Abstract
We have previously shown that the cAMP signaling pathway controls major aspects of embryonic red blood cell (RBC) function in avian embryos (Glombitza et al, Am J Physiol 271:R973, 1996; and Dragon et al, Am J Physiol 271:R982, 1996) that are important for adaptation of the RBC gas transport properties to the progressive hypercapnia and hypoxia of later stages of avian embryonic development. Data about the ontogeny of receptor-mediated cAMP signaling are lacking. We have analyzed the response of primitive and definitive chick embryo RBC harvested from day 3 to 18 of development towards forskolin, β-adrenergic, and A2 receptor agonists. The results show a strong response of immature definitive and primitive RBC to adenosine A2 and β-adrenergic receptor agonists, which is drastically reduced in the last stage of development, coincident with the appearance of mature, transcriptionally inactive RBC. Modulation of cGMP-inhibited phosphodiesterase 3 (PDE3) has a controlling influence on cAMP accumulation in definitive RBC. Under physiological conditions, PDE3 is inhibited due to activation of soluble guanylyl cyclase (sGC). Inhibition of sGC with the specific inhibitor ODQ decreases receptor-mediated stimulation of cAMP production; this effect is reversed by the PDE3 inhibitor milrinone. sGC is acitivated by nitric oxide (NO), but we found no evidence for production of NO by erythrocyte NO-synthase. However, embryonic hemoglobin releases NO in an oxygen-linked manner that may activate guanylyl cyclase.
HORMONAL SIGNALS THAT control the function of circulating embryonic erythrocytes have been scarcely investigated.1 2 In contrast to adult erythropoiesis of higher vertebrates, in which erythrocytes are released into the circulation as transcriptionally inactive or anucleate red blood cell (RBC) with very limited metabolic capacity, embryonic RBC initially enter the circulation as immature precursor cells with ongoing translational and transcriptional activity. This may enable embryonic RBC to respond to changes of developmental conditions with a broader repertoire for adaptative measures than is possible in the adult.
We have previously shown that, in chick embryos, activation of the cAMP signaling pathway via A2a or β-adrenergic receptors controls major aspects of embryonic RBC function of older chick embryos,1-3 including oxygen affinity and carbonic anhydrase II synthesis. These cAMP-induced changes allow rapid adaptation of RBC gas transport properties to the progressive hypercapnia and hypoxia characteristic for late embryonic development.4 Hypoxia causes release of norepinephrine (NE) into the blood of chick embryos, which then activates the β-adrenergic receptors.2 A second physiological stimulus linked to hypoxia is adenosine.1 Additional experimental evidence suggests that cAMP signaling is also involved in the regulation of cellular pyrimidine nucleotide metabolism during terminal erythroid differentiation, because the key enzyme pyrimidine 5′-nucleotidase is induced by cAMP.3
Basic data about the ontogeny of receptor-mediated cAMP signaling of embryonic RBC are lacking. We have analyzed the response of embryonic erythrocytes from 3- to 18-day-old chick embryos towards stimulation with forskolin as a direct activator of adenylyl cyclase and adenosine A2 and β-adrenergic receptor agonists. During this period, the composition of the circulating erythroid population changes from primitive basophilic erythroblast (day 3) to mature definitive RBC (day 17/18) and covers nearly all stages of late erythroid differentiation. Definitive RBC enter the circulation from day 6 onwards, initially as immature polychromatic erythroblasts with substantial translational and transcriptional activity. Mature definitive RBC appear at the end of the second week of incubation, coincident with this, one observes a progressive restriction of transcriptional and translational activity, which is completed by day 17/18.5 A second aim of the present study was to assess the role of cAMP degradation by phosphodiesterase on receptor-mediated cAMP signaling; basic information on this aspect of cAMP metabolism in immature erythroid cells is also lacking. In several systems, the cAMP response is modulated extensively by the activity of regulated phosphodiesterases such as the cGMP-inhibited phosphodiesterase 3 (PDE3).6Thus, in mammalian blood platelets, the activity of cGMP-inhibited PDE3 has a major influence on intracellular cAMP levels.7 We therefore tested if alterations of guanylyl cyclase activity and PDE3 activity influence embryonic RBC cAMP levels.
The results of the present study show a strong response of immature definitive and primitive RBC to β-adrenergic and adenosine receptor agonists that is lost in the transition from transcriptionally active reticulocyte to mature nucleate erythrocyte. Furthermore, we can show that modulation of cGMP-inhibited PDE3 has a controling influence on RBC cAMP concentration of definitive embryonic RBC. Under physiological conditions, PDE3 is inhibited due to activation of soluble NO-dependent guanylyl cyclase. Experimental data suggest that NO is apparently released from embryonic hemoglobin (Hb) and perhaps other intracellular NO donors, such as S-nitrosoglutathione.
MATERIALS AND METHODS
Fertilized eggs from white leghorn chicken were incubated for 3 to 18 days in a commercial forced draft incubator with automatic rotation at 37.5°C and 60% relative humidity. Blood was taken from the embryos by cutting an extraembryonic blood vessel and aspirating the effluent with a pasteur pipette. Blood was collected into ice-cold buffer of the following composition: 120 mmol/L NaCl, 4 mmol/L KCl, 5 mmol/L glucose, 1.5 mmol/L CaCl2, 50 mmol/L Tris, pH 8.0. After day 10, heparin was added as anticoagulant. For cAMP measurements, RBC were suspended for varying periods of time in a buffer of the following composition: 135 mmol/L NaCl, 4 mmol/L KCl, 5 mmol/L glucose, 1.5 mmol/L MgCl2, 1.5 mmol/L CaCl2, 20 mmol/L HEPES, pH 7.4, at 37°C. The cytokrit of the suspension was adjusted to 10%. For experiments determining the response to forskolin, adenosine A2 receptor and β-adrenergic receptor stimulation cells were preincubated for 15 minutes at 37°C and stimulated with agonist for 5 or 15 minutes with 100 μmol/L forskolin, 10 μmol/L NE, or 10 μmol/L 5′-(N-cyclopropyl)-carbamidoadenosine (CPCA) as adenosine receptor agonist; the concentrations were chosen to achieve maximum stimulation of β-adrenergic and A2 receptors, respectively.1,2 CPCA was used as A2a receptor agonist, because with embryonic RBC, it is as effective as the A2a-specific agonist CGS 21680.1 At the end of the incubation, a small sample was taken for Hb determination. The reaction was stopped by addition of ice-cold ethanol, and the mixture was left on ice for at least 5 minutes. This was followed by centrifugation of the sample at 13,000g for 5 minutes at 4°C. The supernatant was transferred into an Eppendorf test tube and dried overnight at 50°C. The dried samples were stored at −20°C. Before the measurements, the samples were mixed for 2 minutes with 40 μL ice-cold perchloric acid, sonicated for 1 minute, and neutralized with 10 μL 2 mol/L KOH. The precipitate was removed by centrifugation and the clear supernatant used for cAMP measurements with the fluorometric enzymatic test of Sugiyama and Lurie,8 using a Perkin Elmer LB 50 fluorescence spectrometer with microplate reader (Perkin Elmer, Norwalk, CT). All measurements were performed in triplicate; cAMP concentrations were calculated as picomoles of cAMP per milligram of Hb. For experiments analyzing the effect of guanylyl cyclase and PDE3 activity on cyclic AMP production, erythrocytes were preincubated for 15 minutes with either 0.5 to 30 μmol/L 1 H-[1,2,4] oxydiazolo[4,-a] quinoxaline-1-one (ODQ), a specific inhibitor of soluble guanylyl cyclase (sGC); 20 μmol/L Ly 83583 (6-Anilino-5,8-quinolinequinone), an inhibitor of soluble and particulate guanylyl cyclase; or 20 μmol/L milrinone [1,6-Dihydro-2-methyl-6-oxo-(4′-bipyridine)-5-carbonitrile], a specific PDE3 inhibitor and were subsequently stimulated for 15 minutes with NE at the indicated concentration, in the continued presence of the respective inhibitor. To test the effect of Hb oxygenation on cAMP signaling, RBC samples suspended in measuring buffer were preequilibrated for 25 minutes at 37°C in a radiometer micro-tonometer unit with either water vapor saturated air or with different oxygen/nitrogen mixtures ranging from 1% oxygen to 8% oxygen, which were provided by a Wösthoff gas mixing pump. After 10 minutes of preequilibration, ODQ (30 μmol/L) was added as indicated and the samples were incubated for an additional 15 minutes. The cells were then stimulated for 15 minutes with 10 μmol/L NE. Samples were processed for cAMP measurements as described above.
Chemicals.
Enzymes for cAMP determination were obtained from Boehringer Manheim (Manheim, Germany); all other reagents were purchased from Sigma Chemicals (St Louis, MO).
Statistics.
Mean values were tested for statistical difference using thet-test for paired or unpaired samples; P < .05 was considered significant.
RESULTS
Basal cAMP concentration and response to forskolin for erythrocytes harvested between 3 to 17 days of incubation.
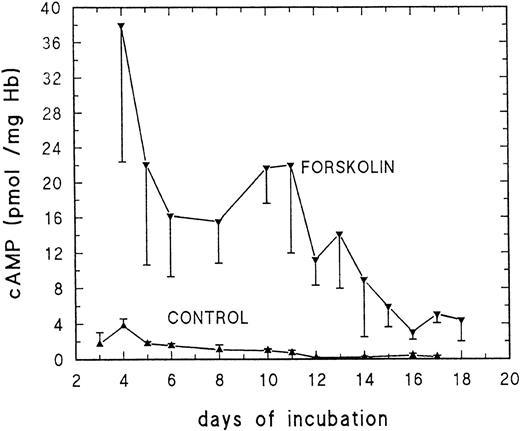
Cyclic AMP levels of unstimulated erythrocytes showed only moderate changes during development. Basal values ranged from a peak value of 4 pmol/mg Hb at day 4 to 0.2 to 0.4 pmol/mg Hb beween days 12 and 17 (Fig 1). Stimulation with forskolin (100 μmol/L) for 5 minutes increased cAMP approximately 20-fold (Fig 1) between days 3 and 11. The ability of forskolin to stimulate adenylyl cyclase activity decreases markedly after day 11/12.
Basal and forskolin-stimulated cAMP production of embryonic RBC at different stages of development. Embryonic RBC were stimulated for 5 minutes with 100 μmol/L forskolin at pH 7.4 and 37°C. For details, see Materials and Methods. Data are the mean values and SD derived from at least 5 determinations at each point.
Basal and forskolin-stimulated cAMP production of embryonic RBC at different stages of development. Embryonic RBC were stimulated for 5 minutes with 100 μmol/L forskolin at pH 7.4 and 37°C. For details, see Materials and Methods. Data are the mean values and SD derived from at least 5 determinations at each point.
Effect of β-adrenergic and adenosine A2 receptor stimulation.
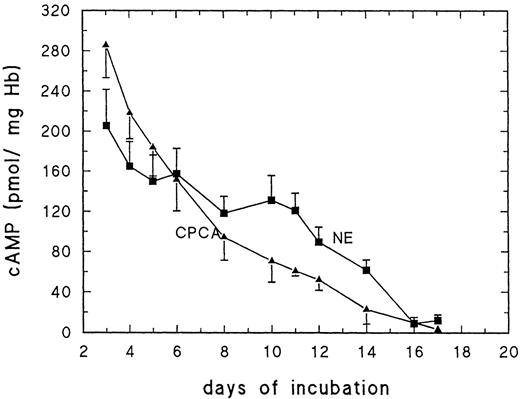
Figure 2 shows the erythrocyte cAMP level after 5 minutes of stimulation with 10 μmol/L NE or 10 μmol/L CPCA with cAMP concentrations related to mg Hb (Fig 2). Between day 3 to about day 13/14, both NE and CPCA cause a large increase of RBC cAMP. Basal cAMP levels are increased 50- to 100-fold within 5 minutes (Fig2). After day 13/14, the response decreases rapidly for NE. In contrast, the response to CPCA decreases gradually after day 6 and in a marked fashion after day 12. Note that both NE as well as CPCA are far more effective stimulators of cAMP production than forskolin and that maximum values for cAMP are at least 5 times higher than what is observed with forskolin.
Effect of adrenergic effector NE (10 μmol/L) and adenosine A2 receptor agonist CPCA (10 μmol/L) on cAMP production of embryonic RBC harvested from 3- to 17-day-old chick embryos; data for day 11 to 17 are taken from Dragon et al.3 Results are the mean values and SD of at least 5 determinations.
Effect of adrenergic effector NE (10 μmol/L) and adenosine A2 receptor agonist CPCA (10 μmol/L) on cAMP production of embryonic RBC harvested from 3- to 17-day-old chick embryos; data for day 11 to 17 are taken from Dragon et al.3 Results are the mean values and SD of at least 5 determinations.
Role of sGC and cGMP inhibited PDE3 in the control of RBC cAMP.
We first analyzed the effect of guanylyl cyclase inhibition on NE-stimulated cAMP production with RBC from day 11 using the nonspecific inhibitor Ly 83 583. After 5 minutes of stimulation, the following results (mean values from 3 experiments) were obtained: control, 0.4 pmol cAMP/mg Hb; NE (10 μmol/L), 55.3 pmol cAMP/mg Hb; NE + 20 μmol/L Ly 83583, 32.4 pmol cAMP/mg Hb; 100 μmol/L dibutyryl-cGMP + NE + Ly 83583, 42.8 pmol cAMP/mg Hb.
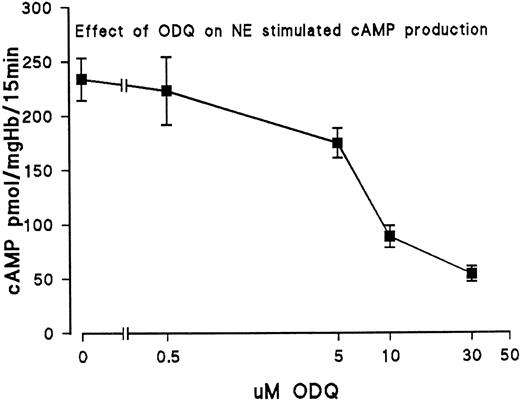
Because Ly 83583 is a nonspecific inhibitor of guanylyl cyclase, we next tested the effect of ODQ, a specific inhibitor of sGC, on cAMP levels. The dose-response curve for inhibition of NE-induced cAMP accumulation by ODQ in day-11 RBC is shown in Fig 3. With 30 μmol/L ODQ, cyclic AMP was reduced by nearly 80%, from 234 pmol cAMP/mgHb/15 minutes (10 μmol/L NE) to 54 pmol cAMP/mg Hb/15 minutes (10 μmol/L NE + 30 μmol/L ODQ). In addition, we tested the effect of ODQ on cells stimulated with physiological concentrations of NE (10 and 30 nmol/L) encountered in late development.2 With 30 nmol/L NE, cAMP increased to 76.7 pmol/mg Hb/15 minutes (standard deviation [SD], 5.6 pmol/mg Hb) compared with 45.0 pmol/mg Hb/15 minutes (SD, 7.9 pmol/mg Hb) in cells treated with 30 nmol/L NE + 30 μmol/L ODQ; when cells were stimulated with 10 nmol/L NE, we found that ODQ decreased cAMP from 13.3 pmol/mg Hb to 9.3 pmol/mg Hb (mean values from 3 experiments). On the other hand, we found no effect of ODQ on basal cAMP levels of unstimulated cells (0.9 pmol/mg Hb). Likewise, the addition of cGMP to unstimulated cells (0.5 mmol/L) had no effect on basal cAMP (control, 0.8 pmol cAMP/mg Hb; +0.5 mmol/L cGMP, 1.1 pmol/mg Hb; mean values from n = 5). However, cGMP increased cAMP levels of cells stimulated with 10 μmol/L NE by approximately 20% (control with 10 μmol/L NE, 254.1 pmol cAMP/mg Hb [SD, 21.0 pmol/mg Hb; n = 5]; 10 μmol/L NE + 0.5 mmol/L cGMP, 303.4 pmol cAMP/mg Hb [SD, 16.8 pmol/mg Hb; n = 5]). The results suggest that alterations of cGMP concentration are important for the regulation of cAMP in embryonic RBC stimulated by NE (or other agents) activating the cAMP pathway; moreover, the marked effect of ODQ suggests that sGC is the major cGMP-producing enzyme of the embryonic RBC.
Inhibition of sGC with ODQ reduces NE-stimulated cAMP production of RBC from day-11 embryos. The ordinate gives cAMP production obtained with 10 μmol/L NE. Erythrocytes from day-11 embryos were incubated for 15 minutes at 37°C with 10 μmol/L NE and varying concentrations of ODQ. Data are the mean values and SD of 3 experiments.
Inhibition of sGC with ODQ reduces NE-stimulated cAMP production of RBC from day-11 embryos. The ordinate gives cAMP production obtained with 10 μmol/L NE. Erythrocytes from day-11 embryos were incubated for 15 minutes at 37°C with 10 μmol/L NE and varying concentrations of ODQ. Data are the mean values and SD of 3 experiments.
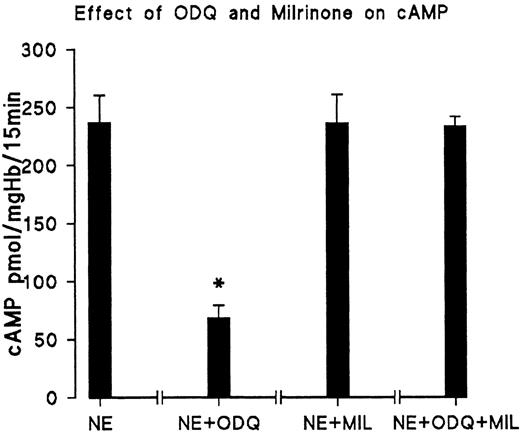
We next tested if the reduction of the cAMP level by ODQ is due to activation of PDE3. The results are shown in Fig 4. Milrinone, a specific inhibitor of PDE3, reverses the antagonistic effect of ODQ on cAMP formation. On the other hand, addition of milrinone together with NE did not significantly increase cAMP (Fig 4) compared with the control with NE; therefore, the PDE3 of the controls must be largely inhibited. The results show that changes of PDE3 activity have a very large effect on RBC cAMP, indicating cGMP-dependent inhibition of PDE3 as a major point of control. The finding that neither ODQ nor added cGMP had an influence on basal cAMP levels of unstimulated cells from day 11 is presumably due to the fact that the basal cAMP concentration (∼0.1 μmol/L) is much lower than the reported Km of about 0.5 μmol/L for PDE3.9
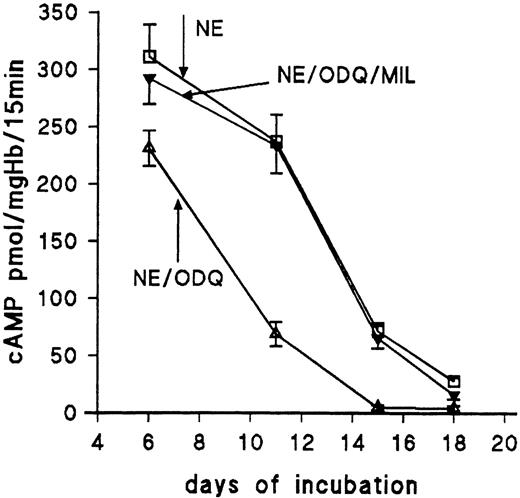
PDE3 inhibitor milrinone reverses ODQ-induced decrease of cAMP. RBC from day 11 were incubated for 15 minutes at 37°C with 10 μmol/L NE (control); 10 μmol/L NE + 30 μmol/L ODQ; 10 μmol/L NE and 20 μmol/L milrinone (MIL); and 10 μmol/L NE, 30 μmol/L ODQ, and 20 μmol/L milrinone, respectively. Data are the mean values and SD from 5 experiments in each case. *Significant difference from control (P < .01).
PDE3 inhibitor milrinone reverses ODQ-induced decrease of cAMP. RBC from day 11 were incubated for 15 minutes at 37°C with 10 μmol/L NE (control); 10 μmol/L NE + 30 μmol/L ODQ; 10 μmol/L NE and 20 μmol/L milrinone (MIL); and 10 μmol/L NE, 30 μmol/L ODQ, and 20 μmol/L milrinone, respectively. Data are the mean values and SD from 5 experiments in each case. *Significant difference from control (P < .01).
Effect of ODQ and milrinone on RBC cAMP production between days 6 and 18.
Because the composition of the circulating RBC population changes completely during development, we tested the effect of ODQ and milrinone on NE-stimulated cAMP formation between days 6 and 18 (Fig 5). At day 6, more than 90% of the circulating erythrocytes are primitive RBC; at day 18, more than 90% are mature definitive RBC.5 The data show that, at all stages, ODQ decreases NE-induced cAMP. However, primitive RBC from day 6 are only moderately affected by ODQ (Fig 5), whereas definitive RBC reduce cAMP by between 77% (day 11) and greater than 95% (days 15 and 18). At day 15, RBC stimulated with NE have 70 pmol cAMP/mg Hb, and those incubated with NE and ODQ have only 2.9 pmol/mg Hb. Thus, in those stages of development when definitive RBC are physiologically stimulated by NE and adenosine,1 2 inhibition of PDE3 is a prerequisite for a significant net increase of cellular cAMP. Milrinone reverses the effect of ODQ at all stages.
Effect of ODQ and milrinone on NE-stimulated cAMP accumulation of RBC from 6- to 18-day-old chick embryos. RBC were stimulated for 15 minutes with 10 μmol/L NE in the absence and presence of ODQ (30 μmol/L) and milrinone (20 μmol/L), respectively. Data are the mean values and SD from 3 to 5 experiments in each case.
Effect of ODQ and milrinone on NE-stimulated cAMP accumulation of RBC from 6- to 18-day-old chick embryos. RBC were stimulated for 15 minutes with 10 μmol/L NE in the absence and presence of ODQ (30 μmol/L) and milrinone (20 μmol/L), respectively. Data are the mean values and SD from 3 to 5 experiments in each case.
Effect of milrinone and ODQ on intracellular cAMP degradation.
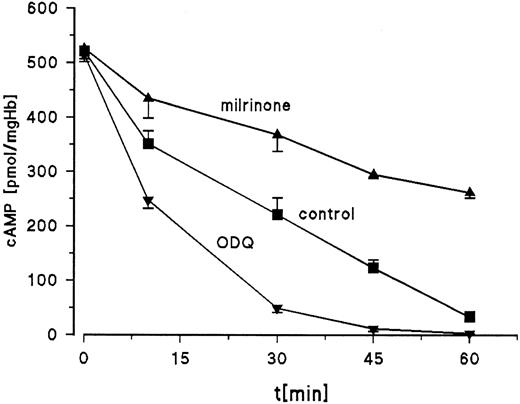
To test the effect of ODQ and milrinone on intracellular cAMP degradation, cells were first incubated for 2 hours with 10 μmol/L NE, rapidly washed 3 times to remove NE, and resuspended in fresh buffer to which ODQ and milrinone were added as indicated. Cyclic AMP concentrations were measured over 60 minutes after the addition of ODQ or milrinone. The data are presented in Fig6. After 2 hours of stimulation with NE, the cAMP concentration was approximately 520 pmol cAMP/mg Hb. In the controls, half of the initial cAMP is degraded by 25 minutes, whereas in the presence of ODQ, the half-life for cAMP is reduced to less than 10 minutes and basal cAMP values of unstimulated cells are reached by 45 minutes. With milrinone, we still find 230 pmol cAMP/mg Hb at the end of the 1-hour incubation period, compared with approximately 35 pmol cAMP/mg Hb for the control. These results support the important role of PDE3 and sGC activity in the regulation of cAMP signal strength.
Effect of ODQ and milrinone on cAMP degradation. RBC from day-11 chick embryos were incubated for 2 hours at 37°C with 10 μmol/L NE, washed, and resuspended in fresh buffer (control) or buffer with 30 μmol/L ODQ or 20 μmol/L milrinone. Cyclic AMP measurements started with addition of ODQ or milrinone and were performed over 60 minutes. Data are the mean values and SD from 3 experiments.
Effect of ODQ and milrinone on cAMP degradation. RBC from day-11 chick embryos were incubated for 2 hours at 37°C with 10 μmol/L NE, washed, and resuspended in fresh buffer (control) or buffer with 30 μmol/L ODQ or 20 μmol/L milrinone. Cyclic AMP measurements started with addition of ODQ or milrinone and were performed over 60 minutes. Data are the mean values and SD from 3 experiments.
Effect of NO synthase inhibitor L-NMMA on cAMP production.
The embryonic RBC must produce sufficient cGMP during short-term incubation (30 minutes at 37°C) to keep PDE3 activity fairly low, because milrinone per se does not increase cAMP formation in the presence of NE. Because sGC is activated by NO, we tested if embryonic erythrocytes contain NO-synthase (NOS) activity. Erythrocytes from day 11 were preincubated for 15 or 120 minutes with the NOS inhibitor L-NMMA (0.5 mmol/L) and subsequently stimulated for 15 minutes with 10 μmol/L NE in the continued presence of the NOS inhibitor. We found no effect of NOS inhibition on NE-stimulated cAMP production (control with 10 μmol/L NE, 247 pmol cAMP/mg; 15 minutes of preincubation with L-NNMA, 245.0 pmol cAMP/mg Hb; 120 minutes of preincubation with L-NNMA, 237.1 pmol cAMP/mg Hb; mean values from n = 5 in each case). The results show that guanylyl cyclase activity was unchanged in the presence of L-NNMA.
Effect of Hb oxygenation and sodium nitroprusside (SNP) on cAMP formation.
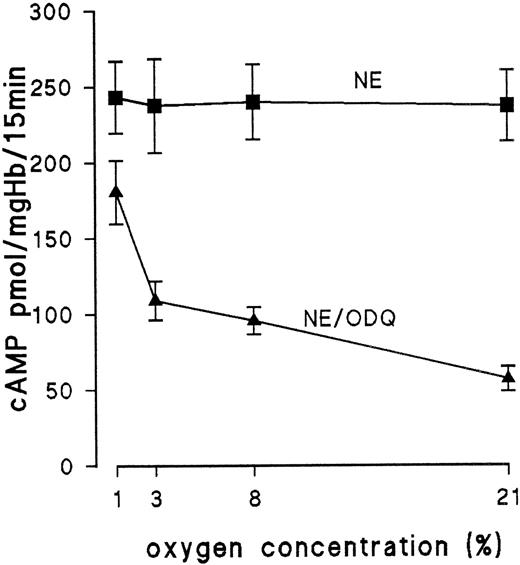
Because we have no evidence for endogenous NO production by NO synthase, other intracellular sources should provide NO. Recent work has shown that Hb is an NO donor under certain conditions: NO binds not only to the heme group of Hb, but upon oxygenation it can be transferred from the heme group to cysteine β-93, forming S-nitrosohemoglobin.10,11 The structural transition from R to T conformation accompanying deoxygenation leads to release of NO from the S-nitroso group.10,11 To test if Hb of embryonic RBC releases NO in an oxygenation-dependent manner, we investigated the effect of partial deoxygenation on ODQ-induced inhibition of cAMP formation. Because ODQ binds competetively to the heme of guanylyl cyclase,12 increased occupancy of the heme of guanylyl cyclase by NO (due to NO release during deoxygenation) should decrease the inhibitory effect of ODQ. Erythrocytes from day-11 embryos were preequilibrated for 25 minutes before the addition of NE either with 1%, 3%, or 8% oxygen in nitrogen or with air. After 10 minutes of the preequilibration period, ODQ was added. The results are shown in Fig 7. Control samples incubated only with NE were unaffected by deoxygenation. In the presence of ODQ, deoxygenation markedly affected cAMP production (Fig 7). In blood samples equilibrated with 1% oxygen, ODQ decreased NE-stimulated cAMP formation only by 28%, whereas in air-equilibrated samples, ODQ decreased cAMP formation by 76% (with 8% oxygen, we observed a 60% reduction; with 3% oxygen, we observed a 52% reduction). Similar results were obtained with RBC from day 15 (data not shown).
Influence of Hb oxygenation on ODQ-induced decrease of cAMP level of RBC from day 11. RBC from day-11 embryos were preequilibrated for 25 minutes before addition of NE (10 μmol/L for 15 minutes) with either air, 1% oxygen, 3% oxygen, or 8% oxygen. After 10 minutes, 30 μmol/L ODQ was added to 1 group of samples. Data are the mean values and SD from 3 to 7 experiments.
Influence of Hb oxygenation on ODQ-induced decrease of cAMP level of RBC from day 11. RBC from day-11 embryos were preequilibrated for 25 minutes before addition of NE (10 μmol/L for 15 minutes) with either air, 1% oxygen, 3% oxygen, or 8% oxygen. After 10 minutes, 30 μmol/L ODQ was added to 1 group of samples. Data are the mean values and SD from 3 to 7 experiments.
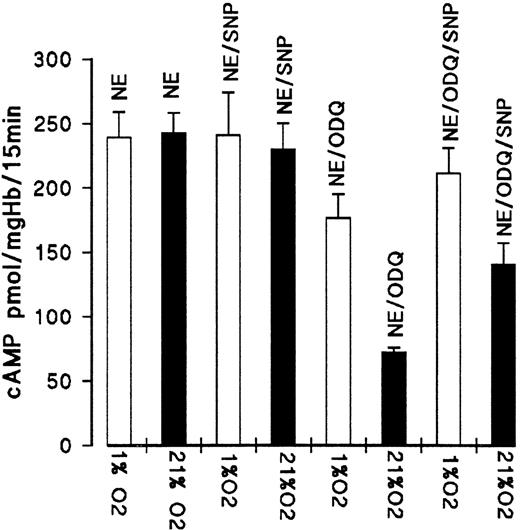
We then tested if the addition of SNP (100 μmol/L) as an external NO donor alters the effect of oxygenation and deoxygenation on cAMP formation. As shown in Fig 8, the addition of SNP at the start of the 25-minute prequlibration period increased the formation of cAMP in air-incubated erythrocytes in the presence of ODQ by approximately 70 pmol/mg Hb (from 72.6 to 141 pmol/mg Hb).The effect was significantly lower for partially deoxygenated RBC (1% oxygen equilibration). With SNP, cAMP increased by approximately 35 pmol/mg Hb compared with the controls with ODQ (from 176.5 to 211 pmol/mg Hb). SNP had no effect on the cAMP formation in the absence of ODQ. These results suggest that embryonic Hb is a substantial store for NO and that the NO release is dependent on Hb oxygen saturation and most effective when oxygen pressure falls to oxygen pressure of 20 mm Hg or less, values that are commonly found in the intraembryonic circulation.13 However, even in the oxygenated state, sufficient NO must be liberated (from Hb or other compounds such as S-nitrosoglutathione) to account for the inhibition of PDE3 during short-term incubation.
Effect of SNP on ODQ-dependent decrease of cAMP level of oxygenated and deoxygenated RBC from day 11. RBC suspensions were preequilibrated in air or 1% oxygen for 25 minutes before the addition of NE (10 μmol/L for 15 minutes) in the absence or presence of 100 μmol/L SNP; ODQ (30 μmol/L) was added after 10 minutes of preequilibration. Data are the mean values and SD from 4 experiments.
Effect of SNP on ODQ-dependent decrease of cAMP level of oxygenated and deoxygenated RBC from day 11. RBC suspensions were preequilibrated in air or 1% oxygen for 25 minutes before the addition of NE (10 μmol/L for 15 minutes) in the absence or presence of 100 μmol/L SNP; ODQ (30 μmol/L) was added after 10 minutes of preequilibration. Data are the mean values and SD from 4 experiments.
DISCUSSION
Developmental changes of cAMP signaling in primitive and definitive RBC.
The results of the present study demonstrate that, for most of embryonic life, RBC adenylyl cyclase from chick embryo is functionally coupled to β-adrenergic receptors and adenosine receptors (A2). Basophilic primitive erythroblasts from day 3 embryos show, within 5 minutes, at least 50-fold stimulation of their basal cAMP production in the presence of NE or CPCA, and similar or even higher degrees of activation (up to 100-fold increase over basal cAMP) are observed until day 12/13, despite the fact that the composition of the RBC population in the circulation has changed profoundly. Such high degrees of stimulation by β-adrenergic or other receptors linked to adenylyl cyclase (measured in the absence of added PDE inhibitor) have so far not been reported for erythroid cells. In primitive RBC, the effect of adenosine receptor stimulation is slightly larger than β-adrenergic activation. On the other hand, after day 7, when the majority of the circulating RBC are definitive erythrocytes, β adrenergic stimulation of cAMP production is stronger and the effect of CPCA gradually decreases. This suggests some reorganization of the signaling system upon transition from primitive to definitive erythrocytes. The definitive RBC present in the embryonic circulation during the second week of development are markedly different from adult RBC. Although postmitotic, they are still transcriptionally active and have an approximately 5- to 6-fold higher respiratory rate than RBC from adult chick.14 Mature definitive RBC first appear around day 12 to 14, and by day 18, the amount of reticulocytes in the circulation has decreased to less than 10%.5 The rapid decrease in cAMP production at this stage (receptor stimulated or forskolin stimulated) closely correlates with the declining population of transcriptionally active reticulocytes in the embryonic circulation. In comparison to NE or CPCA, forskolin is a weak effector. Nevertheless, the response of the cells is remarkably constant between day 3 until about day 12, followed by a significant reduction of forskolin-stimulated adenylyl cyclase activity, which parallels the decrease of NE- or CPCA-mediated stimulation of adenylyl cyclase.
Although we have previously clarified the physiological role of cAMP signaling in immature definitive RBC of the chick embryo,1-3 one can only speculate about the function of adenosine and β-adrenergic receptors in circulating primitive RBC. The yolk of the freshly laid egg contains substantial amounts of catecholamine, which are taken up by the chick embryo once the embryonic circulation is established, ie, after approximately 48 to 55 hours of incubation.15 Between days 2 and 4/5 of chick embryonic development, uptake of extraembryonic catecholamine is probably the major source of catecholamine supply for the embryo.15,16 During this period, primitive embryonic erythroblasts will be exposed to catecholamine in the yolk sac and the vitelline circulation. This could account for our previous results demonstrating that primitive RBC from day 4 had significantly increased CAII17 and pyrimidine-5′-nucleotidase activity3 when compared with definitive RBC of days 8 to 12,3,17 because both proteins are upregulated by cAMP.1 3
Transient stimulation of immature primitive RBC by catecholamine may therefore help to increase the speed of terminal differentiation of the circulating primitive RBC. NE has also been implicated as indispensible for early mammalian development; lack of NE caused intrauterine death that was attributed to cardiovascular failure.18 Our results suggest that the maturation and function of primitive erythrocytes could also be affected. Taken together, the data indicate that, in the avian embryo, the main targets of receptor-mediated cAMP signaling are immature primitive or definitive RBC (basophilic erythroblast to reticulocytes). The rapid decrease of cAMP production in the last week of development correlates with the appearance of mature definitive erythrocytes.
It is difficult to compare our results with those of other studies that have investigated cAMP signaling in immature erythroid cells. There are no comparable data for mamalian embryonic RBC. Most work analyzing cAMP signaling in intact mammalian erythroid cells has been performed with transformed erythroleukemia cell lines, which appear less restricted in differentiation potential than normal erythroid progenitors (see Porzig et al19).
Porzig et al19 analyzed the response of normal human adult erythroid progenitors (eg, burst-forming units-erythroid [BFUe] to colony-forming units-erythroid [CFUe]) derived from peripheral stem cells towards a variety of hormonal agonists. The progenitor cells responded to adenosine, PGE, and less so to isoproterenol with increased cAMP production. Even in the presence of PDE inhibitor adenosine, as the most potent effector, gave only moderate (8-fold) stimulation of cAMP production. In late precursor cells (normocytes and reticulocytes), cAMP signaling was also analyzed in a few cases, but only very weak responses to receptor agonists were observed in these cells.19
Role of PDE3 and sGC in the control of cAMP production.
In the present investigation, we have tested to which extent the cAMP signal is modulated by cAMP degradation and have concentrated on the impact of cGMP regulated PDE3. Our data show that embryonic erythroid cells possess a cGMP-inhibitable PDE3. Using the specific inhibitors ODQ (for sGC) and milrinone (for PDE3), we established the presence of PDE3 and sGC in primitive as well as definitive RBC. Primitive and definitive RBC differ in their response to sGC inhibition by ODQ. In primitive RBC, we observed a 25% reduction of NE-stimulated cAMP formation in the presence of ODQ, whereas in definitive RBC, the signal is reduced by 77% (day 11) to approximately 95% (day 15).
Activation of sGC in embryonic RBC.
Our data suggest that PDE3 is at least partially inhibited under our experimental conditions: during short-term incubation, milrinone was not able to increase NE-dependent cAMP formation in the absence of ODQ. Inhibition of PDE3 requires continuous activity of sGC, which is activated by NO. We found no evidence for NO synthase activity. L-NMMA, which inhibits all known NOS isozymes, had no effect on cAMP levels. Therefore, other intracellular sources must provide NO. NO can form S-nitrosocompounds with suitable receptor molecules such as glutathione,10,11 and there is evidence for the existence of oxylabile S-nitrosogroups formed at cys β-93,10 11 an invariant residue in all mammalian and avian Hbs.
Our own data give some indirect evidence that the erythrocyte contains NO donors that activate sGC. The addition of SNP as an NO donor had no effect on the cAMP accumulation in oxygenated or deoxygenated RBC stimulated with 10 μmol/L NE. This result is compatible with the assumption that sGC is continously activated by intracellular NO donors so that additional NO from an external source will have little effect. On the other hand, SNP increased cAMP production of cells that were stimulated with 10 μmol/L NE in the presence of ODQ. Because ODQ binds competitively to the heme of sGC, increased free NO due to addition of SNP will reduce the inhibitory effect of ODQ. Our results also give some evidence that NO is released in in an oxygen-linked manner from embryonic Hb: increasing free NO on deoxygenation should diminish the antagonistic effect of ODQ. The results are in agreement with this prediction: at the lowest oxygen concentration, ODQ inhibited cAMP formation only by 28%, compared with 76% in the air-incubated controls. Likewise, SNP stimulated cAMP accumulation to a larger extent in oxygenated RBC treated with ODQ than in deoxygenated RBC. Although further work is needed to test the hypothesis mentioned above, namely that RBC sGC is activated by intracellular NO donors such as Nitrosoglutathione or S-nitrosohemoglobin, it is clear that continued activation of sGC provides a key element for cAMP signaling of embryonic RBC under physiological conditions. The release of NE and adenosine under hypoxic conditions and subsequent stimulation of RBC adenylyl cyclase via adenosine and β-adrenergic receptors promote complex changes of RBC metabolism that rely on transcriptional activation and de novo protein synthesis subsequent to stimulation of cAMP formation.1,2 This requires that intracellular cAMP levels must be elevated over hours rather than minutes.1 2A long-lasting cAMP signal is only possible when PDE3 is inhibited. This creates a direct link between the NO-, cGMP-, and cAMP-signaling pathways. In conclusion, we have shown that cAMP signaling of immature nucleate erythrocytes from chick embryo is embedded in a complex network of stimulatory and inhibitory controls, which makes the embryonic erythrocyte susceptible to influences from other embryonic tissues. This feedback may be necessary to adjust the RBC properties to changing developmental and/or environmental conditions.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Rosemarie Baumann, MD, Physiologisches Institut, Universität Regensburg, 93047 Regensburg, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal