Abstract
Natural killer (NK) cells are large granular lymphocytes capable of efficient killing of virus-infected and tumor cells in a major histocompatibility complex-independent manner. The cytotoxic killing potential of NK cells can be modulated by a variety of factors, including cytokines such as interleukin-12 (IL-12), IL-15, and interferon (IFN). IL-15 also plays an important role in NK cell development and survival. Killing of virally infected cells by NK cells is likely to represent an important antiviral defense mechanism, especially during the early phase of infection when antigen-specific immunity has yet to be generated. In the present work, we studied the potential of IL-15 to act as a modulator of NK cell-mediated antiviral defense. Our results clearly indicate that IL-15 can curtail infections by 3 human herpesviruses: Herpes simplex virus type 1, Epstein-Barr virus, and human herpesvirus 6. The antiviral activity of IL-15 is dose-, time-, and NK cell-dependent. IL-15–treated NK cells showed an increased killing potential against a variety of cells, including virus-infected target cells. Lastly, using highly purified cell population, we report that IL-15 triggers the synthesis of IFN-γ from both CD4+ and NK cells, which can act in both autocrine and paracrine fashion to modulate NK cells cytotoxic potential. In conclusion, IL-15 is a cytokine that can contribute to the establishment of an antiviral state in 2 ways: first by increasing the killing ability of NK cells and second by stimulating the synthesis and secretion of IFN.
DURING THE EARLY PHASE of a viral infection, the nonspecific immune defense mechanisms play a crucial role in limiting viral spread. These mechanisms include interferon (IFN) production, activation of the complement cascades, and activation of phagocytes and natural killer (NK) cells. The role of NK cells is best evidenced in patients having reduced or absent NK cell activity that suffer from multiple and recurrent herpesvirus infections1-7 and in infections of experimental animal models.8-10 NK cells are large granular lymphocytes capable of killing tumor and virus-infected cells, independently of major histocompatibility complex (MHC) restriction.11,12 A number of cytokines have been shown to modulate NK cell activity. For example, the cytotoxic potential of NK cells is augmented after exposure to IFN-α and IFN-β,13-15 whereas the secretion of IFN-γ by NK cells is induced after treatment with interleukin-12 (IL-12).16,17 IL-15 is another cytokine that has a major impact on NK cell biology. IL-15 shares many biological properties with IL-2, such as a growth factor for activated T cells and the activation of NK and cytotoxic T lymphocytes (CTL).18-23 IL-15 is produced by a variety of cell types, including monocytes/macrophages (MO/Mø), bone marrow stromal cells, keratinocytes, dendritic cells, and synovial-derived cells from patients with rheumatoid arthritis.24-31 T cells are not a source of IL-15.22,28 Although the primary sequence of IL-15 does not share homologies with IL-2, its modeled 3-dimensional structure closely resembles that of IL-2, a member of the 4-α-helix bundle family of cytokines.22 Structural and biological similarities can be partly attributable to receptor subunit sharing between IL-2 and IL-15. In fact, both cytokines bind and signal through the IL-2 receptor β chain (CD122) and the common γ chain (CD132).21,22,32 A third component of the IL-15 receptor, the α chain, binds IL-15 with very high affinity (1011 mol/L−1) and makes up the third moiety of the heterotrimeric IL-15 receptor.
The effects of IL-15 on cells of the immune system have been relatively well studied. However, the role of IL-15 in antimicrobial defense is much less documented. Several reports have shown an increase in IL-15 mRNA or protein in response to lipopolysaccharide or after bacterial or viral infections.22,26-28 These results suggest that this cytokine may play an important role in the generation of an effective immune response against invading pathogens. The general concept is that IL-15 is rapidly produced by MO/Mø in response to aggression, and then it acts primarily on NK cells, but also on activated T cells, which renders them more responsive to IL-2 (via upregulation of IL-2Rα chain).33 Later, once activated T cells produce IL-2, it becomes the principal T-cell growth factor. IL-15 also has T-cell chemoattractant properties, recruiting T lymphocytes to the site of inflammation.34 To learn more about the biological properties of IL-15, we undertook a study to determine the potential antiviral activity of this cytokine. Our results indicate that IL-15 has an antiviral activity against human Herpesviruses, but its effectiveness is dependent on the presence of NK cells.
MATERIALS AND METHODS
Cell lines and culture conditions.
The HSB-2, K562, and B95-8 cell lines were obtained from the American Type Culture Collection (Rockville, MD). HSB-2 is an immature human T-cell line susceptible to HHV-6 infection, K562 is an erythroleukemia cell line sensitive to NK cell lysis, and the B95-8 cells are chronically Epstein-Barr virus (EBV)-infected B cells of monkey origin. Lymphoblastoid cell lines (LCL) were obtained after immortalization of peripheral blood B lymphocytes by the B95-8 strain of EBV. All cell lines were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 50 U/mL of penicillin, 50 μg/mL of streptomycin, 30 μg/mL of gentamicin, and 10 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer, all purchased from GIBCO BRL (Gaithersburg, MD).
Peripheral blood mononuclear cell (PBMC) isolation and cell population purification.
PBMC were obtained from healthy donors after centrifugation of venous blood over Ficoll-Hypaque gradients obtained from Pharmacia Biotech Inc (Piscataway, NJ). Cells were washed 3 times in phosphate-buffered saline (PBS). In some experiments, PBMC were depleted of NK cells (CD16+/CD56+) using anti-CD16 and anti-CD56 monoclonal antibodies (MoAbs) and goat antimouse IgG-coated magnetic beads according to the manufacturer's technical guidelines (Immunotech Inc, Westbrook, ME). Effectiveness of NK cell depletion from PBMC was determined by flow cytometry. No significant changes in remaining cell populations (T cells, B cells, and monocytes) were observed. Human CD4+ T cells were enriched by negative selection using a cocktail of antibodies (anti-CD8, -CD14, -CD16, -CD19, -CD56, and -glycophorin A; StemCell Technologies, Vancouver, British Columbia, Canada). NK cells were also enriched by negative selection using a cocktail of antibodies (anti-CD3, -CD4, -CD14, -CD19, -CD66b, and -glycophorin A; StemCell Technologies). Human CD8+ T cells were enriched by negative selection using an antibody cocktail (anti-CD4, -CD14, -CD16, -CD19, -CD56, and -glycophorin A). CD4, CD8, and NK cell enrichment procedures consistently yielded population of cells expressing (98%) the CD4, the CD8, or the CD56 marker, respectively.
Virus production.
HHV-6 (GS strain) was propagated in HSB-2 cells as described previously.35 The HHV-6 titer, expressed as the 50% tissue culture infective dose, was determined by scoring the number of HSB-2 cells exhibiting cytopathic effect (CPE). The virus stock had a titer of 106 50% tissue culture infective doses/mL. The mock-infected control was prepared from uninfected HSB-2 culture supernatant and processed as for the infected supernatant.
EBV was produced from the chronically infected B95-8 cell line.36 Briefly, B95-8 cells were seeded at 2 × 105 cells/mL and phorbol 12-myristate 13-acetate (TPA)-activated (20 ng/mL) to induce the EBV replicative cycle. When cell viability was less than 10%, supernatant was harvested and passed through a 0.45-μm membrane filter, and virions were pelleted by centrifugation at 25,000g for 45 minutes. Viral pellets were resuspended in RPMI-1640. Infectivity and titer of preparations were determined by anti-Epstein-Barr nuclear antigen (EBNA) immunofluorescence after infection of PBMC.
Herpes simplex virus type 1 (HSV-1) was produced from infected Vero cells. Briefly, Vero cells were infected with HSV-1 (multiplicity of infection [moi], 0.01) and monitored regularly for CPE. When greater than 85% of the culture was destroyed, supernatant was filtered and stored frozen at −80°C. Titer of preparations was determined by plaque assay formation on Vero cells.
Infection of cells and culture conditions.
In the case of infection by HHV-6, PBMC were activated with phytohemagglutinin (PHA; 1 μg/mL) obtained from Sigma (St Louis, MO) for 2 days before infection. Activated cells were then pelleted by centrifugation, infected with HHV-6 (moi, 0.1) for 2 hours at 37°C, and subsequently washed twice with PBS to remove unadsorbed virions. Cell density was adjusted to 106 cells/mL in complete medium supplemented with 5 U/mL of IL-2 (Boehringer Mannheim, Indianapolis, IN). Selected cultured were further complemented with 10 to 50 ng/mL of IL-15 or IL-6 (R&D Systems, Minneapolis, MN). In some experiments, HSB-2 cells were infected with HHV-6 (moi, 0.1) for 2 hours at 37°C, washed twice with PBS, and resuspended at 4 × 105 cells/mL.
In the cases of EBV and HSV-1, freshly isolated PBMC were infected with EBV (104 TFU/106 cells) or HSV-1 (moi, 0.05) for 2 hours at 37°C, washed with PBS, and resuspended in complete medium with or without 25 to 50 ng/mL of IL-15. In some experiments, cultures were supplemented immediately after infection with 10 μg/mL of anti–IFN-α and anti–IFN-γ neutralizing MoAbs (Serotec Inc, Raleigh, NC). In previous experiments, 10 μg/mL of these antibody preparations was proven to effectively neutralize the antiviral activity of 1,000 pg/mL of recombinant IFN-α or IFN-γ (data not shown).
Flow cytometry and immunofluorescence.
HHV-6 infection of cells was monitored by flow cytometry. Briefly, cells were harvested, washed in PBS, pelleted by centrifugation, and surface-stained for HHV-6 antigen expression using the anti-gp106 MoAb (Advanced Biotechnologies Inc, Columbia, MD). After 1 hour at 4°C, cells were washed with PBS and labeled with fluorescein isothiocyanate (FITC)-labeled goat antimouse antibodies for 45 minutes at 4°C. After washing with PBS, cells were fixed with 1% paraformaldehyde in PBS and analyzed using the FACScalibur flow cytometer (Becton Dickinson, Mountain View, CA). Results were calculated after the accumulation of 10,000 events using the CellQuest software (Becton Dickinson).
For EBV and HSV-1, infectivity was determined by standard immunofluorescence. Briefly, cells were harvested, washed with PBS, and deposited on glass microscope slides. Cells were fixed in cold acetone for 5 minutes, air-dried, and stained with MoAb H62 against HSV-1 (BioSource International, Camarillo, CA) or with a positive reference serum for EBV. Statistics were calculated using the Student'st-test, and the results were considered significant whenP < .05.
DNA slot blot analysis.
Cells were harvested and washed in PBS, and genomic DNA was isolated as described.28 Genomic DNA at 5, 1, and 0.2 μg was transferred to nylon membranes with the use of a slot blot apparatus (Schleicher and Schuell, Keene, NH). After UV cross-linking of the DNA, the membranes were incubated for 1 hour at 68°C in prehybridization buffer (6× SSC, 0.1% sodium dodecyl sulfate [SDS], 1× Denhardt's solution, and 100 μg/mL salmon sperm DNA) and then hybridized overnight at 68°C in prehybridization buffer containing the pZVH14 32P-labeled HHV-6 probe.37 Membranes were washed twice with 1× SSC, 0.1% SDS and once at 68°C with 0.1× SSC, 0.1% SDS before being exposed to x-ray films.
IFN-α and IFN-γ determination.
To determine the effects of IL-15 on IFN synthesis, PBMC were either treated with IL-15 (50 ng/mL), EBV, HSV-1, or in combination of IL-15 with virus for varying periods of time. Cell-free supernatants were collected at time points and stored frozen at −80°C until assayed for IFN-α (sensitivity, 25 pg/mL; Biosource International) and IFN-γ (sensitivity, 4 pg/mL; Pharmingen, Mississauga, Ontario, Canada) by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instructions (Pharmingen). Kinetics of IFN-γ synthesis from purified CD4+, CD8+, and CD56+ cell populations after IL-15 (50 ng/mL) stimulation were determined by ELISA (Pharmingen). Statistics were calculated using the Student's t-test, and the results were considered significant when P < .05.
NK cell cytotoxicity assay.
Purified CD56+ NK cells (98%) were cultured (106 cells/mL) in absence or presence of IL-15 (50 ng/mL) for 3 days. Two million target cells (K562, LCL, HSB-2, and HSB-2 infected with HHV-6) were labeled by incubation with 200 μCi of sodium chromate (DuPont, Mississauga, Ontario, Canada) for 1 hour at 37°C. After 3 washes with PBS, target cells (5 × 103 cells) were mixed with effector NK cells (in triplicate) at ratios ranging from 0.1:1 to 10:1 in V-bottomed wells and were incubated for 16 hours at 37°C in a CO2incubator. Plates were centrifuged and the radioactivity of supernatant (0.1 mL) was determined using a γ-counter (LKB, Uppsala, Sweden). Data are expressed as the percentage of cytotoxicity after calculation using the following formula: (cpm experimental − cpm spontaneous)/(cpm maximum – cpm spontaneous) × 100.
RESULTS
Several cytokines, such as IFN and CC-chemokines, have been shown to possess, in addition to their immunomodulatory activities, antiviral activities. In an effort to learn more about the immunobiological properties of IL-15, we studied its ability to affect the viral growth of HSV-1 (a member of the α herpesvirinae), HHV-6 (a member of the β herpesvirinae), and EBV (as a representative of the γ herpesvirinae).
Effects of IL-15 on infection of PBMC by HHV-6.
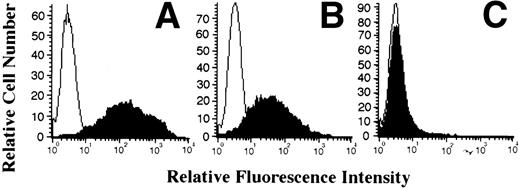
PBMC from normal individuals were activated with PHA for 48 hours before infection with HHV-6, since productive infection of resting cells by this virus is not very efficient. After infection, cells were either cultured in absence or presence of recombinant IL-15 (10 or 50 ng/mL). After 5 to 7 days postinfection, cells were monitored for HHV-6 antigen expression by flow cytometry. As shown in Fig 1A, in the absence of IL-15, more that 95% of the cells were positive for HHV-6 antigen 7 days postinfection (PI), with a mean fluorescence intensity (MFI) of 135. In the presence of 10 ng/mL of IL-15, the percentage was reduced minimally to 85%, whereas the MFI decreased more than half to 65 (Fig 1B). Moreover, in the presence of 50 ng/mL of IL-15, HHV-6 antigen expression was drastically reduced to a minimum, with less than 10% of the cells found to be expressing HHV-6 antigens (Fig 1C). To determine whether this effect was specific to IL-15, we performed the same type of experiment using IL-6, another monocyte-derived cytokine. In contrast to IL-15, cultures treated with 50 ng/mL of IL-6 supported viral replication to the same extent as those without IL-6 (data not shown).
Effect of IL-15 on infection of PBMC by HHV-6. PBMC were PHA-activated, infected with HHV-6 for 2 hours, and cultured in the absence (A) or presence of 10 ng/mL (B) or 50 ng/mL (C) recombinant IL-15 (rIL-15). On day 7 postinfection, cells were harvested and tested for cell surface expression of HHV-6 antigen by flow cytometry. Results are presented as overlay histograms, with the black histograms representing cells stained with anti–HHV-6 MoAb, whereas the white histograms represent cells stained with an irrelevant MoAb. (D) depicts the results obtained when HHV-6–infected cells, treated or not with IL-15, were analyzed for HHV-6 DNA content. Genomic DNA was hybridized with the 32P-labeled HHV-6 pZVH14 probe.
Effect of IL-15 on infection of PBMC by HHV-6. PBMC were PHA-activated, infected with HHV-6 for 2 hours, and cultured in the absence (A) or presence of 10 ng/mL (B) or 50 ng/mL (C) recombinant IL-15 (rIL-15). On day 7 postinfection, cells were harvested and tested for cell surface expression of HHV-6 antigen by flow cytometry. Results are presented as overlay histograms, with the black histograms representing cells stained with anti–HHV-6 MoAb, whereas the white histograms represent cells stained with an irrelevant MoAb. (D) depicts the results obtained when HHV-6–infected cells, treated or not with IL-15, were analyzed for HHV-6 DNA content. Genomic DNA was hybridized with the 32P-labeled HHV-6 pZVH14 probe.
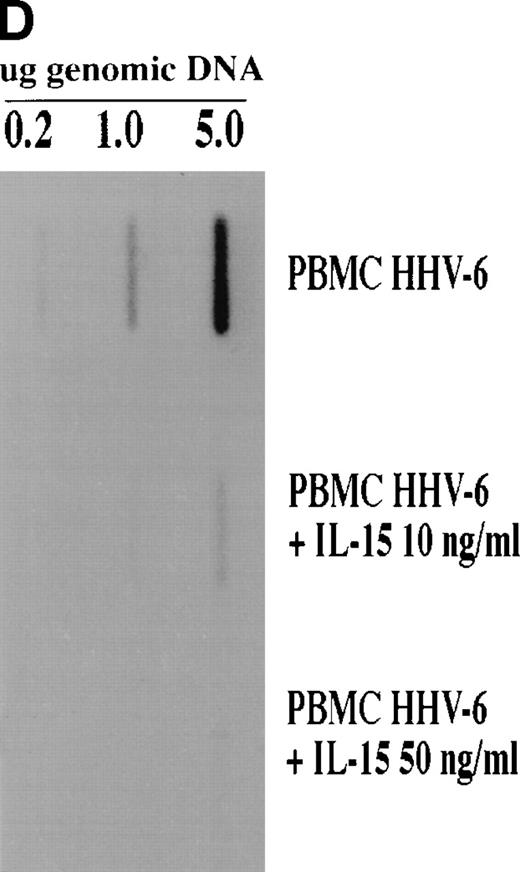
The inhibitory effects of IL-15 on HHV-6 expression were further confirmed by analyzing the HHV-6 DNA content in the various cultures. At day 5 PI, cells were harvested and genomic DNA was isolated. The DNA was probed for HHV-6–related sequences using the pZVH14 probe. As shown in Fig 1D, a reduction in HHV-6 DNA was observed in both IL-15–treated cultures, with the most striking effect observed with the 50 ng/mL dose, for which near complete elimination of HHV-6 genomic DNA is observed, in accordance with the antigen expression results presented above (Fig 1C).
Effects of IL-15 on infection of NK-depleted PBMC and enriched CD4+ T-cell cultures by HHV-6.
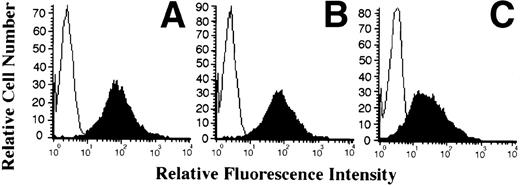
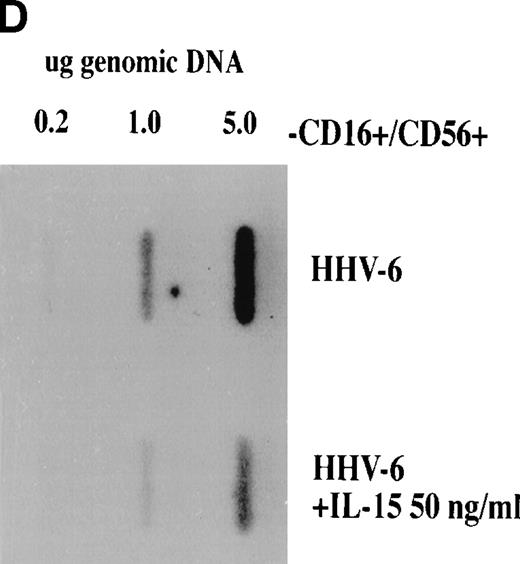
To determine whether IL-15 is acting directly or indirectly on the HHV-6 replicative cycle, we tested IL-15 activity in NK-depleted PBMC cultures as well as in enriched CD4+ T-cell cultures. We opted to deplete NK cells from PBMC cultures (<0.5% residual CD16+/CD56+ cells), because one of the effects of IL-15 is to increase the killing potential of NK cells, an effect that may account for the observed decrease in the number of HHV-6–infected cells. NK-depleted PBMC were therefore infected with HHV-6 and cultured in the absence or presence of IL-15 (10 or 50 ng/mL). Both HHV-6 antigen expression (Fig2A through C) and HHV-6 genomic DNA (Fig 2D) were monitored as described above. As with unfractionated PBMC, successful HHV-6 infection of NK-depleted PBMC was observed, with more than 95% of the cells infected by day 7 PI (Fig 2A). The addition of 10 ng/mL of IL-15 to the cultures had no effect on HHV-6 antigen expression (Fig 2B), with more than 95% of the cells expressing HHV-6 antigen and with an MFI comparable to that of cultures with no IL-15. In the presence of 50 ng/mL of IL-15, more that 81% of the cells were found to express HHV-6 antigens (Fig 2C), which is in striking contrast with unfractionated PBMC, for which less that 10% of the cells were infected (Fig 1C). This result was also confirmed by viral DNA analysis. Genomic DNA was extracted from untreated and IL-15–treated (50 ng/mL) cultures and probed for HHV-6. As shown (Fig 2D), a strong hybridization signal is observed in untreated HHV-6–infected cultures, with a slightly reduced signal in IL-15–treated cultures. Again, this is contrast to unfractionated PBMC, for which virtually no hybridization signals could be detected in IL-15–treated (50 ng/mL) cultures (Fig 1D). The effects of IL-15 on HHV-6 infection of purified (>95%) CD4+ T cells was also tested. No difference between IL-15–treated (50 ng/mL) and nontreated cultures were noticed, with more than 95% of the cells infected with HHV-6 (data not shown).
Effect of IL-15 on infection of NK-depleted PBMC by HHV-6. PHA-activated NK-depleted PBMC were infected with HHV-6 and cultured in the absence (A) or presence of 10 ng/mL (B) or 50 ng/mL (C) rIL-15. HHV-6 antigen expression and HHV-6 DNA content (D) were determined as described in Materials and Methods and as in the legend to Fig 1.
Effect of IL-15 on infection of NK-depleted PBMC by HHV-6. PHA-activated NK-depleted PBMC were infected with HHV-6 and cultured in the absence (A) or presence of 10 ng/mL (B) or 50 ng/mL (C) rIL-15. HHV-6 antigen expression and HHV-6 DNA content (D) were determined as described in Materials and Methods and as in the legend to Fig 1.
To determine whether NK cells needed a direct contact with their targets for inhibition of viral growth or whether they secreted an inhibitory soluble factor in response to IL-15, 2 types of experiments were conducted. First, PBMC depleted of NK cells were infected with HHV-6 and cocultured with unfractionated, uninfected PBMC. Both populations were physically separated by a 0.45-μm filter. The cultures were supplemented with 50 ng/mL of IL-15 and monitored for HHV-6 antigen expression by flow cytometry. Results indicate that HHV-6–infected cells were not affected or lysed by the release of soluble factors from PBMC, suggesting that a direct contact between the NK cells and the infected target cell is necessary for inhibition of viral growth (data not shown). Second, the possibility exists that a contact between the NK cells and their targets may be necessary for the induction and release of factors with antiviral properties. To verify this hypothesis, purified NK cells (98% CD56+) were cultured with IL-15 (50 ng/mL) for 2 days and subsequently mixed with K562 or HHV-6–infected HSB-2 cells. After 8 hours, the supernatants were harvested and tested for their potential cytolytic and antiviral activity. The supernatants were not found to contain soluble factors susceptible of causing lysis of K562 cells and neither were they found to inhibit HHV-6 growth (data not shown). These results suggest that IL-15 enhances the direct NK cell killing of infected cells and that the release of soluble antiviral mediator by such cells is marginal.
We next determined the effect of time of addition of IL-15 to the cultures on HHV-6 expression. PBMC were infected with HHV-6 for varying times before the addition of IL-15 (50 ng/mL) and were monitored for antigen expression by flow cytometry on day 7 PI. In the absence of IL-15, more than 90% of the cells were infected with HHV-6 (Fig 1 and data not shown). The addition of IL-15 on day 0 (same day as infection) resulted in a complete elimination of the HHV-6–infected cells from the cultures, whereas the addition of IL-15 on the second day PI resulted in partial suppression of HHV-6 growth, with 26% of the cells being infected. The addition of IL-15 on day 4 PI was unable to prevent HHV-6 infection, with more than 87% of the cells being infected (data not shown).
Effects of IL-15 on HSV-1 infection of PBMC.
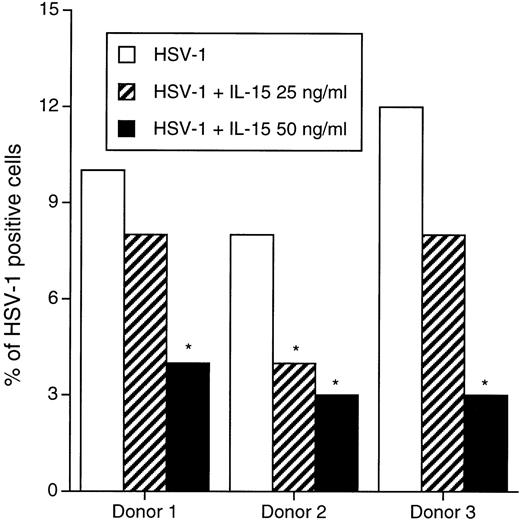
Having determined that IL-15 can prevent the spread of HHV-6 in PBMC, we next determined whether this effect is limited to HHV-6 or can be extended to other viruses. A similar experimental procedure to that of HHV-6 was used, with the exception that resting, freshly isolated PBMC were used to monitor the effect of IL-15 on HSV-1 infection. PBMC from 3 healthy donors were infected with HSV-1 and cultured in the absence or presence of IL-15 (25 or 50 ng/mL) for 5 days before evaluating the percentage of HSV-1–infected cells by conventional immunofluorescence assay using the anti–HSV-1 H62 MoAb. As shown in Fig 3, 10% to 12% of the cells were positive for HSV-1 antigens in the absence of IL-15. In the presence of 25 ng/mL IL-15, a low decrease in the percentage of infected cells is observed, with 4% to 8% of the cells being infected with HSV-1. Finally, a 60% to 75% reduction in cells expressing HSV-1 antigens is recorded in culture treated with 50 ng/mL rIL-15.
Effects of IL-15 on infection of PBMC by HSV-1. PBMC from 3 healthy blood donors were infected with HSV-1 and cultured in the absence or presence of 25 to 50 ng/mL. On day 4 PI, cells were analyzed by standard immunofluorescence with anti–HSV-1 MoAb. The percentage of infected cells was calculated after counting a minimum of 500 cells. *P < .05.
Effects of IL-15 on infection of PBMC by HSV-1. PBMC from 3 healthy blood donors were infected with HSV-1 and cultured in the absence or presence of 25 to 50 ng/mL. On day 4 PI, cells were analyzed by standard immunofluorescence with anti–HSV-1 MoAb. The percentage of infected cells was calculated after counting a minimum of 500 cells. *P < .05.
Effects of IL-15 on EBV infection of PBMC.
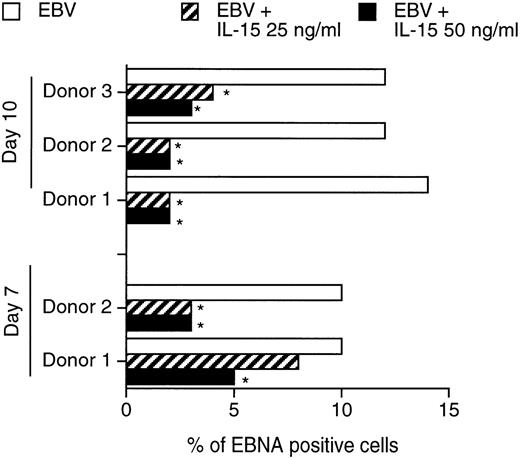
Unlike HHV-6 and HSV-1, EBV does not lytically infect PBMC, but rather immortalizes B lymphocytes. We therefore tested whether IL-15 may play a role in the control of EBV infection of PBMC. Resting PBMC from 3 healthy donors were infected with the B95-8 transforming strain of EBV and cultured in the absence or presence of IL-15 (25 or 50 ng/mL) for 7 and 10 days, at which times the percentage of EBV antigen-positive cells was determined by immunofluorescence. By day 7, in cultures without IL-15, EBV infected 10% of the cells (Fig 4). The percentage of EBV-infected cells decreased by more than half to 3% to 5% in the presence of 50 ng/mL IL-15. On day 10 PI, 12% to 14% of the cells were infected with EBV. However, in presence of IL-15, a reduction in the percentage of EBV-infected cells, ranging from 75% to 85%, was observed. As a control experiment, treatment of cultures with 50 ng/mL IL-6 had no effect on the percentage of EBV-infected cells (data not shown). The effects of IL-15 on the chronically EBV-infected B95-8 cell line were also studied. Cells were TPA-activated to induce EBV replication and cultured in the absence or presence of IL-15 (50 ng/mL). EBV gp350 antigen expression was moni- tored by flow cytometry 4 days after TPA activation and were found not to be influenced by IL-15, suggesting that IL-15 does not directly inhibit the EBV replicative cycle (data not shown).
Effects of IL-15 on infection of PBMC by EBV. PBMC from 3 healthy blood donors were infected with EBV and cultured in the absence or presence of 25 to 50 ng/mL rIL-15. On days 7 and 10, the percentages of EBV-infected cells were determined using the ACIF test, with the use of a human reference serum. Results were calculated after counting a minimum of 500 cells. *P < .05.
Effects of IL-15 on infection of PBMC by EBV. PBMC from 3 healthy blood donors were infected with EBV and cultured in the absence or presence of 25 to 50 ng/mL rIL-15. On days 7 and 10, the percentages of EBV-infected cells were determined using the ACIF test, with the use of a human reference serum. Results were calculated after counting a minimum of 500 cells. *P < .05.
Effects of IL-15 on IFN-α and IFN-γ secretion by PBMC in response to viral infections.
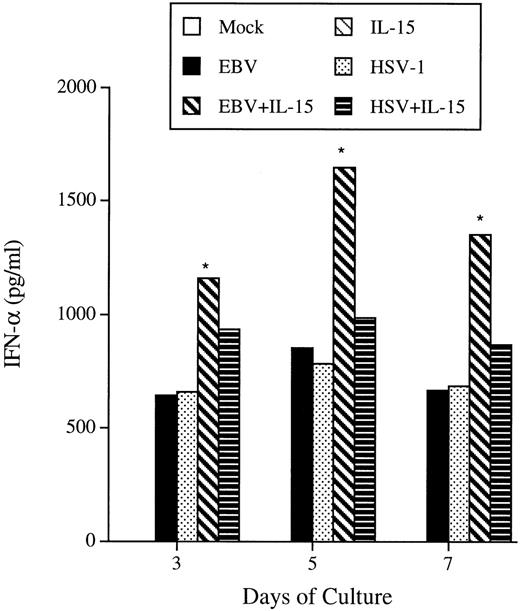
Knowing that many cytokines can modulate the cytolytic activity of NK cells, including IFN, we studied the effects of IL-15 on IFN-α and IFN-γ secretions by PBMC. Our results indicate that IL-15 per se is not able to induce the secretion of IFN-α from PBMC (Fig 5). Viral infection of PBMC by EBV and HSV-1 leads to significant levels of secreted IFN-α on day 3 PI that last up to day 7 PI. A combination of EBV and IL-15 leads to the secretion of more than twice IFN-α into the culture medium.
IFN- production in response to IL-15 and viral infections. PBMC were either treated with rIL-15 (50 ng/mL), EBV, HSV-1, or a combination of virus plus IL-15. On the indicated days, cell-free supernatants were harvested and tested for the presence of IFN- using commercial ELISA kits. Results are representative of 3 donors. *P < .05.
IFN- production in response to IL-15 and viral infections. PBMC were either treated with rIL-15 (50 ng/mL), EBV, HSV-1, or a combination of virus plus IL-15. On the indicated days, cell-free supernatants were harvested and tested for the presence of IFN- using commercial ELISA kits. Results are representative of 3 donors. *P < .05.
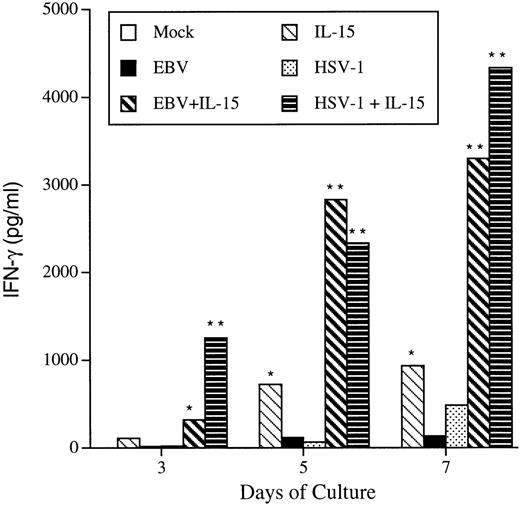
In contrast to IFN-α, IL-15 was able to induce the secretion of IFN-γ from PBMC (Fig 6). IFN-γ could be detected (110 pg/mL) in the culture supernatant of IL-15–treated PBMC on day 3, with increasing IFN-γ levels produced on days 5 (725 pg/mL) and 7 (934 pg/mL). Both EBV and HSV-1 were found to induce low levels of IFN-γ, with maximal secretion observed at day 7 PI. However, combining EBV or HSV-1 with IL-15 lead to a synergistic induction of IFN-γ, reaching levels as high as 4,000 pg/mL (Fig 6). By combining virus and IL-15, IFN-γ is detected to significant levels as early as the first day of culture, with a 3-fold synergistic effect observed later during infection.
IFN-γ production in response to IL-15 and viral infections. PBMC were either treated with rIL-15 (50 ng/mL), EBV, HSV-1, or a combination of virus plus IL-15. On the indicated days, cell-free supernatants were harvested and tested for the presence of IFN-γ using commercial ELISA kits. Results are representative of 3 donors. *P < .05; **P < .02.
IFN-γ production in response to IL-15 and viral infections. PBMC were either treated with rIL-15 (50 ng/mL), EBV, HSV-1, or a combination of virus plus IL-15. On the indicated days, cell-free supernatants were harvested and tested for the presence of IFN-γ using commercial ELISA kits. Results are representative of 3 donors. *P < .05; **P < .02.
Effects of IL-15 on IFN-γ secretion by purified cell populations.
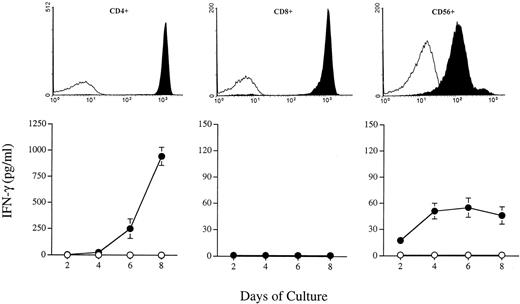
Knowing that IL-15 can induce the synthesis and release of IFN-γ from PBMC, we sought to identify the cell type capable of secreting IFN-γ in response to IL-15 stimulation. Highly enriched (98%) CD4+, CD8+, and CD56+ cells were cultured in absence or presence of IL-15 (50 ng/mL) for 8 days. Supernatants were collected every 2 days, starting on the second day of culture, and were assayed for IFN-γ production. As shown in Fig 7, both CD4+ and CD56+ cells were capable of producing IFN-γ in response to IL-15. During the first 4 days of culture, CD56+ cells produced 3 to 5 times more IL-15 than CD4+ cells. However, at later time points, CD4+ cells were found to produce significantly more IFN-γ (∼15 times more) than CD56+cells whose IFN-γ production appeared to have reached a plateau. In contrast to CD4+ and CD56+, CD8+cells were not found to secrete any detectable IFN-γ in response to IL-15 stimulation.
IFN-γ production by purified cell populations in response to IL-15 stimulation. CD4+ T cells, CD8+ T cells, and CD56+ NK cells were purified from PBMC by negative selection as described in Materials and Methods. The top panels represent the flow cytometry analyses of purified cell population (all 98% pure; black histograms) using anti-CD4 (left panel), anti-CD8 (middle), and anti-CD56 (right) antibodies. The isotype control antibody is represented by the white histograms. The bottom panels represent kinetics of IFN-γ production by corresponding cell population after IL-15 stimulation. Cells were seeded at 106/mL in RPMI medium supplemented or not with IL-15. Cell-free supernatants were collected every 2 days starting on day 2 and were tested by ELISA for IFN-γ production. (○) Mock; (•) 50 ng/mL IL-15. Results are expressed as picograms per milliliter of IFN-γ (mean ± SD of triplicate cultures).
IFN-γ production by purified cell populations in response to IL-15 stimulation. CD4+ T cells, CD8+ T cells, and CD56+ NK cells were purified from PBMC by negative selection as described in Materials and Methods. The top panels represent the flow cytometry analyses of purified cell population (all 98% pure; black histograms) using anti-CD4 (left panel), anti-CD8 (middle), and anti-CD56 (right) antibodies. The isotype control antibody is represented by the white histograms. The bottom panels represent kinetics of IFN-γ production by corresponding cell population after IL-15 stimulation. Cells were seeded at 106/mL in RPMI medium supplemented or not with IL-15. Cell-free supernatants were collected every 2 days starting on day 2 and were tested by ELISA for IFN-γ production. (○) Mock; (•) 50 ng/mL IL-15. Results are expressed as picograms per milliliter of IFN-γ (mean ± SD of triplicate cultures).
Modulation of NK cell killing potential by IL-15.
We have so far shown that IL-15 is able, in presence of NK cells, to limit the viral infection of 3 distinct human herpesviruses. In addition, IL-15 induces the release of IFN-γ from both NK and CD4+ T cells. To clearly establish that it is the increased killing ability of NK cells that is mainly responsible for the antiviral effects of IL-15, we performed an additional experiment. Purified NK cells (98% CD56+) were cultured for 3 days in the absence or presence of IL-15 (10 or 50 ng/mL). NK cells were then mixed with 4 different target cells and tested for their ability to efficiently cause cell lysis (Fig 8). Our results indicate that IL-15–treated cells are much more potent killers than are NK cells that have not been stimulated with IL-15. This increased killing is observed not only with the K562 reference target cells (Fig 8A), but also with an EBV-immortalized lymphoblastoid cell line (Fig 8B) and the HSB-2 T-cell line (Fig 8C). Not surprisingly, HHV-6–infected HSB-2 cells (Fig 8D) are more susceptible to NK cell lysis when compared with uninfected HSB-2 cells. This can be observed for NK cells that were treated or not with IL-15. However, the observation that IL-15 caused a significant increased in lysis of infected cells that is much higher than that of NK cells that were not stimulated with IL-15 is important. Taken together, these results suggest that an increase in killing potential of IL-15–treated NK cells is responsible for the IL-15 antiviral activity.
Increased killing of targets cells by IL-15–stimulated NK cells. Purified CD56+ NK cells (98%) were cultured in the absence (○) or presence of IL-15 ([□] 10 or [•] 50 ng/mL) for 3 days. NK cells were washed with PBS and mixed, in triplicate, with 51Cr-labeled target cells at effector:target ratios varying between 0.1:1 and 10:1. Cells were incubated in V-bottomed wells for 16 hours at 37°C, after which the supernatants (0.1 mL) were analyzed for radioactivity using a γ-counter. Results are expressed as the mean of triplicate cultures ± SD. (A) K562 target cells; (B) EBV-immortalized lymphoblastoid cell line; (C) HSB-2 target cells; (D) HHV-6–infected HSB-2 target cells.
Increased killing of targets cells by IL-15–stimulated NK cells. Purified CD56+ NK cells (98%) were cultured in the absence (○) or presence of IL-15 ([□] 10 or [•] 50 ng/mL) for 3 days. NK cells were washed with PBS and mixed, in triplicate, with 51Cr-labeled target cells at effector:target ratios varying between 0.1:1 and 10:1. Cells were incubated in V-bottomed wells for 16 hours at 37°C, after which the supernatants (0.1 mL) were analyzed for radioactivity using a γ-counter. Results are expressed as the mean of triplicate cultures ± SD. (A) K562 target cells; (B) EBV-immortalized lymphoblastoid cell line; (C) HSB-2 target cells; (D) HHV-6–infected HSB-2 target cells.
DISCUSSION
The control and elimination of viral agents is achieved by both specific and nonspecific defense mechanisms. The specific arm of the immune response includes the generation of antigen-specific antibodies, T-helper cell expansion and recruitment, and activation of CD8+ T lymphocytes capable of lysing infected cells in an MHC class 1-restricted manner. The nonspecific arm includes phagocytes, complement proteins, cytokines, and NK cells. The nonspecific immune mechanisms play a pivotal role in the outcome of an infection, because they represent the first line of defense against invading pathogens. Early cytokine secretion may act directly as antiviral agents, such as the IFNs, tumor necrosis factor-α (TNF-α), and IL-12.38-43 In addition, cytokines such as IFNs and IL-2 can act indirectly by promoting the proliferation and enhancing the cytolytic functions of effector cells such as NK or CTLs.13,14,44-46 In 1994, IL-15, a cytokine having biological properties similar to that of IL-2, was identified.20,22 One such attribute of IL-15 is its ability to generate lymphokine-activated killers (LAK) from NK cells.19-22 IL-15 was also shown to be secreted in response to various intracellular infectious agents and important for proper NK cell activity.27,28,47-49 Furthermore, IL-15 plays an important role in the in vitro survival of NK cells by preventing or delaying apoptosis.50 Knowing this, we became interested in determining whether IL-15 may play a role in the control of a viral infections. The results presented here show that IL-15 can curtail infections by herpesviruses belonging to the α (HSV-1), β (HHV-6), and γ (EBV) herpesvirinae subfamilies. IL-15 does not act as an antiviral agent per se, but rather is dependent on the presence of NK cells for its antiviral effectiveness. In fact, the removal of CD16+/CD56+ NK cells from cultures eliminated the antiviral activity of IL-15. This is further supported by the inability of IL-15 to inhibit infection of purified CD4+ T cells infected by HHV-6. Inhibition of viral infection is IL-15 dose-dependent, with maximal activity in the range of 25 to 50 ng/mL. Furthermore, the time at which IL-15 is added to the cultures is important for maximal activity. If added more than 24 hours after the initiation of the cultures, a dramatic loss of action is noticed. This may represent the inability of NK cells to efficiently eliminate infected cells once infection was allowed to be fully established. In addition, the report that NK cells undergo rapid (within 24 hours) programmed cell death in vitro in the absence of IL-2 or IL-15 may suggest an additional hypothesis for this observation.50
IL-15 treatment of PBMC did not induce the secretion of IFN-α in contradistinction to IFN-γ, which is secreted in response to IL-15. Our results are in agreement with those of others who have reported an induction of IFN-γ secretion after IL-15 stimulation.23,30,50,51 Interestingly, when IL-15 was combined with EBV or HSV-1, the IFN-γ productions were synergistically enhanced by 3- to 4-fold. The observed synergy between virus and IL-15 in IFN-γ production could be the result of autocrine and paracrine stimulation from endogenously produced cytokines such as IL-12. IFN-γ can, in turn, stimulate or prime monocytes/macrophages to secrete IL-1252-54 and TNF-α,55-58 which can further activate NK cells. In fact, both EBV and HSV-1 were reported to induce the secretion of IL-12.59,60 IL-12 is a cytokine previously reported to act synergistically with IL-15 in IFN-γ induction.61 It is likely that many of these cytokines can contribute to boost the NK cells' antiherpesvirus activity. However, surprisingly, neutralization of secreted IFN-α had little impact on the ability of IL-15 to restrict EBV and HHV-6 infection. This is most likely due to the fact that infections took place before IL-15 stimulation and IFN secretion. IFN-α is most effective in establishing an antiviral state in cells that have yet to be infected. In addition, EBV establishes a latent infection and does not produce new viral progeny that would be more susceptible to IFN's action upon infection of new target cells.
Of interest is the fact that IL-15–activated NK cells can control infection by herpesviruses having very different biological properties. HSV-1 has a very wide tropism and kills its target cell rapidly within 1 to 3 days. HHV-6 infects mostly cells of hematopoietic lineage, with an intermediate replication time of 3 to 5 days. Lastly, unlike HSV-1 and HHV-6, EBV has a narrow cell tropism, infecting mainly B lymphocytes and epithelial cells of the oropharynx. Furthermore, EBV-infected B cells are immortalized and can survive indefinitely in vitro. Cells infected with these herpesviruses were shown previously to be efficiently recognized and killed by NK cells.62-77Increasing the cytotoxic potential of NK cells may therefore represent a useful strategy to help combat infections by this group of viruses. Another mean by which IL-15 may promote antiviral activities is through the induction and secretion of molecules having direct antiviral properties. In fact, Oliva et al78 and Fehniger et al79 reported that IL-15–stimulated NK cells, through the secretion of C-C chemokines such as macrophage inflammatory proteins (MIP) and RANTES, were capable of inhibiting HIV-1 infection.
Overall, the results presented indicate that IL-15 can reduce herpesvirus infection through the activation of NK cells resulting in a more efficient killing of the infected cells. Boosting of the innate immune response by IL-15 during primary herpesvirus infections may therefore prove valuable clinically to help reduce viral spread and associated complications. The use of animal models experimentally infected with herpesviruses will enable the testing of the in vivo therapeutic efficacy of IL-15 and determine whether the potential for selected clinical application for this cytokine exists.
ACKNOWLEDGMENT
The authors acknowledge the excellent technical expertise of Suzie Arsenault.
Supported in part by a grant from the Medical Research Council of Canada (MRCC) to L.F. L.F. and J.G. are both scholars from the MRCC.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Louis Flamand, PhD, Laboratory of Virology, Rheumatology and Immunology Research Center Local T1-49, Centre de Recherche du CHUL, 2705 Laurier Blvd, Sainte-Foy, Quebec, Canada G1V 4G2; e-mail: louis.flamand@crchul.ulaval.ca.

![Fig. 8. Increased killing of targets cells by IL-15–stimulated NK cells. Purified CD56+ NK cells (98%) were cultured in the absence (○) or presence of IL-15 ([□] 10 or [•] 50 ng/mL) for 3 days. NK cells were washed with PBS and mixed, in triplicate, with 51Cr-labeled target cells at effector:target ratios varying between 0.1:1 and 10:1. Cells were incubated in V-bottomed wells for 16 hours at 37°C, after which the supernatants (0.1 mL) were analyzed for radioactivity using a γ-counter. Results are expressed as the mean of triplicate cultures ± SD. (A) K562 target cells; (B) EBV-immortalized lymphoblastoid cell line; (C) HSB-2 target cells; (D) HHV-6–infected HSB-2 target cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/12/10.1182_blood.v94.12.4210/5/m_blod42407008x.jpeg?Expires=1765890040&Signature=dc5mGqBAkv06G--BNmfpRHmcduSZCswJGoudX5BmapxQbEhYd~j6RObH3qt2aIGlVjsMsjtYdQ1PfA1GRueE6II~P7~1yUlJpmwVZ~1PVoaZqK2Lcwdeeo~k9UlfiPR6JTRlzYciy3oO7lcr6rONktaUUrEyYj7I30utn1ouG02fOi3xRsTCHhC4tx-6KfGVcANla9rv3bwWXRrrY-sghqR8HA0LF8LXHdPo0p~oLutUKGb87xkqmEW6fQoBrnSLaKNTyzkCPmNCdwOOY-PBpad6OtMcgjWrHZew6tEhuYqqpkJN8brYhFBMUqND0oLljvMHfRpsNb7OOQdi74HcbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal