Abstract
The conformation of the A1 domain of von Willebrand factor (vWF) is a critical determinant of its interaction with the glycoprotein (GP) Ib/V/IX complex. To better define the regulatory mechanisms of vWF A1 domain binding to the GPIb/V/IX complex, we studied vWF-dependent aggregation properties of a cell line overexpressing the GPIb, GPIbβ, and GPIX subunits (CHO-GPIbβ/IX cells). We found that CHO-GPIbβ/IX cell aggregation required the presence of both soluble vWF and ristocetin. Ristocetin-induced CHO-GPIbβ/IX cell aggregation was completely inhibited by the recombinant VCL fragment of vWF that contains the A1 domain. Surprisingly, the substitution of heparin for ristocetin resulted in the formation of CHO-GPIbβ/IX cell aggregates. Using monoclonal antibodies blocking vWF interaction with GPIb/V/IX or mocarhagin, a venom metalloproteinase that removes the amino-terminal fragment of GPIb extending from aa 1 to 282, we demonstrated that both ristocetin- and heparin-induced aggregations involved an interaction between the A1 domain of vWF and the GPIb subunit of the GPIb/V/IX complex. The involvement of heparin in cell aggregation was also demonstrated after treatment of heparin with heparinase that abolished CHO-GPIbβ/IX cell aggregation. These results indicated that heparin was able to induce vWF-dependent CHO-GPIbβ/IX cell aggregation. In conclusion, we demonstrated that heparin is capable of positively modulating the vWF interaction with the GPIb/V/IX complex.
AFTER BLOOD VESSEL injury, von Willebrand factor (vWF) is the only multimeric protein involved in platelet adhesion to the extracellular matrix (ECM) under conditions of high shear stress. vWF is synthesized by platelets and endothelial cells and is found in their storage vesicles, in plasma, and in vascular ECM. Mature subunits of vWF containing 2050 amino acids (aa) are assembled in multimers ranging in molecular weight from 500 kD to more than 10,000 kD. Extremely large multimers of vWF are the most effective ones for promoting platelet adhesion. To initiate platelet adhesion, vWF binds to the glycoprotein (GP) Ibα subunit, which is disulfide-linked to GPIbβ and noncovalently associated with GPIX. The platelet GPIb/V/IX complex also includes GPV noncovalently bound to GPIb-IX.1 Circulating plasma vWF does not interact spontaneously with platelets. Binding to the GPIb/V/IX complex requires conformational changes of the A1 domain of vWF, which can be achieved in vivo by immobilization of vWF onto the endothelial ECM or by high shear stress conditions and in vitro by nonphysiological substances such as ristocetin or botrocetin. Two different types of vWF sequences, the regulatory sites on one hand and the direct binding sites on the other, are involved in binding to GPIbα and are located in the A1 domain. The asymmetric charge distribution of the A1 domain as well as the high level of negative charges on GPIbα itself suggests that exposure of the GPIbα-binding site on vWF is dependent on electrostatic interactions. Ristocetin binds to 2 discontinuous, proline-rich, negatively charged regions (aa 474-488 and 695-708) flanking the disulfide bridge of the A1 domain. Botrocetin, a protein isolated from the venom of the snake Bothrops jararaca, binds to predominantly positively charged sequences in the A1 loop (aa 514-542, 539-553, 569-583, and 629-643).2,3 Interestingly, both inducers may act through different mechanisms.4Binding of ristocetin to vWF may relieve the effect of inhibitory sites responsible for maintaining vWF in an inactive conformation, thus indirectly inducing vWF binding to GPIbα.4,5 In contrast, botrocetin favors direct binding of vWF to GPIbα without a need for relieving inhibitory sites. Additional binding sequences of vWF to GPIbα, involved independently of the inducer, are located in a sequence between aa 596-616.5
Heparin is a glycosaminoglycan with anticoagulant properties related to its ability to accelerate the inhibition of thrombin by antithrombin-III.6 Besides its therapeutic use, heparin and related sulfated glycosaminoglycans may be important in vivo, because they are present in ECM and on cell surfaces. The significance of vWF binding to heparin in physio-pathological conditions remains to be established. Heparin has been reported to inhibit ristocetin-induced platelet agglutination or vWF binding to platelets and to reduce platelet deposition on injured arteries.7-9 However, the major heparin binding sequence on vWF is located within the A1 loop and overlaps a vWF binding site to botrocetin.10-12 Moreover, we have previously reported that a monoclonal antibody (MoAb) 724 to vWF is able to inhibit its binding to heparin and to platelet GPIb/V/IX in the presence of botrocetin.13 Interestingly, binding of MoAb 724 to vWF increased the extent of shear-induced platelet aggregation and of platelet aggregation induced by low ristocetin concentrations, an effect potentially related to a shift of soluble vWF from an inactive conformation to an active one.14
The aim of the present work is to define the regulatory mechanisms controlling the binding of the vWF-A1 domain to the GPIb/V/IX complex and to determine the importance of the A1 domain conformation. To this end, we compared the effect of ristocetin and heparin on the aggregation properties of a stable CHO cell line overexpressing the GPIb/V/IX receptor (CHO-GPIbαβ/IX cells) in the presence of fluid-phase vWF. Our work indicates that heparin binding to vWF may positively modulate the interaction of vWF with GPIbα. This indicates that heparin may participate in conformational changes of the A1 domain that allow optimal vWF binding to the GPIb/V/IX complex.
MATERIALS AND METHODS
Plasma vWF and VCL recombinant fragment.
Human vWF was isolated from plasma high-purity concentrates (provided by the Laboratoire Français du Fractionnement et des Biotechnologies, Les Ulis, France).15 The amount of vWF:Ag was measured by enzyme-linked immunosorbent assay.13Analysis of vWF subunit and its multimeric pattern was performed as described,16 showing a single band of molecular weight (MW) 250 kD by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis under reducing conditions and a whole range of multimers by agarose gel electrophoresis. Molar concentration of vWF was calculated with an MW of the vWF subunit of 250 kD. VCL, a monomeric recombinant vWF fragment extending between aa 504-728, was expressed in Escherichia coli and purified on carboxymethyl-Sepharose17 (kind gift of Dr L. Garfinkel, Biotechnology General, Rehovot, Israel). VCL was reconstituted from its lyophilized form in phosphate-buffered saline (PBS), pH 7.4, with ice-cold sterile water to a concentration of 1.5 mg/mL (60 μmol/L). Analysis of VCL by 3.5% to 20% gradient SDS-polyacrylamide gel electrophoresis showed a single band of 25 kD. Molar concentration of VCL was calculated with an MW of 25 kD.
Radiolabeling of proteins.
VCL or vWF was labeled with Na125I (Amersham, Les Ulis, France) using Iodo-Gen (Pierce Chemical Co, Rockford, IL) as described.18 Specific radioactivity was 0.04 μCi/μg for125I-VCL and between 3 and 5.2 μCi/μg for125I-vWF. Labeled proteins were stored at 4°C and used within 1 week.
Binding of radiolabeled vWF or VCL to platelet GPIb/V/IX or heparin.
Binding of vWF to the GPIb/V/IX receptor was performed using final concentrations of 2 nmol/L (0.5 μg/mL) 125I-vWF, 108 paraformaldehyde-fixed platelets/mL, 1 mg/mL ristocetin (abp, New York, NY), or 0.5 μg/mL purified botrocetin, as previously described.19 Binding of vWF or VCL to unfractionated heparin (from porcine intestinal mucosa; Sigma, La Verpillière, France) immobilized on aminoethyl-agarose beads (Sigma) was performed as reported, using final concentrations of 3% heparin-agarose beads (vol:vol) and 2 nmol/L (0.5 μg/mL)125I-vWF, as reported.20 For competition with unlabeled ligand, vWF or VCL was added to these mixtures. Lower heparin-agarose concentration (0.6%, vol:vol) was used for125I-VCL (1 μg/mL, 40 nmol/L) binding studies. After 1 hour of incubation, duplicate aliquots of the mixtures were centrifuged on 25% sucrose in 20 mmol/L MES-Tris, pH 6, 100 mmol/L NaCl containing 0.5% bovine serum albumin (BSA; Calbiochem, La Jolla, CA) to separate bound from free ligand. Radioactivity was counted in a γ-counter (MultiGamma; Pharmacia, LKB Biotechnology, St Quentin-en-Yvelines, France). The percentage of bound radioactivity was calculated as (bound/[free + bound]) × 100 radioactivity.
Antibodies.
We used MoAbs reported as blocking the interaction of vWF with platelet GPIb/V/IX. We selected 2 MoAbs to vWF: MoAb 328 inhibiting vWF binding to GPIb/V/IX in the presence of ristocetin and shear-induced platelet adhesion or aggregation14,21,22 or MoAb 724, which blocks vWF binding to GPIb/V/IX in the presence of botrocetin, as well as heparin or botrocetin binding to vWF.13,21 We also used MoAbs to GPIb/V/IX, SZ2 (Immunotech, Marseille, France), and 6D1 (kindly provided by Dr B. Coller, Mount Sinai School of Medicine, New York, NY) directed against the amino-terminal 45-kD domain of GPIbα,23,24 as well as MoAb SZ1 to the GPIb/V/IX complex, which binds to a conformation-sensitive epitope on GPIX (provided by Dr C. Ruan, Suzhou Medical College, Suzhou, People's Republic of China).25 These MoAbs were used as purified IgGs at 20 μg/mL. As control, nonimmune purified IgG was tested in comparison with specific antibody.
Cell culture.
Chinese hamster ovary (CHO) cells defective in the dihydrofolate reductase gene (CHOdhfr−; American Type Culture Collection, Rockville, MD) were grown in Iscove's medium (Eurobio, Les Ulis, France) supplemented with 10% fetal calf serum (FCS; Boehringer Mannheim, Meylan, France), 2 mmol/L glutamine, 100 μmol/L hypoxanthine, and 10 μmol/L thymidine (Eurobio). After cell transfection with full-length cDNAs encoding Ibα, Ibβ, and GPIX or Ibβ and GPIX, 2 stable cell lines were selected expressing either the CHO-GPIbαβ/IX (in this study referred to as CHO-GPIbαβ/IX cells) or the GPIbβ/IX complex (CHO-GPIbβ/IX cells).25Both cell lines were cultured in minimum essential medium (αMEM; Eurobio) containing 10% FCS, 2 mmol/L glutamine, and 400 μg/mL G418 (GIBCO, Cergy Pontoise, France). CHO-GPIbβ/IX cells were cultured in the presence of 100 μmol/L methotrexate (Sigma). Cells were detached with 0.5 mmol/L EDTA for 10 minutes at 37°C. Flow cytometry experiments were performed in a FACScan flow cytometer (Becton Dickinson, Le-Pont-de-Claix, France) as previously described26 and confirmed the expected surface expression of GPIbα on CHO-GPIbαβ/IX cell line using MoAb 6D1 and of GPIbβ and GPIX on CHO-GPIbαβ/IX and CHO-GPIbβ/IX cell lines using MoAb SZ1. Binding properties to vWF of CHO-GPIbαβ/IX cells were similar to those of CHO-GPIbαβ/V/IX, another cell line expressing the GPIbαβ/V/IX complex, thus indicating little difference according to the presence of GPV.
Enzymatic treatment.
To confirm the involvement of the GPIbα subunit, enzymatic treatment of CHO-GPIbαβ/IX cells was performed using enzymes with known cleavage sites within the extracellular domain of GPIbα. Mocarhagin, a cobra venom metalloproteinase generating the amino-terminal fragment of GPIbα (aa 1 to 282), was a kind gift of Dr M. C. Berndt (Baker Medical Research Institute, Prahran Victoria, Australia) and was incubated with cells at 10 μg/mL.27 The neutrophil proteinase elastase, generating an amino-terminal fragment of GPIbα extending from aa 1 to 296, was a kind gift of Dr M. Chignard (INSERM U485, Institut Pasteur, Paris, France) and was added to the cells at a concentration of 200 nmol/L, followed by neutralization by eglin C (10 μg/mL).28 Trypsin (50 μg/mL; GIBCO) was added to the cell suspension, followed by a 12.5-fold excess (wt/wt) of soybean trypsin inhibitor (Sigma). Confluent cells were washed with PBS and incubated with the enzymes for 10 minutes at 37°C in PBS, except for mocarhagin, which was incubated in PBS containing 1 mmol/L CaCl2. Cells were washed with PBS before conducting the functional assays.
Aggregation assay.
CHO-GPIbαβ/IX cell aggregation was performed on a table-top rotary shaker according to the method of Dong et al,29 with some modifications. Two hundred microliters of CHO-GPIbαβ/IX cells (106 cells/mL) in PBS containing 1.2 mmol/L CaCl2 was added to 48-well plastic plates (ATGC, Noisy-le-Grand, France) in the presence of purified vWF (0.5 to 40 nmol/L) and either ristocetin (0.2 to 2 mg/mL) or heparin (10 to 250 μg/mL). In some cases, cells were preincubated with inhibitors for 10 minutes before the aggregation assay. In some experiments, heparin was degraded by treatment with 2 U/mL heparinase I (EC 4.2.2.7; Sigma) in 5 mmol/L NaCOOCH3, 1 mmol/L CaCl2, 50 mmol/L Tris, pH 7, for 18 hours at 37°C. Cell aggregation was performed for 20 minutes at room temperature by subjecting the plates to a constant rotary shaking of 6 cycles/sec on a table-top shaker (Rotatest 95220; Bioblock, Illkirch, France). This orbital motion induced mechanical forces resulting in increased bond formation between cells, without exerting any measurable shear stress. Cell aggregation was observed by light microscopy in an inverted microscope (Axiovert 135; Carl Zeiss, Göttingen, Germany) and microphotographs were taken using a Yashica 108 camera (Kyocera, Tokyo, Japan) at a 10-fold magnification. To quantitate cell aggregation, we counted nonaggregated cells defined as singlets, doublets, and triplets. Between 1,000 and 3,500 cells were counted on 5 microphotographs taken on contiguous fields. Results were expressed relative to the number of nonaggregated cells obtained in the absence of vWF (n0). We calculated the percentage of aggregated cells as ([n0 − n]/n0) × 100, where n represents the number of nonaggregated cells in the presence of vWF.
Statistical analysis.
Mean values of percentages of cell aggregation and their standard errors (SEM) were calculated from 3 to 4 experiments performed in duplicate. The statistical significance of differences between means was evaluated using the Student's t-test for paired samples;P values less than .05 were considered significant.
RESULTS
GPIb/V/IX and heparin binding properties of vWF and VCL.
The isolated A1 domain of vWF has been reported to bind GPIb/V/IX both in the absence and presence of modulators. As a model of the vWF-A1 domain, we used the recombinant VCL fragment of vWF that contains the A1 domain. By analyzing its inhibitory effect on 125I-vWF binding to platelet GPIb/V/IX, we confirmed that VCL was a potent inhibitor of ristocetin-induced binding of 125I-vWF to platelet GPIb/V/IX with an IC50 (concentration able to inhibit 50% of binding) of 0.34 μmol/L, in agreement with a reported value of 0.26 μmol/L.17 Interestingly, a 3-fold lower concentration of unlabeled vWF (0.1 μmol/L) was required to inhibit 50% of125I-vWF binding to platelet GPIb/V/IX. Heparin inhibited the ristocetin-induced 125I-vWF binding to platelet GPIb/V/IX with an IC50 of 6 μg/mL, a value in concordance with published data.9 Although the VCL fragment overlaps a heparin-binding domain, few data are available on its heparin binding properties. Figure 1 shows some heparin-binding properties of vWF and VCL. We found that unlabeled vWF inhibited 125I-vWF binding to heparin-agarose beads with an IC50 of 0.6 μmol/L. VCL was an efficient competitor of125I-vWF binding to heparin-agarose with an IC50 of 1.8 μmol/L (Fig 1A). These data showed the need for a 3-fold higher concentration of VCL compared with vWF to inhibit 125I-vWF binding to heparin. Interestingly, inhibition of 125I-VCL binding to heparin-agarose beads by unlabeled vWF or VCL yield IC50 values of 0.8 and 2 μmol/L, respectively, similar to those obtained for inhibition of 125I-vWF binding to heparin (Fig 1B).
Binding of 125I-vWF and 125I-VCL to heparin. Heparin-agarose beads were incubated for 1 hour at 20°C with 125I-vWF (A) or 125I-VCL (B) in the presence of varying concentrations of unlabeled vWF (▪) or VCL (□). The mean ± SEM of percentage of bound radioactivity was calculated as described in Materials and Methods for 3 separate experiments.
Binding of 125I-vWF and 125I-VCL to heparin. Heparin-agarose beads were incubated for 1 hour at 20°C with 125I-vWF (A) or 125I-VCL (B) in the presence of varying concentrations of unlabeled vWF (▪) or VCL (□). The mean ± SEM of percentage of bound radioactivity was calculated as described in Materials and Methods for 3 separate experiments.
vWF-dependent aggregation of CHO-GPIbαβ/IX cells.
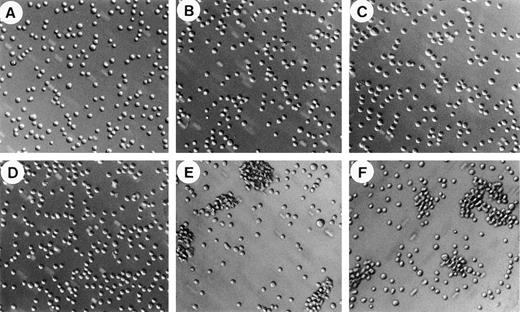
To further characterize the regulatory mechanism of soluble vWF binding to the GPIb/V/IX complex, we studied the effect of vWF on the aggregation of CHO-GPIbαβ/IX cells expressing the GPIbαβ/IX complex. CHO-GPIbαβ/IX cell aggregation was performed essentially as described by Dong et al29 using ristocetin or heparin and a rotary shaker. When shaken at 6 cycles per second, CHO-GPIbαβ/IX cells in suspension were unable to aggregate in the absence of vWF (Fig 2A). Most cells remained nonaggregated as single cells, and only a few doublets or triplets were visible. The addition of vWF was not sufficient to induce CHO-GPIbαβ/IX cell aggregation, as shown by the fact that only isolated cells were present (Fig 2B). In addition, we found that ristocetin by itself was not responsible for cell aggregation in the absence of vWF (Fig 2C). In contrast, the addition of both vWF and ristocetin induced the formation of large cell aggregates, with very few isolated cells remaining (Fig 2E). These cell aggregates had different sizes, including a majority of large aggregates containing 35 to 45 CHO-GPIbαβ/IX cells. Surprisingly, the substitution of heparin for ristocetin also resulted in the formation of large aggregates (Fig 2F). Heparin-induced cell aggregation was characterized by the presence of large aggregates of a similar size as ristocetin-induced aggregates. The aggregatory effect of heparin required the presence of vWF associated with shaking forces, because heparin alone was not able to induce cell aggregation in the absence of vWF (Fig 2D). In addition, in the absence of rotary shaking, neither heparin nor ristocetin was able to induce aggregation of cells incubated with vWF. Thus, both ristocetin and heparin were able to induce vWF-dependent CHO-GPIbαβ/IX cell aggregation.
CHO-GPIbβ/IX cell aggregation. CHO-GPIbβ/IX cells (106 cells/mL) were exposed to rotary shaking at 6 cycles per second for 20 minutes either in the absence of vWF (A, C, and D) or in the presence of vWF (B, E, and F) to which either buffer (A and B), ristocetin (C and E), or heparin (D and F) was added. Microphotographs of cell aggregation were taken at a 10-fold magnification. vWF-dependent cell aggregation was seen in the presence of ristocetin or heparin.
CHO-GPIbβ/IX cell aggregation. CHO-GPIbβ/IX cells (106 cells/mL) were exposed to rotary shaking at 6 cycles per second for 20 minutes either in the absence of vWF (A, C, and D) or in the presence of vWF (B, E, and F) to which either buffer (A and B), ristocetin (C and E), or heparin (D and F) was added. Microphotographs of cell aggregation were taken at a 10-fold magnification. vWF-dependent cell aggregation was seen in the presence of ristocetin or heparin.
Ristocetin-induced aggregation of CHO-GPIbαβ/IX cells.
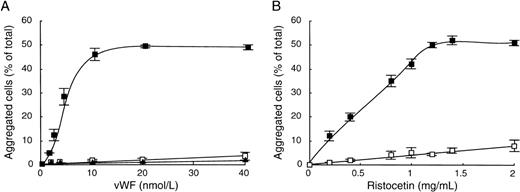
To better understand the similarities between both agonists on vWF-mediated aggregation of CHO-GPIbαβ/IX cells, we first characterized the ristocetin-induced cell aggregation. To this end, we determined dose-response curves of cell aggregation as a function of vWF or ristocetin concentrations. The influence of vWF concentration was studied in the presence of 1.4 mg/mL of ristocetin (Fig 3A). We found that CHO-GPIbαβ/IX cell aggregation increased with the vWF concentration, reaching a plateau at 10 nmol/L of vWF, corresponding to 48.5% ± 0.5% aggregated cells. In contrast, CHO cells expressing the GPIbβ/IX complex and nontransfected cells (CHO dhfr−) did not aggregate in the presence of ristocetin, even at vWF concentrations as high as 40 nmol/L. To determine whether ristocetin had a dose-dependent effect on cell aggregation, CHO-GPIbαβ/IX cells were shaken in the presence of 10 nmol/L vWF and different ristocetin concentrations (Fig3B). Cell aggregation increased with the concentration of ristocetin, tending to a plateau of 52.0% ± 0.6% aggregated cells for 1.4 mg/mL ristocetin. In contrast, in the absence of vWF, hardly any cell aggregation occurred, because it did not exceed 8.4% ± 2.8% aggregated cells at the highest ristocetin concentration (2 mg/mL). These results indicate that cell aggregation depends on vWF and ristocetin and requires the GPIbα subunit.
vWF-dependent ristocetin-induced CHO-GPIbβ/IX cell aggregation. (A) Aggregation of CHO-GPIbβ/IX (▪), CHO-GPIbβ/IX (□), or CHOdhfr− (▴) cells was performed in the presence of various concentrations of vWF (0 to 40 nmol/L) by adding 1.4 mg/mL ristocetin. (B) CHO-GPIbβ/IX cell aggregation was performed in the absence (□) or in the presence (▪) of 10 nmol/L vWF and various concentrations of ristocetin (0 to 2 mg/mL). After 20 minutes of shaking at 6 cycles per second, microphotographs were taken to measure the percentages of aggregated cells relative to the total number of cells. The mean ± SEM of 4 separate experiments was reported. Cell aggregation was dependent on the concentration of vWF and ristocetin and required the GPIb subunit.
vWF-dependent ristocetin-induced CHO-GPIbβ/IX cell aggregation. (A) Aggregation of CHO-GPIbβ/IX (▪), CHO-GPIbβ/IX (□), or CHOdhfr− (▴) cells was performed in the presence of various concentrations of vWF (0 to 40 nmol/L) by adding 1.4 mg/mL ristocetin. (B) CHO-GPIbβ/IX cell aggregation was performed in the absence (□) or in the presence (▪) of 10 nmol/L vWF and various concentrations of ristocetin (0 to 2 mg/mL). After 20 minutes of shaking at 6 cycles per second, microphotographs were taken to measure the percentages of aggregated cells relative to the total number of cells. The mean ± SEM of 4 separate experiments was reported. Cell aggregation was dependent on the concentration of vWF and ristocetin and required the GPIb subunit.
Heparin-induced aggregation of CHO-GPIbαβ/IX cells.
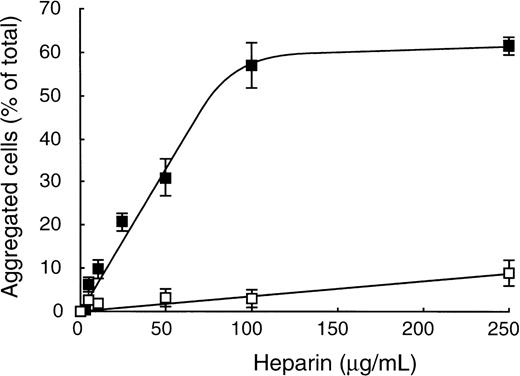
To characterize this new role of heparin as an inducer of vWF binding to GPIbα, we analyzed the influence of heparin concentrations on CHO-GPIbαβ/IX cell aggregation. In the presence of vWF, cell aggregation increased as a function of heparin concentrations, reaching a plateau of 57.0% ± 5.1% aggregated cells at 100 μg/mL heparin (Fig 4). In the absence of vWF and in the presence of increasing doses of heparin, cell aggregation did not exceed 9.1% ± 3.0% at 250 μg/mL heparin. CHO-GPIbαβ/IX cell aggregation specifically involved the GPIbα subunit, because the association of vWF with heparin did not allow the aggregation of CHO-GPIbβ/IX cells or CHO dhfr-cells (data not shown). To confirm the involvement of GPIbα, we performed enzymatic degradation of GPIbα by treatment of CHO-GPIbαβ/IX cells (Table 1). We found that trypsin or elastase treatment of CHO-GPIbαβ/IX cells completely abolished both ristocetin- and heparin-induced cell aggregations (Table 1). The involvement of the vWF-binding domain of GPIbα was further demonstrated by the potent inhibitory effect on cell aggregation of mocarhagin, a cobra venom metalloproteinase that removes an amino-terminal fragment of GPIbα extending from aa 1 to 282 (Table1). Enzymatic treatment of GPIbαβ/IX cells by trypsin, elastase, or mocarhagin was as effective in removing the vWF-binding domain of GPIbα, as shown by the absence of fluorescence staining with MoAb 6D1 or SZ2, whereas untreated CHO-GPIbαβ/IX cells were positively labeled with these MoAbs (data not shown).
vWF-dependent heparin-induced CHO-GPIbβ/IX cell aggregation. CHO-GPIbβ/IX cell aggregation was performed in the presence (▪) or in the absence (□) of 10 nmol/L vWF by adding varying concentrations of heparin (0 to 250 μg/mL) at a rotary shaking of 6 cycles per second. The mean ± SEM of 4 separate experiments is shown. Heparin-induced dose-dependent CHO-GPIbβ/IX cell aggregation in the presence of vWF.
vWF-dependent heparin-induced CHO-GPIbβ/IX cell aggregation. CHO-GPIbβ/IX cell aggregation was performed in the presence (▪) or in the absence (□) of 10 nmol/L vWF by adding varying concentrations of heparin (0 to 250 μg/mL) at a rotary shaking of 6 cycles per second. The mean ± SEM of 4 separate experiments is shown. Heparin-induced dose-dependent CHO-GPIbβ/IX cell aggregation in the presence of vWF.
Inhibition of vWF-Dependent CHO-GPIbβ/IX Cell Aggregation
| Inhibitor . | CHO-GPIbαβ/IX Aggregation (%) . | ||
|---|---|---|---|
| Buffer . | Ristocetin . | Heparin . | |
| None | 2.2 ± 0.5 | 60.3 ± 5.8 | 50.2 ± 4 |
| Trypsin | 2.9 ± 0.9 | 0.9 ± 0.1* | 3.3 ± 1.9* |
| Elastase | 3.5 ± 0.9 | 3 ± 0.5* | 4.3 ± 2.2* |
| Mocarhagin | 2.6 ± 1.0 | 1 ± 1* | 7.5 ± 4.5† |
| Inhibitor . | CHO-GPIbαβ/IX Aggregation (%) . | ||
|---|---|---|---|
| Buffer . | Ristocetin . | Heparin . | |
| None | 2.2 ± 0.5 | 60.3 ± 5.8 | 50.2 ± 4 |
| Trypsin | 2.9 ± 0.9 | 0.9 ± 0.1* | 3.3 ± 1.9* |
| Elastase | 3.5 ± 0.9 | 3 ± 0.5* | 4.3 ± 2.2* |
| Mocarhagin | 2.6 ± 1.0 | 1 ± 1* | 7.5 ± 4.5† |
CHO-GPIbαβ/IX cell aggregation was assessed at 6 cycles per second in the presence of 10 nmol/L vWF and either buffer, 1.4 mg/mL ristocetin or 100 μg/mL heparin. Cells were treated with trypsin (50 μg/mL), elastase (200 nmol/L), or mocarhagin (10 μg/mL). The percentage of aggregated cells was calculated and the mean ± SEM was determined in 3 separate experiments. Statistical significance of differences between means was evaluated using the Student'st-test for paired samples by comparing aggregation in the presence of enzyme versus the control corresponding sample in the absence of enzyme.
P < .0025.
P < .05.
The involvement of heparin in cell aggregation was also demonstrated after treatment of heparin with heparinase. Degradation of heparin significantly abolished vWF-dependent cell aggregation, resulting in 8.0% ± 5.7% aggregated cells, compared with 30.2% ± 1.3% aggregated cells induced by 50 μg/mL heparin (P < .0025). Because vWF-dependent CHO-GPIbαβ/IX cell aggregation has been reported in the absence of ristocetin at a higher rotary shaking frequency of 10 cycles per second,29 we investigated the heparin effect in this condition. We found a 14.4% aggregation in the presence of vWF that was increased 3-fold in the presence of 50 μg/mL heparin, reaching 47.2% aggregated cells. These results indicate the ability of heparin to act as a positive modulator of vWF binding to CHO-GPIbαβ/IX cells.
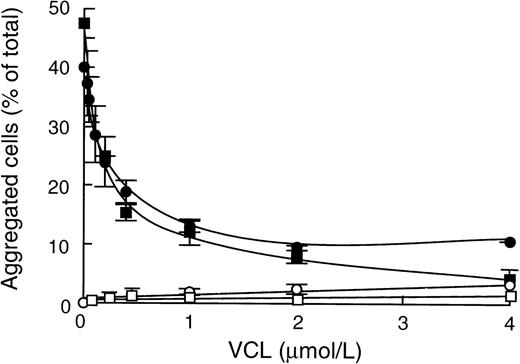
The involvement of the A1 domain of vWF was further established by showing the inhibitory effect of the VCL fragment on ristocetin- or heparin-induced CHO-GPIbαβ/IX cell aggregation (Fig 5). We found that VCL fragment completely blocked ristocetin-induced cell aggregation at 10 μmol/L and that its IC50 was 0.3 μmol/L (Fig 5). As expected, in the presence of ristocetin and in the absence of vWF, VCL was unable to aggregate CHO-GPIbαβ/IX cells due to its monomeric structure. Our results indicate that VCL competes with vWF for binding to GPIbα. Interestingly, significant inhibition of heparin-induced aggregation was observed when VCL was added as a soluble compound, leading to 50% inhibition in the presence of 0.4 μmol/L (Fig 5).
Effect of VCL on vWF-dependent ristocetin- or heparin-induced CHO-GPIbβ/IX cell aggregation. After preincubation of CHO-GPIbβ/IX cells with increasing concentrations of VCL, cell aggregation was performed at 6 cycles per second in the presence of 1.4 mg/mL ristocetin (□, ▪) or in the presence of 50 μg/mL heparin (•, ○). Solid symbols are samples incubated in the presence of 10 nmol/L vWF; open symbols are samples incubated without vWF. The mean ± SEM was obtained from 3 experiments. The VCL fragment competed with vWF for binding to GPIb.
Effect of VCL on vWF-dependent ristocetin- or heparin-induced CHO-GPIbβ/IX cell aggregation. After preincubation of CHO-GPIbβ/IX cells with increasing concentrations of VCL, cell aggregation was performed at 6 cycles per second in the presence of 1.4 mg/mL ristocetin (□, ▪) or in the presence of 50 μg/mL heparin (•, ○). Solid symbols are samples incubated in the presence of 10 nmol/L vWF; open symbols are samples incubated without vWF. The mean ± SEM was obtained from 3 experiments. The VCL fragment competed with vWF for binding to GPIb.
To determine the involvement of the vWF-A1 domain and the GPIbα subunit in both ristocetin- and heparin-induced cell aggregations, we analyzed the effect of MoAbs that block the interaction of vWF with the GPIbα subunit (Table 2). Cell aggregations were performed in the presence of 10 nmol/L vWF and induced by either 1.4 mg/mL ristocetin or 100 μg/mL heparin. In the presence of a control antibody, ristocetin- and heparin-induced cell aggregations reached 50.7% ± 0.9% and 47.8% ± 1.5% aggregated cells, respectively. Compared with control antibody, MoAbs 328 (anti-vWF) and 6D1 (anti-GPIbα) completely inhibited ristocetin-induced cell aggregation, which reached 5% aggregated cells. Furthermore, MoAb 328 or 6D1 inhibited by 80% heparin-induced cell aggregation, as shown by values of 8.5% ± 2.5% and 10.6% ± 2.7% aggregated cells, respectively. Interestingly, whereas MoAb 724 had no inhibitory effect on ristocetin-induced aggregation, it resulted in a 63% reduction of heparin-induced aggregation that reached 17.8% ± 1.6% aggregated cells (Table 2). Our results indicate that both ristocetin- and heparin-induced aggregations involve an interaction between the A1 domain of vWF and the GPIbα subunit of the GPIb/V/IX complex. These results suggest that heparin may act as a positive modulator of the interaction of vWF with the GPIbα subunit.
Effect of MoAbs on vWF-Dependent CHO-GPIbβ/IX Cell Aggregation
| MoAb . | CHO-GPIbαβ/IX Aggregation (%) . | ||
|---|---|---|---|
| Buffer . | Ristocetin . | Heparin . | |
| None | 4.2 ± 0.8 | 51.0 ± 1.0 | 48 ± 4.9 |
| Control antibody | 3.1 ± 0.9 | 50.7 ± 0.9 | 47.8 ± 1.5 |
| 6D1 (anti-GPIbα) | 3.5 ± 1.2 | 5.3 ± 2.0* | 8.5 ± 2.5* |
| 328 (anti-vWF) | 5.8 ± 0.8 | 5.7 ± 3.7* | 10.6 ± 2.7† |
| 724 (anti-vWF) | 7.8 ± 0.8 | 50.3 ± 1.2 | 17.8 ± 1.6† |
| MoAb . | CHO-GPIbαβ/IX Aggregation (%) . | ||
|---|---|---|---|
| Buffer . | Ristocetin . | Heparin . | |
| None | 4.2 ± 0.8 | 51.0 ± 1.0 | 48 ± 4.9 |
| Control antibody | 3.1 ± 0.9 | 50.7 ± 0.9 | 47.8 ± 1.5 |
| 6D1 (anti-GPIbα) | 3.5 ± 1.2 | 5.3 ± 2.0* | 8.5 ± 2.5* |
| 328 (anti-vWF) | 5.8 ± 0.8 | 5.7 ± 3.7* | 10.6 ± 2.7† |
| 724 (anti-vWF) | 7.8 ± 0.8 | 50.3 ± 1.2 | 17.8 ± 1.6† |
CHO-GPIbαβ/IX cell aggregation was assessed at 6 cycles per second in the presence of 10 nmol/L vWF and either buffer, 1.4 mg/mL ristocetin or 100 μg/mL heparin. Cells were either preincubated with 20 μg/mL of control MoAb, 328, 724, or 6D1. The percentage of aggregated cells was calculated and the mean ± SEM was determined in 3 separate experiments. Statistical significance of differences between means was evaluated using the Student's t-test for paired samples by comparing aggregation in the presence of inhibitor versus the control corresponding sample in the absence of inhibitor.
P < .0025.
P < .05.
Interactions between heparin and ristocetin: Effect on cell aggregation.
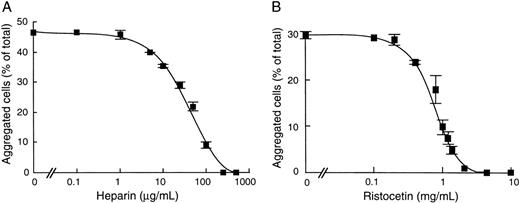
Because ristocetin and heparin are known to bind different domains of vWF,4 10 we investigated whether these compounds could act independently to enhance cell aggregation. To this end, we studied the effect of heparin on ristocetin-induced cell aggregation and conversely of ristocetin on heparin-induced cell aggregation (Fig 6). Our results showed that addition of heparin resulted in a complete inhibition of ristocetin-induced cell aggregation at 250 μg/mL, with a corresponding IC50 value at 50 μg/mL heparin (Fig 6A). Conversely, ristocetin dose-dependently inhibited heparin-induced cell aggregation, with an IC50 of 0.84 mg/mL (Fig 6B). In contrast, in separate assays of 125I-vWF binding to heparin-agarose beads, we found that ristocetin neither inhibited vWF binding to solid-phase heparin nor abrogated the inhibitory effect of soluble heparin as a competitor of vWF binding to solid-phase heparin (Table 3). These results suggest that ristocetin does not prevent heparin binding to vWF and that its inhibitory effect on heparin-induced cell aggregation is not due to a direct neutralization between the positive charges of ristocetin and the negative charges of heparin. Taken together, our results suggest that heparin and ristocetin each induce different active conformations of vWF. However, their combination may result in a conformation that does not favor cell aggregation.
Effect of heparin and ristocetin on vWF-dependent CHO-GPIbβ/IX cell aggregation. After incubation of CHO-GPIbβ/IX cells with increasing concentrations of heparin or ristocetin, cell aggregation was performed at 6 cycles per second in the presence of 10 nmol/L vWF and was induced by 1.4 mg/mL ristocetin (A) or 50 μg/mL heparin (B). The mean ± SEM was performed on 4 separate experiments. Heparin inhibited the ristocetin-induced CHO-GPIbβ/IX cell aggregation and ristocetin inhibited the heparin-induced CHO-GPIbβ/IX cell aggregation.
Effect of heparin and ristocetin on vWF-dependent CHO-GPIbβ/IX cell aggregation. After incubation of CHO-GPIbβ/IX cells with increasing concentrations of heparin or ristocetin, cell aggregation was performed at 6 cycles per second in the presence of 10 nmol/L vWF and was induced by 1.4 mg/mL ristocetin (A) or 50 μg/mL heparin (B). The mean ± SEM was performed on 4 separate experiments. Heparin inhibited the ristocetin-induced CHO-GPIbβ/IX cell aggregation and ristocetin inhibited the heparin-induced CHO-GPIbβ/IX cell aggregation.
Effect of Ristocetin or Heparin on 125I-vWF Binding to Heparin-Agarose Beads
| Competitor . | vWF Bound to Heparin (% of total) . | |
|---|---|---|
| Ristocetin (mg/mL) . | Heparin (μg/mL) . | |
| 0 | 0 | 50.4 |
| 0 | 50 | 5.3 |
| 0 | 100 | 3.1 |
| 0.35 | 0 | 51 |
| 0.7 | 0 | 49 |
| 1.4 | 0 | 49 |
| 1.4 | 50 | 7 |
| 1.4 | 100 | 4.1 |
| Competitor . | vWF Bound to Heparin (% of total) . | |
|---|---|---|
| Ristocetin (mg/mL) . | Heparin (μg/mL) . | |
| 0 | 0 | 50.4 |
| 0 | 50 | 5.3 |
| 0 | 100 | 3.1 |
| 0.35 | 0 | 51 |
| 0.7 | 0 | 49 |
| 1.4 | 0 | 49 |
| 1.4 | 50 | 7 |
| 1.4 | 100 | 4.1 |
125I-vWF binding to heparin-agarose beads was performed in the presence of varying fluid-phase heparin or ristocetin concentrations or of 1.4 mg/mL ristocetin and varying heparin concentrations. The percentage of bound vWF is shown as bound/total radioactivity as described in Materials and Methods.
DISCUSSION
In the present study, we demonstrated a new role of heparin as a promoter of vWF-GPIbα interaction. Using ristocetin as a reference activator of CHO-GPIbαβ/IX cell aggregation in the presence of mechanical forces, we demonstrated that heparin induced an interaction of the A1 domain of vWF with the GPIbα subunit.
Although binding of vWF to heparin is well established, its physiological significance remains completely unknown.8,9The proximity of the heparin- and GPIbα-binding sites within the vWF A1 domain prompted us to investigate the role of heparin in an aggregation assay. To rule out an effect of vWF binding to the activated αIIbβ3 platelet receptor, which can be expressed when platelets undergo even minimal activation during isolation procedures, we performed our study using a stable CHO cell line expressing the GPIbα subunit.25 This CHO-GPIbαβ/IX cell line was previously reported to aggregate in response to mechanical forces and ristocetin or at high shaking frequencies in the absence of ristocetin.29 We have confirmed these findings in both conditions, and, to improve the sensitivity for quantitating a positive modulation of the GPIbα-vWF interaction, we have selected an intermediate shaking frequency of 6 cycles per second. We demonstrated that aggregation was induced by ristocetin in a dose-dependent manner. This interaction required the GPIbα subunit, as indicated by the abrogating effect of MoAb 6D1 or trypsin, thus confirming previous findings.29 We also used GPIbα-specific proteinases such as elastase and mocarhagin to show the involvement of the GPIbα subunit.27,28 Furthermore, we have extended these data by demonstrating the involvement of the vWF A1 domain in ristocetin-induced cell aggregation, because MoAb 328, an anti-vWF that inhibits binding to GPIb in both static and high shear conditions,14,21 was able to completely inhibit cell aggregation. In addition, a recombinant vWF fragment containing the A1 domain (VCL fragment) blocked ristocetin-induced CHO-GPIbαβ/IX cell aggregation with an IC50 of 0.3 μmol/L. These results are in good agreement with the reported 50% inhibition of ristocetin-induced platelet aggregation by 0.24 μmol/L VCL.17
Ristocetin is a modulator of vWF A1 domain binding to platelet GPIb/V/IX and acts by modifying the equilibrium between inhibitory sites and direct GPIbα binding sites.4 5 The inhibitory sites cooperate to maintain an inactive conformation that prevents GPIbα binding to vWF and include 2 acidic regions (497-511 and 687-698) and a basic region (540-578) corresponding to type 2B von Willebrand disease (vWD) mutations that lead to an increased affinity for GPIbα. When bound to vWF, ristocetin may relieve the inhibitory sites and, thus, may be comparable to an endogenous activator that stimulates binding of vWF to GPIbα.
We have recently demonstrated that, upon binding to MoAb 724, soluble vWF undergoes a conformational transition from an inactive state to an active state that becomes sensitive to intermediate shear rates or to low ristocetin concentrations, thus inducing GPIb-dependent platelet aggregation.14 This MoAb has also been described as an inhibitor of vWF binding to heparin.13 Therefore, we investigated whether heparin may act as a positive modulator of vWF interaction with GPIb/V/IX. Our results showed that, in the presence of vWF, heparin can substitute for ristocetin and promote CHO-GPIbαβ/IX cell aggregation. The arguments for a potentiating effect of heparin on the interaction of vWF with GPIbα were the following. Cell aggregation was induced by heparin in a dose-dependent manner. In addition, MoAb 6D1, as well as 3 enzymes involved in the cleavage of the extracellular amino-terminal part of GPIbα (trypsin, mocarhagin, and elastase), completely blocked heparin-induced cell aggregation, clearly establishing the involvement of vWF binding to GPIbα. This was confirmed by the absence of aggregation of control cells lacking the GPIbα subunit. Furthermore, the involvement of heparin on vWF interaction with GPIbα was shown by the abrogation of CHO-GPIbαβ/IX cell aggregation after enzymatic degradation of heparin by heparinase. Altogether, these results demonstrated that heparin reproduced the activating effect of ristocetin on vWF interaction with GPIbα subunit.
Interestingly, we found that anti-vWF MoAbs 328 and 724 directed to the A1 domain were able to inhibit heparin-induced aggregation to a significant extent (by 80% and 60%, respectively). However, this inhibition of heparin-induced aggregation contrasted with the opposite effect of both MoAbs on ristocetin-induced aggregation, which was completely suppressed by MoAb 328, whereas it remained unaffected by MoAb 724. It is likely that the absence of effect of MoAb 724 is related to its inability to prevent the interaction with ristocetin, whereas its effect on heparin-induced aggregation may be simply explained by an inhibition of vWF binding to heparin.13However, the inhibitory effect of MoAb 328 on heparin-induced aggregation was intriguing, because this MoAb is known as an inhibitor of vWF binding to GPIb/V/IX in the presence of ristocetin and of shear-induced platelet adhesion or aggregation, but has no effect on heparin binding to vWF.14,21,22 Our data thus suggested that, in the presence of mechanical forces, heparin could induce CHO-GPIbαβ/IX cell aggregation by increasing the interaction between vWF and GPIbα. This putative activating effect of heparin is further supported by recent data on the crystal structure of the vWF A1 domain, indicating that 2 heparin binding sites are found at the lower surface of a GPIbα-binding groove consisting of 2 adjacent α-helices and a β-sheet.30 31 Interestingly, the type 2B vWD mutations leading to increased GPIb binding are clustered on this lower surface. This suggests that heparin-binding sites are important for the stability of the GPIbα-binding sites.
Our data are in favor of a complex model through which heparin and ristocetin promote vWF-GPIbα interaction by mutually exclusive mechanisms and by binding to distinct sites on vWF. Ristocetin and heparin bind to different domains of the vWF A1 domain, because heparin interacts with aa 565-587 and 642-645.12,32 To investigate whether their simultaneous addition would favor a shift toward a highly active conformation, we studied the effect of heparin on ristocetin-induced cell aggregation and conversely of ristocetin on heparin-induced cell aggregation. Surprisingly, we found that heparin inhibited ristocetin-induced cell aggregation with an IC50 value of 50 μg/mL heparin. This result was previously observed by Sobel et al,8 who showed an IC50 of 24 μg/mL heparin on ristocetin-induced vWF binding to fixed platelets. Interestingly, we found that ristocetin also inhibited heparin-induced cell aggregation. Because both compounds have opposite charges, it was of importance to rule out a direct neutralization of their charges through electrostatic interactions. In the absence of GPIb, no effect of ristocetin was observed on direct vWF binding to heparin-agarose beads. These results suggested that, when added simultaneously, heparin and ristocetin were unable to act in concert so as to induce cell aggregation. Whether ristocetin could sterically hinder the accessibility of heparin and impair its binding to the vWF-GPIb complex remains to be elucidated.
Most in vitro studies have considered heparin as an inhibitor of platelet aggregation.8,9 Our results provide an explanation for the inhibitory effect by heparin on ristocetin-induced platelet aggregation. However, because in vivo no ristocetin is available, one could question the contribution of heparin as an inhibitor of vWF-GPIbα interaction. Because we used heparin concentrations ranging from 10 to 100 μg/mL (0.1 to 1 U/mL) within the plasma concentration range obtained in vivo after therapeutic doses of heparin, we could hypothesize that, in vivo, heparin might have an activating effect on platelet aggregation. Heparin-induced thrombocytopenia (HIT) is one of the most severe side effects of heparin therapy, often associated with thrombocytopenia and thrombosis, and has been attributed to the pathogenic effect of an IgG that activates platelets in the presence of heparin via the complex platelet factor 4-heparin or to the role of some chemokines-binding auto-antibodies.33 Beside HIT, evidence for in vivo platelet activation in the presence of heparin has been recently reported.34 It is far beyond the scope of this in vitro study to determine whether the reported activating effect of heparin on vWF-GPIbα interaction may be involved in the pathogenesis of HIT. In the cellular model that we have used, we have avoided the presence of the αIIbβ3 platelet receptor, which is responsible for stable aggregation, either through binding to fibrinogen in the absence of elevated shear stress or through an interaction with vWF that can be demonstrated under high shear conditions. Therefore, future studies will have to address whether heparin may lead to platelet activation through a vWF-dependent mechanism.
ACKNOWLEDGMENT
The authors thank Dr B. Coller and Dr C. Ruan for providing antibodies, Dr L. Garfinkel for providing the recombinant VCL fragment, Dr M.C. Berndt for the gift of mocarhagin, and Dr M. Chignard for elastase. Dr D. Pidard is thanked for critical reading of the manuscript.
Supported by EC Biomed2 BMH4-CT-98-3517 (D.B.), SANOFI and Fondation pour la Recherche Médicale fellowship (C.P.), an INSERM grant (N.A.), and a Ministere de la Recherche et de la Technologie grant (G.R.L.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Dominique Baruch, MD, PhD, INSERM U143, Hopital de Bicetre, 84 rue du Général Leclerc, 94276 Bicetre, Cedex, France; e-mail: baruch@infobiogen.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal