminal AT-hooks, might render the MLL protein nonfunctional.
MLL Fusion Proteins That May Affect Chromatin Remodeling
| Fusion . | Karyotype . | Potential Target Pathway . |
|---|---|---|
| All MLL-fusions | Any 11q23 abnormality | SWI/SNF |
| MLL-AF9 | t(9;11)(p22;q23) | SWI/SNF |
| MLL-ENL | t(11;19)(q23;p13.3) | SWI/SNF and/or HAT |
| MLL-CBP | t(11;16)(q23;p13) | HAT |
| MLL-p300 | t(11;23)(q23;q13) | HAT |
| Fusion . | Karyotype . | Potential Target Pathway . |
|---|---|---|
| All MLL-fusions | Any 11q23 abnormality | SWI/SNF |
| MLL-AF9 | t(9;11)(p22;q23) | SWI/SNF |
| MLL-ENL | t(11;19)(q23;p13.3) | SWI/SNF and/or HAT |
| MLL-CBP | t(11;16)(q23;p13) | HAT |
| MLL-p300 | t(11;23)(q23;q13) | HAT |
However, the picture for 11q23 rearrangements is likely not so simple. Two of its fusion partners,122 AF9 in t(9;11)(p22;q23) and ENL in t(11;19)(q23;p13.3), are homologous to proteins that are themselves associated with the SWI/SNF complex.123 Fusion with the DNA-binding AT-hook domain of MLL might result in permanent tethering of a SWI/SNF-like complex to MLL targets, resulting in constitutive activation of the SWI/SNF chromatin remodeling complex. Another speculative model is that the fusions disrupt MLL-target chromatin structure through recruitment of histone acetylase enzymes. For example, ENL contains a transcriptional activation domain that is retained in the MLL-ENL fusion. Through its activation motif, ENL might tether an HAT-containing coactivator complex to MLL target genes.
Switching HATs.
Two other MLL-fusions potentially act through a similar gain-of-function mechanism involving histone acetylase complexes: t(11;16)(q23;p13) and t(11;23)(q23; q13) fuse the upstream sequences ofMLL to CBP124-126 andp300,127 respectively. Most of the domains of CBP and p300 are retained, including those that bind other components of the coactivator complex, as well as the catalytic domain that encodes histone acetylase activity (Fig 4B). For these fusions it is likely that abnormal chromatin remodeling occurs either though constitutive activation of HAT activity or through recruitment of other acetyltransferase components of the coactivator complex.
Histone acetylases are also mutated in the t(8;16)(p11;p13)128 and inv(8)(p11;q13)48chromosomal rearrangements associated with M4 and M5 AML. In the first,CBP is fused to upstream elements of a gene calledMOZ.128 MOZ itself has predicted acetyltransferase activity, although its targets and biological function have not yet been determined. MOZ contains a variant Zn-finger but has not been shown to bind DNA. In the MOZ-CBP fusion the Zn-finger and catalytic domain of MOZ are fused to almost the entire CBP protein. The breakpoint in CBP is similar to that seen in the MLL-CBP fusion (see above). MOZ-CBP therefore has 2 HAT activities.
Similarly, the inv(8) rearrangement fuses the upstream sequences ofMOZ to TIF2.48 TIF2 is a homologue of p/CIP, a component of the CBP/p300 coactivator complex. The domain of p/CIP that mediates direct interaction with nuclear hormone receptors is lost in the MOZ-fusion, but the CBP-interaction domain is retained. TIF2 itself is predicted to have acetyltransferase activity, and, like MOZ-CBP, MOZ-TIF2 retains the acetyltransferase homology domains from each of the fusion partners.48 Like the MOZ-CBP rearrangement, such fusion proteins might result in altered chromatin remodeling of MOZ targets either through a dominant-negative effect, altered substrate specificity of the fusion enzyme, or recruitment of a HAT-containing coactivator complex to MOZ targets. Any of these mechanisms might impair normal chromatin remodeling, leading to aberrant transcription. It is worth noting that the MOZ-CBPt(8;16) and MOZ-TIF2 inv(8) leukemias have virtually identical FAB M5 phenotypes,48 128 suggesting that the 2 fusions have a similar final common pathway.
CHROMATIN THERAPY
Excitement in the field of chromatin structure has been generated with the realization that remodeling mechanisms might be targeted in therapeutic strategies. Preliminary studies, mostly in the in vitro setting, have focused on inhibition of histone deacetylase, in part because HDAC1 was one of the first enzymes identified in nucleosomal remodeling, because its function is best understood, and because it is the only candidate for which specific inhibitors have been identified.
Butyric acid, or butyrate, a physiologic byproduct of colonic bacterial fermentation, was the first identified of the HDAC inhibitors.129-131 It functions as a competitive inhibitor of HDAC, perhaps by mimicking the normal substrate (butyrate is a 4-carbon molecule, whereas acetyl groups are 2-carbon). In micromolar concentrations, butyrate is not specific for HDAC: it also inhibits phosphorylation and methylation of nuclear proteins as well as DNA methylation. Its analog phenylbutyrate132 133 acts in a similar manner.
More specific for HDAC than butyrate or its analogs are trichostatin A134,135 (TSA) and trapoxin136,137 (TPX). TSA, a product of Streptomyces hygropicus, was originally isolated as an antifungal agent. TPX, a cyclic tetrapeptide containing 2 L-phenylalanines, was identified in screens of fungal metabolites that induced morphological reversion of transformed NIH3T3 cells.138 TPX and TSA have emerged as potent inhibitors of the histone deacetylases. TSA reversibly inhibits, whereas TPX irreversibly binds to and inactivates the HDAC enzyme. TSA inhibits histone deacetylation with a Ki of 3.4 nmol/L,135 about 1,000-fold less than butyrate; TPX is even more potent. Unlike butyrate and its analogs, nonspecific inhibition of other enzyme systems has not yet been reported for TSA or TPX. To date, no data are available on the pharmacokinetics or pharmacodynamics of TSA or TPX. Besides TSA, TPX, and butyrate and its derivatives, a number of hybrid polar compounds139,140 have been found to potently inhibit HDAC enzymes, such as suberoylanilide hydroxamic acid and m-carboxycinnamic acid bishydroxamide. Tributyrin,141 a triglyceride with butyrate molecules esterified at the 1, 2, or 3 positions, holds potential as a long-acting, orally administered prodrug.
A major issue concerning the use of such HDAC inhibitors is the potential for modulating chromatin of genes that are not involved in the leukemia or genes in nonleukemic bystander cells. As discussed above, the translocations that cause HDAC to function in inappropriate ways do not truly alter the enzyme itself, but rather bring the normal enzyme into a chromatin environment that it would not otherwise contact. Would HDAC inhibitors have unwanted nonspecific effects that disrupt chromatin of all genes in a cell? Although the explanations are not yet apparent, experience in multiple tissue culture systems has suggested that the effects of HDAC inhibitors are limited. Butyrate, at doses that should inhibit HDAC enzymatic activity, does not kill cells.129 However, the nonspecific effects of butyrate on phosphorylation and methylation make it difficult to draw definite conclusions as to the contribution of HDAC inhibition to its biological effects. TSA has been used in a number of in vitro settings: it was first shown to induce differentiation of MEL cells,142without having significant apoptotic or transforming effects on the cells. TSA inhibition of HDAC has been shown to increase acetylation of only a subset of promoters,143 suggesting that a minority of genes are regulated through HDAC-dependent chromatin remodeling mechanisms. The observation of normal ordered differentiation in APL model systems with a variety of HDAC inhibitors85-88supports the notion that these agents do not have global effects on chromatin structure and gene expression.
HDAC inhibitors in leukemia.
We have already alluded to the relationship between RA responsiveness, the HDAC complex, and the RARα-fusion proteins in APL. APL has become the paradigm for the application of HDAC inhibitors. As described above, HDAC inhibitors synergize with retinoic acid to overcome the PML-RARα and PLZF-RARα induced maturation blockade in cell models.85-88 Pandolfi’s group87 has shown that, similar to patients with APL, PML-RARα transgenic mice responded to RA treatment, whereas PLZF-RARα transgenic mice developed RA-resistant leukemia. In vitro, neither RA nor TSA had significant effects on the PLZF-RARα blasts, but together they induced differentiation. The interpretation of this data is that RA alone failed to release the N-CoR/Sin3/HDAC1 complex from PLZF-RARα; that TSA acted downstream to inhibit the HDAC complex, but did not induce recruitment of a coactivator complex; and that only in the presence of both molecules could the suppressive effects of PLZF-RARα be overcome. These studies are consistent with other reports that suggest that HDAC inhibitors might have a role in APL therapy.85-88 The recent report of blast-cell differentiation and successful remission induction in a patient withPLZF-RARα treated with a combination of ATRA and granulocyte colony-stimulating factor (G-CSF)144 raises speculation as to whether G-CSF might alter HDAC activity.
Besides PLZF-RARα blasts, some PML-RARα–expressing APL cells do not respond to RA alone but may differentiate in response to the combination of RA and an HDAC inhibitor. Several patient samples have been identified to have mutations in the C-terminal region of PML-RARα, near the N-CoR binding domain.145 Warrell’s group at Memorial Sloan-Kettering has attempted to capitalize on such in vitro observations, reporting the use of the HDAC inhibitor phenylbutyrate in a PML-RARα patient who was in her third relapse.146 She had previously been induced with ATRA and chemotherapy, treated in first remission with ATRA and allogeneic bone marrow transplantation, and treated in second relapse with arsenic trioxide after failing reinduction with ATRA or standard chemotherapy. After 11 days on ATRA at 45 mg/m2/d, no change was observed in her bone marrow. At that time, phenylbutyrate was added to her regimen at a dose of 150 mg/kg/d. Immunofluorescence and Western blot analysis of blood and bone marrow mononuclear cells documented a time-dependent increase in histone acetylation (presumably as a result of antagonization of HDAC). Over the next 3 weeks, the doses of both ATRA and phenylbutyrate were increased to 90 mg/m2/d and 210 mg/kg, respectively. A bone marrow performed 2 weeks after the addition of the phenylbutyrate showed a decrease in the percentage of leukemic cells from 23% to 9%. A bone marrow 10 days later showed elimination of leukemic blasts. After a second course of therapy, she achieved a molecular remission (PML-RARα negative) and continued to be in remission after 6 months.
Aside from APL, HDAC inhibitors have a potential role in the treatment of AML1-ETO AML (see above). We and others have proposed that AML1-ETO’s leukemic potential is mediated by the recruitment of an HDAC-containing complex to AML1-responsive promoters.36-38In our own experiments,147 we found that TSA or phenylbutyrate was able to partially reverse transcriptional repression mediated by the ETO moiety of AML1-ETO. In vitro treatment of an AML1-ETO cell line (Kasumi-1) with clinically attainable levels of phenylbutyrate induced partial differentiation and apoptosis. Such results may herald the therapeutic application of HDAC inhibitors for t(8;21) AML in the future.
The clinical use of HDAC inhibitors need not be limited to patients with obvious abnormalities in histone deacetylation pathways. In 1983, continuous infusion of butyrate, at a dose of 500 mg/kg/d, was reported to induce a partial remission in a patient with myelomonocytic leukemia.148 No chromosomal abnormalities were noted in this case. It is possible that this patient did have an unrecognized rearrangement that would have aberrantly recruited a histone deacetylase to an abnormal target or that inhibition of HDAC may have compensated for an underactive HAT. Alternatively, the effects might not have been related to fusion gene-specific effects. For example, phenylbutyrate has been reported to upregulate the B7 costimulatory molecule on AML blasts,149 suggesting a role in immune surveillance.
These exciting results with HDAC inhibitors invigorate the search for other means of modulating chromatin remodeling. Despite the current dearth of specific inhibitors of HAT and SWI/SNF enzymes, the development of inhibitory peptides is a potential avenue that has yet to be pursued. Thanks to insights gained from studies of the molecular biology of leukemia, chromatin therapy may emerge as a potent antileukemia strategy of the future.
ACKNOWLEDGMENT
The authors thank Daniel E. Johnson, Margaret V. Ragni, and Richard A. Steinman for critical reading of the manuscript and Neal Young for encouragement and support.
Supported by National Institutes of Health Grant No. CA67346 and American Institute for Cancer Research Grant No. 98B039 to R.L.R.
REFERENCES
Author notes
Address reprint requests to Robert L. Redner, MD, E1058 Biomedical Science Tower, University of Pittsburgh Medical Center, 211 Lothrop St, Pittsburgh, PA 15213; e-mail: redner+@pitt.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal