Abstract
Interleukin-2 (IL-2) is a cytokine that induces the proliferation of certain IL-2 receptor expressing quiescent cells. Human IL-2 was fused to the amino-terminus of amphotropic murine leukemia virus (MLV) envelope glycoproteins. Retroviral vectors were pseudotyped with both the IL-2 chimeric envelope and the wild-type amphotropic MLV envelope. The chimeric IL-2 glycoproteins were incorporated on retroviral vectors and the IL-2–displaying vector particles could bind specifically to cell surface IL-2 receptors. In addition, the IL-2–displaying vectors could infect proliferating cells through amphotropic receptors irrespective of whether the cells expressed the IL-2 receptor. IL-2–displaying vector particles could also transiently stimulate the cell cycle entry and proliferation of several IL-2–dependent cell lines. Finally, retroviral vectors displaying IL-2 could efficiently transduce G0/G1-arrested cells expressing the IL-2 receptor at a 34-fold higher efficiency compared with vectors with unmodified envelopes. This new strategy, whereby C-type retroviral vector particles display a ligand that activates the cell cycle of the target cells at the time of virus entry, may represent an alternative to lentivirus-derived retroviral vectors for the infection of quiescent cells. In addition, upon infection of an heterogeneous population of nonproliferating cells, MLV-retroviral vectors that display cytokines/growth factors will allow the transgene of interest to be integrated specifically in quiescent cells expressing the corresponding cytokine/growth factor receptor.
GENE THERAPY PROTOCOLS will benefit from the design of vectors that permit the transfer of therapeutic genes directly to target cells and tissues in vivo. This strategy requires that the therapeutic transgene should be efficiently and accurately delivered and stably expressed in the target cells. To date, retroviral vectors derived from murine leukemia viruses have been used in the majority of gene transfer protocols,1 because, in addition to the simplicity of their manipulation, the transgene is stably integrated into the target cell DNA. However, these vectors suffer a number of drawbacks that need to be overcome before they can be of use for in situ gene transfer approaches. In particular, in a quantitative and specific manner, they have to be optimized such that they reach the target cells and are integrated in the genome of cells that are often quiescent.
Integration of murine retroviruses requires cell division, and in nonproliferating cells the block in the integration process occurs at the level of entry of the viral preintegration complex into the nucleus. This complex, containing the proviral DNA and an assembly of viral proteins necessary for its integration in the host DNA, can only enter the nucleus after the disruption of the nuclear membrane, ie, during mitosis.2 This characteristic is not common to all retroviruses. Indeed, lentiviruses are able to infect certain types of quiescent cells.3 Several characteristics specific to the lentivirus subfamily, including the expression of certain viral accessory proteins such as vpr, as well as distinct features harbored by their matrix and integrase proteins appear to play an important role in their ability to infect quiescent cells, particularly macrophages.4-8
Strategies to obtain retroviral vectors capable of infecting quiescent cells have therefore been based on the development of lentivirus-derived packaging cell lines and vectors or, alternatively, on the introduction of human immunodeficiency virus (HIV) determinants into murine leukemia virus (MLV) retroviral vectors to permit their integration in quiescent cells. Several groups have reported the generation of lentivirus-derived vectors that are capable of infecting quiescent cells,7 but there are still concerns regarding the safety of these vectors for human gene therapy. Furthermore, some experiments suggest that not all quiescent cells can be transduced by lentiviral vectors. For example, HIV cannot integrate in certain cells blocked at the Go phase of the cell cycle (such as T lymphocytes in vivo), and the cells must be in an active metabolic state to permit completion of reverse transcription and integration.3 9-11
An alternative strategy for the stable transduction of quiescent cells is to engineer growth factor domains onto the surface of the retroviral vector particles so that they can stimulate receptors on the target cell surface, inducing transient proliferation of the target cells at the time of gene delivery.
We describe here the construction of a novel chimeric MLV retroviral envelope glycoprotein containing the interleukin-2 (IL-2) polypeptide. IL-2 is a cytokine that induces the proliferation of certain IL-2 receptor-expressing quiescent cells. The IL-2 chimeric envelopes were coexpressed in retroviral vector particles with wild-type envelopes to allow infection to proceed through the natural retroviral receptor. We found that IL-2 receptor-expressing target cells were transiently activated when they were incubated with retroviral vectors displaying the IL-2 chimeric envelope glycoprotein. Moreover, the IL-2 receptor-positive target cells were transduced at a 34-fold higher efficiency by vectors pseudotyped with the IL-2 chimeric envelope than by vectors carrying wild-type amphotropic envelopes.
MATERIALS AND METHODS
Cell lines.
The TELCeB6 cell line12 was derived from the TELac2 line13 after transfection and clonal selection of TE671 cells (ATCC CRL8805; American Type Culture Collection, Rockville, MD) containing a plasmid expressing Moloney murine leukemia virus (MoMLV) gag and pol proteins. TELCeB6 cells produce noninfectious viral core particles carrying an nlsLacZ reporter retroviral vector, whereas TELac2 cells express only the nlsLacZ reporter retroviral vector. TELCeB6 were grown in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, France) supplemented with 10% fetal bovine serum.
The F7 cell clone (kind gift of T. Taniguchi), derived from the BAF-BO3 bone marrow murine pro-B–cell line,14expresses the three IL-2 receptor subunits (α, β, and γ) as well as bcl-2, an antiapoptotic protein.15 Proliferation of F7 cells is dependent on the addition of exogenous either IL-3 or IL-2 cytokines. F7 cells were grown in RPMI 1640 (Life Technologies) supplemented with 10% fetal bovine serum and 2 ng/mL of recombinant human IL-2 (R&D Systems, France).
The Bclp75 and W4E9 cells were derived from the IL-3–dependent BAF3 cell line, murine pro-B–cell line.16 They have been rendered responsive to IL-2. The Bclp75 cells express the human β IL-2 receptor subunit as well as Bcl-2. The W4E9 cells express high-affinity human IL-2 receptors (α and β IL-2 receptor subunits). These cells were grown in DMEM supplemented with 10% fetal bovine serum and 0.1% IL-3–conditioned medium.
The IL-2–dependent T-helper murine cell line HT-2 (kind gift of S. Zurawski) was grown in RPMI 1640 supplemented with 10% fetal bovine serum and 10−10 mol/L of murine IL-2 (mIL-2).17
Kit225 cells (kind gift of P. Lecine) are an IL-2–dependent T-cell line derived from a patient with chronic T-lymphocytic leukemia.18 They were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mmol/L of L-glutamine, and 20 ng/mL of IL-2.
J3-13 cells (kind gift of T. Taniguchi) are derived from the NIH3T3 murine fibroblasts and express the human IL-2 receptor as well as jak3 Janus kinase that renders them responsive to IL-2.19 J3-13 cells were grown in DMEM (Life Technologies) supplemented with 10% fetal bovine serum.
Plasmids and transfection.
The unmodified wild-type amphotropic 4070A MLV envelope20was encoded by the expression plasmid FBASALF that also expresses the phleo selectable marker conferring resistancy against phleomycin.12
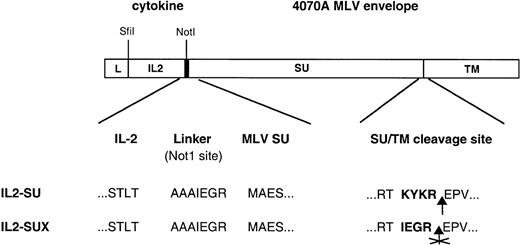
The human IL-2 cDNA (396 bp) was obtained by polymerase chain reaction (PCR) by using the following pair of primers: OUIL2SfiI, 5′-CAT AAT GGC CCA GCC GGC CAT GGC CGC ACC TAC TTC AAG TTC TAC; and OLIL2NotI, 5′-TGT CCA GCG GCC GCA GTT AGT GTT GAG ATG ATG C, which containSfi I and Not I sites at the 5′ and 3′ ends, respectively. The Sfi I/Not I PCR fragment, containing the IL-2 cDNA without the IL-2 signal peptide sequence and stop codon, was cloned in the +1 position of the 4070A MLV envelope glycoprotein in FBASALF (Fig 1) by using the EXA1 adapter plasmid21 that provides Sfi I andNot I sites at the 5′ end of the sequence corresponding to the surface subunit (SU) of the amphotropic envelope glycoprotein. To reduce steric hindrances between IL-2 and the receptor binding domain of the amphotropic envelope, a 7-amino acid linker (FX) was inserted between these two domains. The resulting plasmid FBIL2A1FXSALF codes for a chimeric envelope (IL2-SU).
Schematic representation of envelope expression constructs. The human IL-2 cDNA was fused to the 5′ end of the amphotropic MLV env gene at the first codon of the SU envelope subunit. A schematic diagram of the IL2-SU polypeptide encoded by this chimeric gene is shown. A 7-amino acid-long interdomain spacer was inserted between the cytokine and amphotropic receptor binding domain to reduce steric hindrances and optimize the display of IL-2. In the IL2-SUX chimeric envelope derived from IL2-SU, mutations were introduced in the SU/TM cleavage site to prevent cleavage of the envelope precursor and, thus, SU shedding. The amino acid sequence of the interdomain spacer between IL-2 and the SU protein as well as that of the SU/TM cleavage site are shown. L, leader-signal-peptide; IL2, IL-2; SU, surface envelope subunit; TM, transmembrane envelope subunit.
Schematic representation of envelope expression constructs. The human IL-2 cDNA was fused to the 5′ end of the amphotropic MLV env gene at the first codon of the SU envelope subunit. A schematic diagram of the IL2-SU polypeptide encoded by this chimeric gene is shown. A 7-amino acid-long interdomain spacer was inserted between the cytokine and amphotropic receptor binding domain to reduce steric hindrances and optimize the display of IL-2. In the IL2-SUX chimeric envelope derived from IL2-SU, mutations were introduced in the SU/TM cleavage site to prevent cleavage of the envelope precursor and, thus, SU shedding. The amino acid sequence of the interdomain spacer between IL-2 and the SU protein as well as that of the SU/TM cleavage site are shown. L, leader-signal-peptide; IL2, IL-2; SU, surface envelope subunit; TM, transmembrane envelope subunit.
Two PCR fragments were generated on the matrix FBASALF and cloned in the FBIL2A1FXSALF backbone digested with BamHI and ClaI to introduce a mutation between the SU and TM subunits of the envelope glycoprotein without changing the reading frame. This mutation results in the inactivation of the cleavage site located between the SU and TM subunits. The first PCR fragment (229 bp) was obtained with a 5′ oligonucleotide located upstream of the BamHI restriction site (UpBamHI, 5′-AGG CCT TAT GTA ACA CCA CC) and a 3′ oligonucleotide providing the point mutation and a PvuI restriction site (LowFX, 5′-TCC TTC GAT CGT ACG CTG TTC AAG CTG ACC). The second PCR fragment (554 bp) was generated with a 5′ oligonucleotide overlapping LowFX (UpFX, 5′-AAG TAC GAT CGA AGG AAG AGA GCC AGT ATC ATT GAC C) providing the mutation and a PvuI restriction site, and a 3′ oligonucleotide located downstream of the Cla I restriction site (LowClaI, 5′-AGC CTG GAC TAC TGA GAT CC). The first PCR fragment was digested withBamHI and Pvu I and the second with Pvu I andCla I, and they were cloned together intoBamHI-Cla I–digested FBIL2A1FXSALF plasmid, resulting in the plasmid FBIL2A1SUXSALF. The chimeric envelope encoded by FBIL2A1SUXSALF plasmid has been named IL2-SUX (Fig 1).
Chimeric envelope glycoproteins expression plasmids were either transfected or cotransfected with the expression plasmid FBASA (plasmid encoding the amphotropic 4070A-MLV envelope deprived of selection marker) by calcium phosphate precipitation into TELCeB6 cells, as previously described.22 Transfected cells were selected with phleomycin (50 μg/mL; CAYLA, Toulouse, France), and phleomycin-resistant clones were isolated and screened for the coexpression of both envelopes by Western blot analysis.
Antibodies.
Anti-gp70 (Quality Biotech Inc, Camden, NJ), a goat antiserum raised against the Rausher leukemia virus gp70, was diluted 1/2,000 for Western blots. Anti-CA (Quality Biotech Inc), a goat antiserum raised against the Rausher leukemia virus p30 capsid protein (CA), was diluted 1/10,000 for Western blots. 83A25, a rat monoclonal antibody against MLV SU, was used for binding assays.23 Dichlorotriazinyl amino fluorescein (DTAF)-conjugated affinity-purified F(ab)2 fragment goat antirat IgG (Immunotech, Marseille, France) was diluted 1/50 for binding assays
Immunoblots.
Binding assays.
Target cells (Kit225; 800,000 cells/point) were incubated for 1 hour at 4°C with 3 mL of nontransfected TELCeB6 cell supernatant and 3 mL of viral supernatants supplemented with 8 μg/mL of polybrene. After virus binding, cells were washed twice with PBA (phosphate-buffered saline [PBS] with 2% fetal bovine serum and 0.1% sodium azide) and incubated with 83A25 antibody23 supplemented with 0.1% sodium azide for 30 minutes at 4°C. Cells were washed with PBA and incubated for 30 minutes at 4°C with DTAF-conjugated affinity-purified F(ab)2 fragment goat antirat IgG. Five minutes before the two final washes in PBA and the final resuspension in PBS, cells were counterstained at 4°C with 20 μg/mL of propidium iodide. Fluorescence of living cells was analyzed with a fluorescence-activated cell sorter (FACSCalibur; Becton Dickinson, France). For the competition binding assay with human recombinant IL-2 (rIL-2; R&D Systems), target cells (Kit225) were preincubated for 30 minutes at 37°C with 100 ng of rIL-2 to provoke IL-2 receptor downregulation and then exposed to virus. Cells were then processed as previously described.
[3H]-Thymidine incorporation assay.
Twenty thousand to 40,000 IL-2–dependent cells per well (96-well plates) were cultivated overnight in medium without IL-2. The cells were then stimulated, in triplicate, for 24 hours with IL-2 (50 U; Boehringer Mannheim, Mannheim, Germany) or by adding 70 μL of filtered viral supernatant (confluent producer cells were cultivated overnight in DMEM-0% fetal bovine serum). Four hours before the end of the stimulation, the cells were pulsed with 1 μCi/mL of [3H]-Thymidine. The 96-well plates were passed in a cell collector to harvest cells on filters (cell collector TOMTEC MACH III; Wallac, EG&G Instruments, Finland), and incorporated radioactivity was measured in a β scintillation counter (LS 6000SC; Beckman Instruments, France).
Cell proliferation and quantification of cell viability.
Cell proliferation and cell viability were quantified by a colorimetric assay using the cell proliferation reagent WST-1 as per the manufacturer’s instructions (Boehringer Mannheim). This method is based on the cleavage of the tetrazolium salt WST-1 by mitochondrial dehydrogenases in viable cells. After cultivation of 5,000 F7 cells for 24 hours without IL-2 on 96-well plates, filtered viral supernatants (13 μL, produced from confluent producer cells during 8 hours in DMEM free of serum) or 10 U of recombinant human IL-2 were added. Cell proliferation was quantified several days later. Quantifications were performed after 4 hours of incubation with the cell proliferation reagent, WST-1. The absorbance was measured at 450 nm.
Cell cycle stimulation assays.
F7 cells were arrested in their cycle by IL-2 deprivation when they were at a confluency of 600,000 to 700,000 cells/mL. To remove IL-2, cells were washed once with PBS (Life Technologies) and seeded in 24-well plates at a density of 600,000 to 700,000 cells per well. Cells were deprived of IL-2 for 24 hours. During IL-2 deprivation, the cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum. J3-13 cells, seeded in 24-well plates at confluency, were arrested in their cycle by 48 hours of incubation in DMEM supplemented with 1% fetal bovine serum. F7 cells and J3-13 cells were stimulated for different time periods by adding 1 to 2 mL of filtered viral supernatants, equivalent quantities of ultracentrifuged supernatants, or 2 ng/mL of IL-2.
At the end of the stimulation period, the cells were centrifuged (F7 cells) or trypsinized and centrifuged (J3-13 cells) and then fixed in 70% ethanol-30% PBS. The cells were stored at 4°C until fluorescence-activated cell sorting (FACS) analysis. Just before FACS analysis, the fixed cells were pelleted and resuspended in 1 mL of PBS containing 50 μg of RNase and 10 μg/mL of propidium iodide. The cells were incubated for 30 minutes at 37°C and the cell cycle was then analyzed with an FACS (FACSCalibur; Becton Dickinson).
For the kinetics with F7 cells, after the different times of stimulation, the cells were cultured in medium deprived of IL-2 until a time period of 24 hours (the longest period of stimulation) and then fixed in 70% ethanol-30% PBS to be analyzed.
Infection assays.
The confluent virus producer cells were incubated for 3 days at 32°C. Overnight virus production was performed in a medium deprived of serum. Target cells were seeded in 24-well plates at a density of 5 × 104 cells per well for infection assays on proliferating cells. For assays on nonproliferative cells, cells were cultured in DMEM supplemented with 1% fetal bovine serum for 48 hours once they had reached confluency. Viral supernatant dilutions containing 5 μg/mL of polybrene were added and cells were incubated with viruses for 5 hours at 37°C. Viral supernatant was then removed and cells were incubated in complete medium (for proliferating cells) or in DMEM supplemented with 1% of fetal bovine serum (for nonproliferative cells) for 48 hours. X-Gal staining and viral titer determination were performed as previously described22 and expressed as LacZ infectious units (IU)/mL.
RESULTS
Construction of mutant envelopes.
Two IL-2–displaying envelope glycoproteins were generated. In the first envelope, termed IL2-SU, the human IL-2 was fused at the amino-terminus of the amphotropic MLV SU envelope subunit (Fig 1). This position of insertion was previously shown to allow the functional display of various polypeptides at the surface of virions.22 To optimize its functionality, the IL-2 moiety was separated from the SU by a small linker containing 7 amino acids (Fig 1), as previously described.24 25 In the second recombinant envelope glycoprotein, termed IL2-SUX, the cleavage site between the SU and TM envelope subunits of the IL2-SU chimeric envelope was inactivated by PCR mutagenesis (Fig 1). Thus, in this latter chimera, the IL-2–displaying chimeric SU subunit is covalently attached to the TM subunit of the envelope glycoprotein and is therefore not shed from the surface of the viral particles.
Expression and incorporation of envelopes into virions.
Expression vectors encoding the IL2-SU or IL2-SUX envelope chimeras or the control amphotropic MLV envelopes, A, were transfected into TELCeB6 cells that express MLV gag-pol core particles and an nlslacZ retroviral vector. In a separate set of transfections, plasmids encoding the wild-type amphotropic envelope were cotransfected with either the IL2-SU or IL2-SUX envelope expression vectors. The expression of a wild-type envelope glycoprotein was necessary because retroviruses expressing only IL2-SU or IL2-SUX demonstrated impaired infectivity, whereas retroviruses pseudotyped with a combination of amphotropic and chimeric envelopes were fully infectious.22
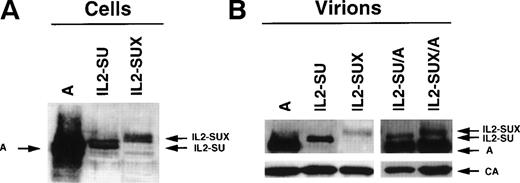
Lysates of transfected-TELCeB6 cells were analyzed for envelope expression using antibodies against MLV SU (Fig 2A). For IL2-SU envelopes, both a precursor and a processed SU product were detected, suggesting that the chimeras underwent normal envelope maturation and were correctly expressed. As expected, a band corresponding to the envelope precursor was detected for the IL2-SUX chimera but was not processed as a mature SU protein. A second band, which was more diffuse and exhibited a lower mobility, was detected and most likely corresponded to a fully glycosylated form of the IL2-SUX envelope glycoprotein. Expression of both chimeric envelopes was approximately 20-fold lower than that of wild-type amphotropic envelopes.
Expression of IL-2 chimeric envelopes. (A) Immunoblots of lysates of TELCeB6 cells expressing wild-type amphotropic envelopes (A), IL2-SU, and IL2-SUX chimeras. (B) Immunoblots of viral pellets obtained by ultracentrifugation of supernatants of TELCeB6 cells expressing wild-type amphotropic envelopes (A), IL2-SU, and IL2-SUX or coexpressing wild-type amphotropic envelopes and either IL2-SU (IL2-SU/A) or IL2-SUX (IL2-SUX/A) chimeras. All blots were stained with an MLV-SU antiserum. The immunoblot of viral pellets was separated at the position of 46-kD marker, and the lower portion of the membrane was stained with a p30-CA antiserum. The positions of the IL-2 chimeric envelope glycoproteins and the wild-type amphotropic SU are indicated.
Expression of IL-2 chimeric envelopes. (A) Immunoblots of lysates of TELCeB6 cells expressing wild-type amphotropic envelopes (A), IL2-SU, and IL2-SUX chimeras. (B) Immunoblots of viral pellets obtained by ultracentrifugation of supernatants of TELCeB6 cells expressing wild-type amphotropic envelopes (A), IL2-SU, and IL2-SUX or coexpressing wild-type amphotropic envelopes and either IL2-SU (IL2-SU/A) or IL2-SUX (IL2-SUX/A) chimeras. All blots were stained with an MLV-SU antiserum. The immunoblot of viral pellets was separated at the position of 46-kD marker, and the lower portion of the membrane was stained with a p30-CA antiserum. The positions of the IL-2 chimeric envelope glycoproteins and the wild-type amphotropic SU are indicated.
To demonstrate incorporation of the chimeric envelope glycoproteins into retroviral vector particles, supernatants from the different transfected-TELCeB6 cells were ultracentrifuged to pellet the vector particles. Pellets were then analyzed on immunoblots for the presence of capsid (p30-CA) and envelope proteins (Fig 2B). Envelope glycoproteins could be detected for both IL2-SU and IL2-SUX mutants, albeit at 20- to 50-fold reduced levels, respectively, compared with wild-type amphotropic envelopes, thus indicating a weaker incorporation of the chimeras into vector particles. As expected, no envelope glycoprotein was detected in pellets of IL2-SU– and IL2-SUX–transfected TElac2 cells that do not express gag and pol proteins (data not shown). Altogether, these results demonstrate that the envelope glycoproteins detected in the pellets of env-transfected TELCeB6 cells were stably associated with gag-pol viral particles. TELCeB6 cells coexpressing wild-type envelope and either of the two chimeric envelope glycoproteins (IL2-SU/A or IL2-SUX/A) exhibited a stronger incorporation of the former in the generated vector particles (Fig 2B), consistent with the weaker expression of the latter (Fig 2A).
IL-2 receptor binding of chimeric envelopes.
Kit225 human cells expressing the three subunits comprising the high-affinity IL-2 receptor were used for binding assays. The cells were incubated with vector-containing supernatants and binding of viral envelopes to the cell surface was analyzed by flow cytometry using antibodies against the MLV SU (Fig 3). To assess binding to the IL-2 receptor, binding assays were performed at 4°C for 1 hour. Under these temperature conditions, binding to the Pit-2 amphotropic receptor is very weak.26 As expected, no binding was detected with vectors carrying wild-type amphotropic envelopes (Fig 3). In contrast, vectors carrying the IL2-SU envelopes bound specifically to IL-2 receptor-positive human cells (Fig 3). Although clearly detectable, binding of vector particles generated with IL2-SUX envelopes was 5 times weaker than that of vectors carrying IL2-SU envelopes (Fig 3). This result is likely due to the weaker expression and incorporation of the former chimera as compared with the latter (Fig 2).
Envelope binding assays to IL-2 receptor-expressing target cells. Kit225 cells were used as an IL-2 receptor-expressing target cell. The background fluorescence was determined by incubating cells with supernatant from nontransfected TELCeB6 packaging cells (white area). Binding assays were performed with viruses pseudotyped with the wild-type amphotropic envelope, A, the IL2-SU, or the IL2-SUX chimeric envelopes. Target cells were either treated (broken line) with recombinant IL-2 (100 ng for 30 minutes at 37°C) or not treated (black area) before binding assays with the various virions.
Envelope binding assays to IL-2 receptor-expressing target cells. Kit225 cells were used as an IL-2 receptor-expressing target cell. The background fluorescence was determined by incubating cells with supernatant from nontransfected TELCeB6 packaging cells (white area). Binding assays were performed with viruses pseudotyped with the wild-type amphotropic envelope, A, the IL2-SU, or the IL2-SUX chimeric envelopes. Target cells were either treated (broken line) with recombinant IL-2 (100 ng for 30 minutes at 37°C) or not treated (black area) before binding assays with the various virions.
To demonstrate the specificity of IL2-SU and IL2-SUX binding to cells, IL-2 receptors on Kit225 cells were blocked by preincubation with recombinant soluble IL-2 (rIL-2). Although this treatment did not affect the binding of control envelope chimeras targeted to a receptor other than the IL-2 receptor (data not shown), there was a consistent decrease in the binding of vectors carrying either IL2-SU or IL2-SUX envelopes (Fig 3). These data indicated that IL-2 was correctly displayed on retroviruses and could specifically retarget the binding of vector particles to cells expressing the IL-2 receptor.
Cell cycle activation of IL-2–dependent cells by IL-2–expressing retroviral envelopes.
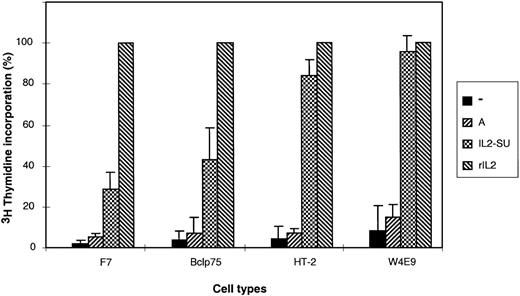
To determine whether retroviruses carrying the IL-2–fusion envelopes could stimulate cell cycle progression, DNA synthesis was measured by3H-thymidine incorporation assays in several G0/G1-arrested IL-2–dependent cell types (Fig 4). Cells incubated with media containing either nonenveloped retroviruses or retroviruses with unmodified amphotropic MLV envelopes did not demonstrate an increase of 3H-thymidine incorporation as compared with cells incubated with unconditioned medium. In contrast, incubation of G0/G1 arrested cells in media containing vectors pseudotyped with IL2-SU chimeric envelopes resulted in a fivefold to 12-fold increase in 3H-thymidine incorporation (Fig 4), indicating that binding of these latter vectors to the IL-2 receptor induced the IL-2 signaling cascade resulting in DNA synthesis. The level of response to IL-2 of the different cell types is related to the number of IL-2 receptors expressed by these cells and to the affinity of these receptors for IL-2.
Induction of DNA synthesis in cells incubated with IL-2–displaying retroviruses. 3H-thymidine incorporation was measured in four different IL-2–dependent cell types: F7, Bclp75, HT-2, and W4E9 cells. Cells, which were arrested in Go/G1 by overnight deprivation of IL-2, were either incubated in 96-well plates for 24 hours in media conditioned with 50 U of recombinant IL-2 (rIL-2), 70 μL of viral supernatant containing nonenveloped retroviruses (−), 70 μL of viral supernatant containing retroviruses coated with amphotropic envelopes (A), or 70 μL of viral supernatant containing retroviruses coated with IL-2 chimeric envelopes (IL2-SU). The levels of 3H-thymidine incorporation are expressed as percentages relative to 3H-thymidine incorporation in rIL-2–stimulated cells. Experiments were performed in triplicate, and the means ± standard deviations are shown.
Induction of DNA synthesis in cells incubated with IL-2–displaying retroviruses. 3H-thymidine incorporation was measured in four different IL-2–dependent cell types: F7, Bclp75, HT-2, and W4E9 cells. Cells, which were arrested in Go/G1 by overnight deprivation of IL-2, were either incubated in 96-well plates for 24 hours in media conditioned with 50 U of recombinant IL-2 (rIL-2), 70 μL of viral supernatant containing nonenveloped retroviruses (−), 70 μL of viral supernatant containing retroviruses coated with amphotropic envelopes (A), or 70 μL of viral supernatant containing retroviruses coated with IL-2 chimeric envelopes (IL2-SU). The levels of 3H-thymidine incorporation are expressed as percentages relative to 3H-thymidine incorporation in rIL-2–stimulated cells. Experiments were performed in triplicate, and the means ± standard deviations are shown.
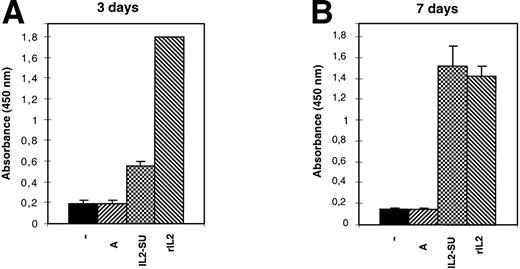
Because 3H-thymidine incorporation is a measure of DNA synthesis but does not provide any information regarding the capacity of the cells to undergo a productive division, we also assessed cell proliferation using the WST-1 reagent. The F7 IL-2–dependent cell line was arrested in GO/G1 and cultured in media containing vector particles carrying wild-type MLV envelopes or IL2-SU chimeric envelopes, and cell proliferation was then quantified 3 and 7 days later. The results shown in Fig 5 indicate that, whereas media containing retroviruses devoid of envelope glycoproteins or carrying wild-type MLV envelopes could not sustain cell multiplication, the IL-2–dependent cells replicated in media containing retroviruses with IL-2 fusion envelopes in a manner similar to that observed in rIL-2–conditioned media (Fig 5). Similarly, viral particles carrying the IL2-SUX chimera alone or viral particles coexpressing wild-type amphotropic envelopes with either IL2-SU or IL2-SUX chimeras could stimulate the cell cycle progression and division of IL-2–responsive target cells (data not shown). Collectively, these data therefore indicated that IL-2–displaying retroviral envelope glycoproteins were fully functional with respect to IL-2 receptor stimulation and activation of the cell cycle leading to DNA synthesis, mitosis, and cytokinesis.
Proliferation of IL-2–dependent cells induced by IL-2–displaying retroviruses. F7 cells were arrested in Go/G1 by overnight deprivation of IL-2. Cell proliferation was then quantified 3 or 7 days later after culture in media conditioned with either 10 U of recombinant IL-2 (rIL-2), 13 μL of viral supernatants containing nonenveloped retroviruses (−), 13 μL of viral supernatants containing retroviruses coated with amphotropic envelopes (A), or 13 μL of viral supernatants containing retroviruses coated with IL-2 chimeric envelopes (IL2-SU). The values shown are the means ± SD of four separate experiments.
Proliferation of IL-2–dependent cells induced by IL-2–displaying retroviruses. F7 cells were arrested in Go/G1 by overnight deprivation of IL-2. Cell proliferation was then quantified 3 or 7 days later after culture in media conditioned with either 10 U of recombinant IL-2 (rIL-2), 13 μL of viral supernatants containing nonenveloped retroviruses (−), 13 μL of viral supernatants containing retroviruses coated with amphotropic envelopes (A), or 13 μL of viral supernatants containing retroviruses coated with IL-2 chimeric envelopes (IL2-SU). The values shown are the means ± SD of four separate experiments.
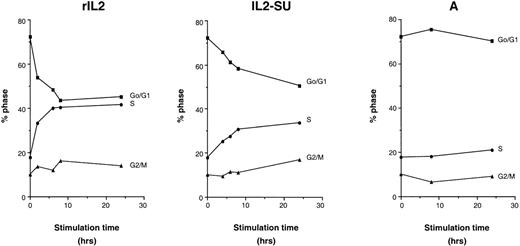
To determine the minimal period of exposure to IL-2–displaying retroviruses required to stimulate the cell cycle, F7 cells were arrested in G0/G1 by overnight IL-2 starvation. At that time, G0/G1-arrested cells were incubated for different time periods with serum-free media containing rIL-2 or, alternatively, with retroviruses harboring wild-type or IL2-SU fusion envelopes. After the stimulation period, cells were washed and reincubated in media without IL-2 until T24 hours, at which time the proportion of cells in the different cell cycle stages, G0/G1, S, and G2/M, was measured by FACS analysis (Fig 6). In contrast to media containing viral particles generated with wild-type MLV envelopes, media containing rIL-2 or containing retroviruses with IL2-SU chimeric envelopes stimulated cell cycle progression of G0/G1-arrested cells (Fig 6). This was demonstrated by the diminution of the percentage of cells in G0/G1 and the concomitant increase in the proportion of cells in the S phase. In addition, these results indicate that only a brief exposure of cells to IL2-SU carrying retroviruses was necessary to stimulate cell division. Indeed, after 6 hours of exposure to IL-2–displaying viruses, the proportion of cells in the G0/G1 phase decreased by about 10%, resulting in an increased number of cells in the S phase (Fig 6).
Stimulation of cell cycle progression in Go/G1-arrested cells by IL-2–displaying retroviruses. F7 cells, arrested in Go/G1 by an overnight IL-2 deprivation, were stimulated for different time periods with 2 ng/mL of recombinant IL-2 (rIL-2), viral supernatants containing retroviruses coated with amphotropic envelopes (A), or viral supernatants containing retroviruses coated with IL-2 chimeric envelopes (IL2-SU). The percentage of cells in the different phases of the cell cycle (Go/G1; S; G2/M) was measured by FACS analysis after propidium iodide staining. At the onset of stimulation, 72.33% of F7 cells were in the Go/G1 phase. Experiments were performed in duplicate and the means are shown.
Stimulation of cell cycle progression in Go/G1-arrested cells by IL-2–displaying retroviruses. F7 cells, arrested in Go/G1 by an overnight IL-2 deprivation, were stimulated for different time periods with 2 ng/mL of recombinant IL-2 (rIL-2), viral supernatants containing retroviruses coated with amphotropic envelopes (A), or viral supernatants containing retroviruses coated with IL-2 chimeric envelopes (IL2-SU). The percentage of cells in the different phases of the cell cycle (Go/G1; S; G2/M) was measured by FACS analysis after propidium iodide staining. At the onset of stimulation, 72.33% of F7 cells were in the Go/G1 phase. Experiments were performed in duplicate and the means are shown.
Retroviral envelope glycoproteins are not very stable structures, because the SU subunits of MLV are not covalently attached to the membrane-bound TM subunit and are easily shed from viral particles or from the surface of the vector producer cells.27 Thus, it is probable that the viral supernatants generated with IL2-SU chimeric envelopes are contaminated by soluble, non–virion-associated, IL2-SU fusion envelopes. This evidence was supported by the finding that supernatants of cells producing vector particles with IL2-SU envelopes could efficiently stimulate GO/G1-arrested target cells, regardless of whether the viral particles were removed from the supernatant by ultracentrifugation (Fig 7). Thus, it is likely that soluble-shed IL2-SU accounted for a significant part of the cell activation measured in our experiments. In addition, the activation observed upon incubation with nonultracentrifugated viral supernatants bearing IL2-SUX envelopes, in which cleavage of the envelope precursor was prevented, was significantly less than that observed with the IL2-SU envelopes (data not shown). This was probably due to the inability of the IL2-SUX envelope to accumulate in the supernatant as soluble material and to its less efficient incorporation on viral particles. Indeed, no activation of cell cycle was observed upon culture of cells in the presence of supernatant in which IL2-SUX/A vector particles were removed by ultracentrifugation (data not shown).
Cell cycle activation by virion-shed and virion-associated IL2-SU chimeras. Go/G1-arrested J3-13 cells were incubated for 24 hours in media conditioned with either rIL-2 (recombinant IL-2; bottom right panel) or with viral supernatants containing retroviruses coincorporating IL2-SU and wild-type amphotropic envelope glycoproteins (top left panel). Viral particles were removed by ultracentrifugation before the addition of supernatants to cultures of Go/G1 arrested J3-13 cells (top right panel). Cells were stained with propidium iodide to assess the DNA content of the treated cells. Before stimulation, 80% of J3-13 cells were in the Go/G1 phase of the cell cycle (bottom left panel).
Cell cycle activation by virion-shed and virion-associated IL2-SU chimeras. Go/G1-arrested J3-13 cells were incubated for 24 hours in media conditioned with either rIL-2 (recombinant IL-2; bottom right panel) or with viral supernatants containing retroviruses coincorporating IL2-SU and wild-type amphotropic envelope glycoproteins (top left panel). Viral particles were removed by ultracentrifugation before the addition of supernatants to cultures of Go/G1 arrested J3-13 cells (top right panel). Cells were stained with propidium iodide to assess the DNA content of the treated cells. Before stimulation, 80% of J3-13 cells were in the Go/G1 phase of the cell cycle (bottom left panel).
IL-2–displaying vector particles efficiently infect GO/G1 arrested cells.
Vectors generated with wild-type amphotropic, IL2-SU, or IL2-SUX envelopes were used to infect IL-2–responsive J3-13 cells. These cells were chosen because they are adherent and are easily infected by wild-type amphotropic retroviruses. As expected, vectors bearing IL2-SUX envelopes were not infectious because of the lack of cleavage between their SU and TM subunits that is necessary for fusion activation.27 In contrast, vectors bearing IL2-SU envelopes were infectious, and although titers reached 103 IU/mL, this titer was more than 4 orders of magnitude lower than that obtained with viruses carrying wild-type amphotropic envelopes (data not shown). As previously shown for other chimeric envelopes, the reduced capacity of IL2-SU envelopes to mediate infection via PiT-2 amphotropic receptors was either due to a steric hindrance of envelope binding/fusion by the displayed polypeptide domain24,25and/or to the sequestration of retroviruses displaying a growth factor/cytokine upon its interaction with the corresponding tyrosine kinase receptor.22 28 To rescue or enhance the infectivity of IL-2–displaying envelope glycoproteins, retroviruses were pseudotyped with both amphotropic envelopes and either of the two IL-2–fusion envelopes. The infectivity of the two resulting retroviruses, IL2-SU/A and IL2-SUX/A, was greater than 106IU/mL on J3-13 proliferating cells, which was only 1 log lower than that of amphotropic-pseudotyped retroviruses (data not shown).
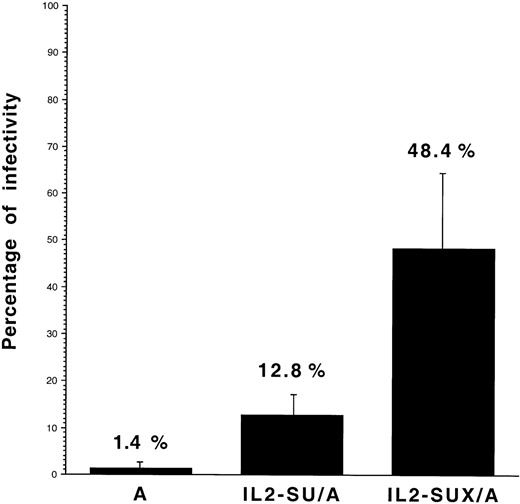
Infectivity of the IL2-SU/A and IL2-SUX/A viruses was then compared with that of control retroviruses carrying wild-type amphotropic envelopes on J3-13 cells that were GO/G1-arrested by serum starvation. A low residual infectivity was detected on the latter cells with the control amphotropic viruses (1.4% ± 1% [n = 8] of the infection obtained on proliferating J3-13 cells) and was most likely due to a small number of J3-13 cells that were not arrested in G0/G1 (Fig 8). In contrast, the relative ability of IL2-SU/A and IL2-SUX/A viruses to infect nonproliferating J3-13 cells was significantly higher than that of control amphotropic retroviruses and reached 12.8% ± 4.2% (n = 8) and 48.4% ± 16.3% (n = 8), respectively (Fig 8). It is likely that the increased ability of IL2-SU/A retroviruses to infect the nonproliferating J3-13 cells was due to cell activation mediated by both soluble-shed IL2-SU and virion-associated IL2-SU envelopes. In contrast, because soluble-shed IL2-SUX envelopes were not found in viral supernatants, our data indicate that viruses carrying IL2-SUX/A envelope glycoproteins activate the cells into which they enter, thus favoring their integration after mitosis.
Infectivity of retroviruses carrying IL-2–fusion envelopes. Retroviral infections were performed on proliferating and Go/G1-arrested J3-13 cells. The latter cells were obtained by 48 hours of incubation in medium containing 1% serum, which resulted in 80% of cells being arrested in the Go/G1 cell cycle phase, as shown by propidium iodide staining. Proliferating or quiescent (Go/G1-arrested) J3-13 cells were infected with lacZ retroviral vectors carrying the indicated envelope glycoproteins. The retroviruses, which were harvested in serum-free medium, were diluted in medium containg 1% fetal calf serum and deposited on the cells. After 5 hours of infection, virus-containing media were removed and replaced by fresh media supplemented with 1% fetal calf serum only. Cells were then cultured for 48 hours before X-gal staining. Results are expressed as the percentage (mean ± SD; n = 8) of titers on quiescent cells relative to titers on proliferating J3-13 cells.
Infectivity of retroviruses carrying IL-2–fusion envelopes. Retroviral infections were performed on proliferating and Go/G1-arrested J3-13 cells. The latter cells were obtained by 48 hours of incubation in medium containing 1% serum, which resulted in 80% of cells being arrested in the Go/G1 cell cycle phase, as shown by propidium iodide staining. Proliferating or quiescent (Go/G1-arrested) J3-13 cells were infected with lacZ retroviral vectors carrying the indicated envelope glycoproteins. The retroviruses, which were harvested in serum-free medium, were diluted in medium containg 1% fetal calf serum and deposited on the cells. After 5 hours of infection, virus-containing media were removed and replaced by fresh media supplemented with 1% fetal calf serum only. Cells were then cultured for 48 hours before X-gal staining. Results are expressed as the percentage (mean ± SD; n = 8) of titers on quiescent cells relative to titers on proliferating J3-13 cells.
DISCUSSION
We report here a novel strategy whereby retroviral particles displaying a polypeptide growth factor can transiently activate cell division at the time of virus entry, allowing integration of a transgene in quiescent cells. This strategy is based on the development of retroviral vectors that bear bifunctional chimeric envelope glycoproteins. Such recombinant envelopes are able to stimulate the proliferation of resting cells via the activation of a specific cytokine or growth factor receptor at the cell surface. This stimulation results in a mitosis that allows the penetration of the retroviral capsid and viral genome into the nucleus with subsequent integration of the transgene into the host cell DNA. Because the cytokine/growth factor harbored by the bifunctional retroviral envelopes is not encoded by the genome of the retroviral vector itself, the activation is only transient. Therefore, the stimulation of the target cells is not expected to result in more than one or two cell divisions and the activated cells should return to their initial nonproliferative state after having integrated the transgene.
Our results provide a proof of concept for the approach that may have utility in various gene transfer applications. In view of the complexity of T-cell activation pathways, we anticipate that the display of IL-2 on the vector particles may not be sufficient to give highly efficient transduction of primary T cells. For example, primary CD4+ T cells, which exhibit increased responsiveness to IL-2 only after preactivation with other stimuli, such as phytohemagglutinin (PHA) or multivalent anti-CD3 and anti-CD28, may not be optimal.10,29 However, we anticipate that it will be easy to generate bifunctional chimeric envelope glycoproteins able to activate various cell surface molecules involved in transduction of a mitogenic signal. Indeed, over the last few years, we have extensively characterized a great number of chimeric envelope glycoproteins generated by amino-terminal extensions of MLV envelopes with various polypeptides,30 such as growth factors,22,28 single-chain antibodies,24,31-33or other ligands.22,25,34 Viral particles carrying these recombinant envelopes could efficiently and easily retarget virion binding and, in some cases, were able to redirect infection, although at low levels.30 Moreover, preliminary results from our laboratory indicate that virions displaying epidermal growth factor (EGF) or stem cell factor (SCF) are also able to activate cells expressing EGF or c-kit receptors, respectively (F.-L.C. and S.J.R., unpublished results). Therefore, we believe that recombinant retroviruses displaying polypeptide growth factors and other cell signaling polypeptides hold promise for several in vivo gene transfer situations in which the target cells are quiescent. For example, airway epithelial cells are important targets for diseases such as cystic fibrosis. However, gene transfer in such primary cells using MLV-derived retroviral vectors can only be achieved if the cells are induced to divide by in vivo or in vitro prestimulation with keratinocyte growth factor.35,36 The display of KGF, a single chain 162 aa-long polypeptide,37 at the surface of retroviral particles through molecular engineering of the viral envelope glycoprotein might allow the generation of MLV-derived retroviral vectors that can activate these airway cells, resulting in efficient integration of the therapeutic transgene.
Several aspects of the current study point to the need for improvements to optimize the strategy for clinical gene transfer applications. Although the incorporation of IL-2–chimeric envelopes on viral particles was reasonably efficient, we found that a substantial part of the IL-2–dependent cell cycle activation was not mediated by IL-2–displaying vector particles, but by soluble IL-2 chimeric SU envelope subunits shed from vector particles or from the packaging cells. Indeed, the association between the SU and the TM retroviral envelope subunits is known to be weak,27resulting in the accumulation of free SU in vector supernatants. To overcome this problem, we designed the IL2-SUX recombinant envelope glycoproteins in which the cleavage site between the SU and the TM subunits was inactivated, preventing shedding of the IL-2–displaying SU subunit. Thus, in the IL2-SUX envelope, the displayed IL-2 polypeptide was covalently attached to the envelope complex and was tightly anchored to the viral particles by the transmembrane anchoring domain located at the carboxy-terminus of the envelope glycoprotein. Despite a lower incorporation of IL2-SUX compared with IL2-SU on viral particles, gene transfer to growth arrested cells was significantly increased upon infection with viral particles pseudotyped with the former envelope. One possible explanation for this phenomenon is that, because the IL2-SUX envelope is always associated with virions, cell activation through interaction with the IL-2 receptor occurred concurrently with viral entry. In contrast, upon infection of cells with virions pseudotyped with IL2-SU, activation of cells with soluble-shed IL2-SU subunits was not necessarily associated with viral entry. Our results suggest that the backbone of the IL2-SUX vector will be superior for the display of other cytokines/growth factors.
Although for some quiescent cells activation of the cell cycle can be achieved using a single cytokine or growth factor, the proliferation of most other resting cell types requires stimulation with two or more cytokines. For example, most gene transfer protocols involving hematopoietic progenitors use a combination of SCF, IL-3, and IL-6 to induce cell proliferation and allow subsequent integration of MLV-derived retroviral vectors.38 Similarly, optimal activation and proliferation of T lymphocytes is obtained by stimulation with anti-CD3 and anti-CD28 antibodies in combination with IL-2.10 Therefore, gene delivery to such cells using the strategy described in this report will require MLV-derived retroviral vectors that display, in addition to wild-type envelope glycoproteins, at least two chimeric envelopes able to activate different cell surface receptors.
In contrast to MLVs, lentiviruses such as HIV are able to infect nonproliferating cells such as macrophages and resting CD4+T cells. However, particularly for primary T lymphocytes, completion of reverse transcription and subsequent integration of the HIV genome does not occur unless the cells have been activated, and it is quite probable that the gp120 envelope glycoproteins of HIV can activate certain target cell types through their interaction with CD4 or CXCR4.39 Morevover, the integration of the HIV genome in resting cells, such as macrophages, requires several accessory proteins, such as vpr, which are specifically encoded by the lentivirus genome.40 Thus, although retroviral vectors derived from HIV seem to exhibit the same biological properties as wild-type HIV, allowing integration of the transgene in the nonproliferating target cells,5 41-43 some particular quiescent cell types may not be transduced by such vectors (D. Trono, personal communication, November 1998). Additionally, because the expression of accessory lentivirus proteins in HIV-derived vectors is best avoided for biosafety, retroviral vectors derived from either HIV or MLV that incorporate stimulating envelope chimeras may represent an interesting alternative for the design of safe gene delivery vectors for in vivo applications.
Finally, because of their inability to integrate in the genome in nonproliferating cells, MLV-derived vectors displaying polypeptide growth factors will facilitate selective gene transfer to cells that express the corresponding cytokine receptor. Indeed, although they penetrate most resting cell types (depending on the presence of the corresponding retroviral receptor), such MLV vectors will only integrate in cells in which they have concomitantly and specifically activated the cell cycle. Therefore, this strategy may also allow the delivery of a transgene in a tissue-specific manner.30
ACKNOWLEDGMENT
The authors are grateful to Jacqueline Marvel and Naomi Taylor for stimulating discussions and for critical reading of the manuscript. We are also grateful to T. Taniguchi, S. Zarawski, and P. Lecine for kindly providing the IL-2 receptor expressing cell lines.
Supported by Agence Nationale pour la Recherche contre le SIDA (ANRS), Association pour la Recherche contre le Cancer (ARC), Association Française de Lutte contre la Mucoviscidose (AFLM), Centre National de la Recherche Scientifique (CNRS), Ministére de l’Enseignement Supérieur et de la Recherche (ACC-SV2), and Institut National de la Santé Et de la Recherche Médicale (INSERM). S.J.R. and F.J.B. were supported by the Medical Research Council. M.M. was supported by a fellowship from the Association Française contre les Myopathies.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to François-Loı̈c Cosset, PhD, U412, ENS de Lyon, 46 allée d’Italie, 69364 Lyon Cedex 07, France; e-mail: Francois-Loic.Cosset@ens-lyon.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal