Abstract
We conducted a long-term prospective study of 89 cancer survivor children who had acquired hepatitis B virus (HBV) and/or hepatitis C virus (HCV) during treatment for neoplasia, the aim being to evaluate the natural history of the diseases and the effect of interferon (IFN) treatment. Patients were followed up for a median period of 13 years (range, 8 to 20); 46 were infected by HBV, 11 by HCV, and 32 coinfected by HBV and HCV. A spontaneous clearance of hepatitis B surface antigen (HBsAg) occurred more frequently in coinfected patients (19%) than in the HBV-infected (2%; P = .004), with an annual seroconversion rate of 2.1% and 0.2%, respectively (P= .008). Loss of hepatitis Be antigen (HBeAg) occurred in 44% of coinfected and in 28% of HBV-infected patients. Clearance of serum HCV-RNA was observed in 34% and 9%, respectively, of coinfected and HCV-infected patients. Seventeen HBV-infected, 4 HCV-infected, and 16 coinfected patients received -IFN treatment. In the HBV group, 6 patients (35%) cleared serum HBV DNA and seroconverted to anti-HBe; in the HCV-group, none cleared HCV-RNA. In the coinfected group, 1 patient cleared both HBV DNA and HCV-RNA, 6 patients cleared serum HCV-RNA alone, and 1 only HBV DNA and HBeAg. Overall, the diseases showed a mild histological course with no evidence of liver cirrhosis. A reciprocal interference on viral replication between HBV and HCV may occur in coinfected patients. Treatment seems to be effective for selected cases and is justified in view of the uncertain prognosis of the disease in these patients.
INFECTIONS BY HEPATITIS B virus (HBV) or hepatitis C virus (HCV) acquired during infancy are usually characterized by persistent viremia and a chronic mild disease,1,2 although cases of childhood cirrhosis and hepatocellular carcinoma (HCC) have been reported.1,3 4During adolescence and even in adulthood, unsuccessful attempts by the host to achieve viral immunoclearance (mainly in HBV patients) and/or the high mutation rate of the viruses (mainly HCV) may lead to a sustained necro-inflammatory activity and to progression to more severe forms of liver disease.
In recent decades and, in particular, before the availability of screening tests for HCV, children with oncological diseases were at risk of acquiring the hepatitis viruses either as a single or as a multiple infection. These viruses were acquired as a consequence of transfusions of blood and blood products, of the direct exposure to contaminated sanitary equipment, or to HBV or HCV carriers in the hospital or the family environment at a time when the immune system was markedly suppressed. Long-term studies of HBV or HCV infection in these patients indicate the presence of a slowly progressive chronic liver disease,5-7 but very little is known about patients with HBV-HCV coinfection. In adults, coinfection appears to be more severe than each infection alone.8
At present, α-interferon (IFN) is the only available treatment for children with chronic HBV or HCV hepatitis. In HBV-infected white children, the response rate to IFN is similar to that observed in adults,9-11 whereas in HCV-infected children, the response rate has not yet been clearly established because it varies in relation to viral and host factors.12,13 Very little is known about the efficacy of IFN treatment of chronic hepatitis acquired during a previous oncological disease.5 14
Presently, in a prospective study, we are following up a group of patients who were treated for malignancy during childood at the Pediatric Oncologic Service, 2nd University of Naples, from 1978 to 1993, and were infected during this period by HBV and/or HCV. In this report, we present the biochemical, virological, and histological events of the infections during a long-term follow-up and later the effects of IFN treatment.
PATIENTS AND METHODS
Study population.
Included in the study were consecutive children diagnosed and treated for either leukemia/lymphoma or solid tumors who during treatment showed an increase in serum aminotrasferases or the presence of hepatitis B surface antigen (HBsAg) in serum. After 1990, all patients were also tested for serum anti-HCV (anti-HCV) antibodies, and those with a positive test were also included in the study. The exclusion criteria from the study were the elevation of aminotrasferases and/or positive HBsAg or, after 1990, anti-HCV in the serum at the time of diagnosis of malignancy. For patients who acquired the neoplasia before 1990, we were unable to establish whether HCV infection was present before malignancy. However, all HCV-infected patients had normal alanine transaminase (ALT) at the time they started chemotherapy and had had no obvious exposure to a potential source of HCV infection.
Both during and after chemotherapy, liver function tests were evaluated at 1 to 3 monthly intervals, as necessary, and serum viral markers every 3 to 6 months.
After 1993, the patients were fully reevaluated for the progression of liver disease and possible treatment. At this time, these patients underwent a complete physical, biochemical, and virological examination that included the determination of serum liver enzymes, markers of HBV, HCV, hepatitis delta virus (HDV), and human immunodeficiency virus (HIV), and, where indicated, serum HBV DNA and HCV-RNA. From this time point, sera were stored at –70°C for subsequent determinations. Quantitative serum HCV-RNA and HCV genotype were determined in all HCV-RNA positive patients. All patients were also evaluated for thyroid function and antinuclear, antimitochondrial, antismooth muscle, and antiliver kidney microsome autoantibodies, as well as serum ceruloplasmin, α1-antitrypsin, and ferritin. Serum liver enzymes were routinely assessed every 3 months and markers of HBV replication (hepatitis Be antigen [HBeAg], HBV DNA) every 6 months, while qualitative and quantitative serum HCV-RNA were tested every year. Liver and spleen doppler-ultrasound examination and serum α-fetoprotein assay were performed every year.
Histological evaluation.
All patients with elevated ALT (>1.5 upper normal levels [UNL] in the preceding 6 months) and active viral replication or with dual infection were considered for liver biopsy. The pathological assessment was performed on sections from formalin-fixed and paraffin-embedded liver biopsy stained with hematoxylin-eosin, trichrome, and Prussian blue reaction. Histological features were scored according to the Knodell Histological Activity Index (HAI) and the Scheuer fibrosis score. We considered scores for necro-inflammatory changes (grading) separately from architectural alterations (staging).15
Treatment.
According to the current predictive criteria of a favorable response to IFN,16,17 we decided to initiate treatment only in patients with HBV or HCV replication and persistently elevated ALT in the last 6 months. Coinfected patients with replication of both viruses were also treated. After the acquisition of informed consent, patients with HBV infection or with dual HBV-HCV infection received α-2a IFN (Roferon; Roche, Basel, Switzerland), 5 megaunits (MU)/m2 3 times weekly for 12 months, while those with HCV infection alone received 3 MU/m2 3 times weekly for 12 months. None of these patients had been treated before with IFN. Treatment was discontinued after 6 months for nonresponder patients.
Response to treatment in the HBV group was defined on the basis of serum HBV DNA and HBeAg clearance at the end of treatment and seroconversion to the hepatitis B e antibody (anti-HBe) and ALT normalization (ALT < 1.5 × UNL) within an additional 12 months. In the HCV group, response was defined on the basis of a sustained serum HCV-RNA negative test and ALT normalization at the end of therapy and 12 months after suspension. In the HBV-HCV group, we considered the anti-HBV and anti-HCV response separately. An increase in serum ALT and return to a viremic status of either HBV (HBeAg and HBV DNA positive) and/or HCV (HCV-RNA positive) was defined as a relapse after the response to treatment.
Treated patients underwent clinical examination and routine laboratory tests twice in the first month of treatment and every month until 6 months after the end of therapy and subsequently every 3 months. In patients with HBV infection, markers of viral replication were tested at months 2 and 6 of therapy and every 6 months thereafter. In patients with HCV infection, viremia was studied before and at 6 and 12 months of treatment and then every year. In untreated patients, biochemical tests were performed every 3 months and virological determination every 6 to 12 months.
Virological markers.
HBsAg, HBeAg, anti-HBe, antidelta, and anti-HIV were tested in serum by commercially available immunoenzymatic assays (EIA; Abbott Laboratories, North Chicago, IL), anti-HCV by enzyme-linked immunosorbent assay (ELISA) II and III generation tests (Ortho Diagnostic System, Raritan, NJ). HBV DNA was assayed by liquid phase hybridization (HBV DNA test, Abbott Laboratories); the sensitivity of the test was 2 pg/mL. Anti-HCV were confirmed by Western blot (CHIRON RIBA HCV 3.0; Chiron Co, Emeryville, CA). HCV-RNA was detected by qualitative (PCR Hepatest C, ViennaLab, Vienna, Austria) and quantitative b-DNA (Quantiplex, version 2, Chiron Co) tests. HCV genotypes were determined by reverse hybridization line probe assay (INNO LIPA HCV assay, 2nd generation; Innogenetics, Zwijnaarde, Belgium).
Statistical analysis.
Statistical analysis of nonparametric data was performed using the Fisher's exact test. Differences in viremia levels among the patient groups were compared with the Mann-Whitney U test. Spontaneous seroconversion rates to anti-HBe and anti-HBs were calculated and the Kaplan-Meier life table method and Wilcoxon's and log rank sum tests were used to compare the curves.18
RESULTS
According to the inclusion criteria, 89 patients from a total of 324 (27%) treated for malignancy acquired HBV and/or HCV infections during treatment, 50 of whom were treated for solid tumor and 39 for leukemia or lymphoma.
The median age of the patients when they entered the study was 4 years (range, 1 to 16). On the basis of viral markers, patients were grouped retrospectively as follows: 46 infected by HBV alone (HBV group), 11 by HCV alone (HCV group), and 32 by HBV and HCV (HBV-HCV group). None of these patients were infected by HDV or HIV.
During chemotherapy, 41%, 45%, and 81% of patients of HBV, HCV, and HBV-HCV groups, respectively, showed biochemical evidence of acute hepatitis. No patient developed signs of acute liver failure. The data of patients on stopping chemotherapy are reported in Table 1.
Data of the 89 Patients at Stopping Chemotherapy
| . | HBV Group . | HCV Group . | HBV-HCV Group . |
|---|---|---|---|
| No. of patients | 46 | 11 | 32 |
| Male/female | 20/26 | 4/7 | 18/14 |
| Median age; yr (range) | 6 (1-13) | 7 (3-16) | 7 (3-14) |
| Patients with elevated ALT* | 20 | 7 | 23 |
| Median ALT × UNL (range) | 2.4 (1.5-6.8) | 2.0 (1.5-21) | 3.0 (1.8-9.2) |
| Transfusion/surgery† | 26/11 | 10/1 | 28/2 |
| . | HBV Group . | HCV Group . | HBV-HCV Group . |
|---|---|---|---|
| No. of patients | 46 | 11 | 32 |
| Male/female | 20/26 | 4/7 | 18/14 |
| Median age; yr (range) | 6 (1-13) | 7 (3-16) | 7 (3-14) |
| Patients with elevated ALT* | 20 | 7 | 23 |
| Median ALT × UNL (range) | 2.4 (1.5-6.8) | 2.0 (1.5-21) | 3.0 (1.8-9.2) |
| Transfusion/surgery† | 26/11 | 10/1 | 28/2 |
ALT ≥ 1.5 × upper normal limit.
Number of patients who received transfusion and/or surgery in each group. In 9 patients of HBV group and in 2 of the HBV-HCV group, no evidence of sources of infection was found.
During a median follow-up of 13 years (range, 8 to 20) after stopping chemotherapy, all patients remained asymptomatic with normal serum bilirubin, albumin, and prothrombin times; all of them had normal thyroid function tests and no evidence of autoimmunity or other possible causes of chronic liver disease.
Analysis of HBV-infected patients.
After chemotherapy, all 46 patients were HBeAg positive, 26 with normal and 20 with elevated ALT (Table 1).
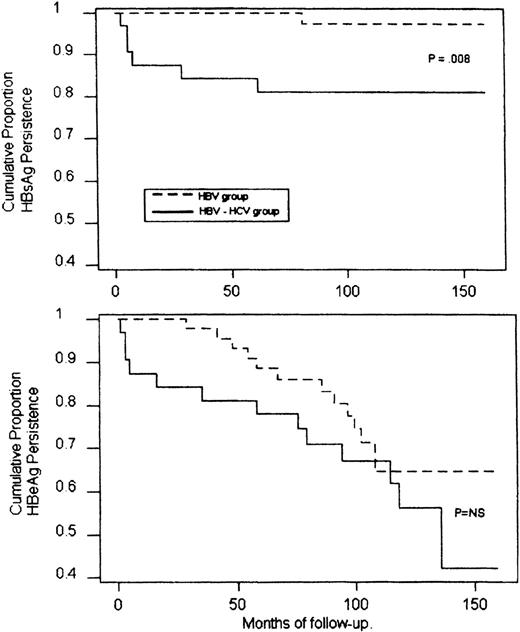
During a median follow-up period of 12 years (range, 9 to 20), 13 patients (28%) cleared HBeAg spontaneously and showed a stable seroconversion to anti-HBe (Fig 1). This event occurred within 3 to 9 years (median, 6) after finishing chemotherapy and was heralded by a transient and occasionally marked elevation of ALT. One of these patients also seroconverted to anti-HBs 7 years after HBeAg clearance. In this group, the calculated mean annual rate of seroconversion to anti-HBe was 3.22% and to anti-HBs, 0.22%. An analysis of the conversion rates according to the Kaplan-Meier life table is reported in Fig2.
Cumulative probability of HBsAg (upper panel) and HBeAg (lower panel) persistance in HBV-infected or HBV-HCV coinfected patients during the follow-up (Kaplan-Meier method and log rank test). NS, not significant.
Cumulative probability of HBsAg (upper panel) and HBeAg (lower panel) persistance in HBV-infected or HBV-HCV coinfected patients during the follow-up (Kaplan-Meier method and log rank test). NS, not significant.
Thirty-three patients showed active viral replication during the follow-up (Fig 1). Patients with persistently normal ALT had a median baseline serum HBV DNA of 273 pg/mL (range, 99 to 560) and those with elevated ALT, 100 pg/mL (range, 5 to 405). All patients with elevated ALT underwent liver biopsy. The median interval between end of chemotherapy and liver biopsy was 9 years (range, 4 to 17). The histological data are presented in Table 2. Fifteen patients showed minimal changes, 6 mild chronic hepatitis, and 1 severe chronic hepatitis.
Histological Data of Patients According to the Type of Infection
| . | HBV Infection* (n = 22) . | HCV Infection* (n = 5) . | HBV-HCV Infected* (n = 23) . | |||
|---|---|---|---|---|---|---|
| HBV DNA+ HCV-RNA− (n = 2) . | HBV DNA− HCV-RNA+ (n = 6) . | HBV DNA+ HCV-RNA+ (n = 13) . | HBV DNA− HCV-RNA− (n = 2) . | |||
| Median HAI† score (range) | 2 (0-10) | 2 (1-4) | 0, 3‡ | 5 (1-6) | 2 (0-8) | 1, 3‡ |
| Median fibrosis score (range) | 1 (0-1) | 1 (0-1) | 0, 1‡ | 1 (0-3) | 1 (0-1) | 1, 1‡ |
| Median steatosis score (range) | 0 (0-1) | 1 (0-3) | 1, 1‡ | 1 (0-1) | 0 (0-2) | 0, 0‡ |
| . | HBV Infection* (n = 22) . | HCV Infection* (n = 5) . | HBV-HCV Infected* (n = 23) . | |||
|---|---|---|---|---|---|---|
| HBV DNA+ HCV-RNA− (n = 2) . | HBV DNA− HCV-RNA+ (n = 6) . | HBV DNA+ HCV-RNA+ (n = 13) . | HBV DNA− HCV-RNA− (n = 2) . | |||
| Median HAI† score (range) | 2 (0-10) | 2 (1-4) | 0, 3‡ | 5 (1-6) | 2 (0-8) | 1, 3‡ |
| Median fibrosis score (range) | 1 (0-1) | 1 (0-1) | 0, 1‡ | 1 (0-3) | 1 (0-1) | 1, 1‡ |
| Median steatosis score (range) | 0 (0-1) | 1 (0-3) | 1, 1‡ | 1 (0-1) | 0 (0-2) | 0, 0‡ |
Median interval between end of chemotherapy and liver biopsy was: HBV group, 9 years (range, 4 to 17); HCV group, 13 years (range, 4 to 20); HBV-HCV group, 9 years (range, 3 to 14).
Histological activity index.
Score of each patient.
Of the 22 patients with a histological evaluation, 17 (9 males and 8 females, median age, 15; range, 7 to 24) were treated with α-2a IFN. Response to treatment is presented in Fig 1. Interestingly, the pretreatment median serum HBV DNA level of the 7 responder patients was 25 pg/mL (range, 5 to 138), whereas that of nonresponders was 356 pg/mL (range, 84 to 400; P = .002). Of the responder patients, 6 cleared HBV DNA and seroconverted to anti-HBe within the treatment period, and 1 seroconverted to anti-HBe within 12 months after suspending treatment. Anti-HBe seroconversion was associated with a normalization of ALT. One patient had a relapse after the initial response to IFN.
Analysis of HCV-infected patients.
Eleven patients were found to be serum anti-HCV positive after chemotherapy. No serum was available for HCV-RNA detection at the time they discontinued chemotherapy. At the end of therapy, 4 patients had normal ALT and 7 elevated ALT (Fig 3).
During the follow-up (median, 14 years; range, 9 to 20), 1 of the 4 patients with normal ALT showed constantly negative serum HCV-RNA over a 9-year period (Fig 3). The other 3 patients had positive serum HCV-RNA.
The 7 patients with elevated off-therapy ALT were serum HCV-RNA positive during follow-up. Two showed a spontaneous biochemical remission over a period of 18 to 24 months, and 5 had persistent biochemical activity. In the 10 patients with positive serum HCV-RNA, the viral load ranged between < 0.2 to 0.7 × 106Eq/mL (median, 0.34 × 106). No differences in the viremia levels were observed between patients with normal and elevated ALT. The HCV genotypes were analyzed in all HCV-RNA positive patients, and the following distribution was observed: 1b, 6 patients; 2a/c, 2 patients; 3a, 1 patient; mixed 2a+1b, 1 patient.
A liver biopsy was performed for 4 of the 5 patients with biochemical activity (for the fifth, both biopsy and IFN treatment were contraindicated because of a psychiatric disorder) and in 1 with persistently normal aminotransferases after a median follow-up period of 13 years (range, 4 to 20) after stopping chemotherapy. Two patients showed mild chronic hepatitis and 3 minimal changes (1 of the latter was the patient with no biochemical activity). A median fibrosis score of 1 was observed in all patients; the steatosis score ranged from 0 to 3 (Table 2).
The four patients with elevated ALT (all females, aged from 7 to 40 years) received IFN treatment. Of these, only 1 patient (genotype 1b, baseline viral load 0.2 × 106 Eq/mL) showed an end-treatment response, but relapsed shortly after stopping IFN.
Analysis of HBV-HCV–infected patients.
During chemotherapy, 32 patients proved to be infected by HBV and were subsequently found to be also anti-HCV positive. Of the 26 patients who presented evidence of acute hepatitis, 14 (54%) showed more than 1 peak of ALT elevation, which occurred at least 6 months apart. In this period, 1 patient spontaneously cleared HBV infection and seroconverted to anti-HBs. He normalized ALT and showed constantly negative serum HCV-RNA during the entire follow-up.
During a median follow-up period of 14 years (range, 8 to 20), another 5 patients spontaneously seroconverted to anti-HBe and 1 also to anti-HBs; they also cleared serum HCV-RNA and normalized ALT (Fig 4). In addition, 8 patients cleared HBeAg, but maintained HCV-RNA in the serum (4 of them also cleared HBV infection and seroconverted to anti-HBs); 5 patients cleared HCV viremia, but showed persistent HBV replication, and 13 still showed active replication of both viruses (Fig 4).
Follow-up events and effect of IFN treatment in HBV-HCV–infected patients.
Overall, a spontaneous recovery from HBV infection occurred in 19% of coinfected patients versus 2% observed in the HBV-infected group (P = . 04), the annual rate of HBsAg seroconversion being 2.1% and 0.2%, respectively (P = .008; Fig 2). A spontaneous clearance of HBV DNA with seroconversion to anti-HBe occurred in 44% of coinfected patients at an annual rate of 5.1%. This prevalence, although higher, did not differ significantly from those observed in the HBV group (Fig 2).
In HBV viremic patients, serum HBV DNA ranged from 5 to 470 pg/mL (median, 129). Clearance of HBV DNA occurred between 3 months and 8 years after stopping chemotherapy. In the patients who cleared HBV infection, anti-HBs seroconversion occurred 1 to 2 years after anti-HBe seroconversion.
The distribution of HCV genotypes in HCV-RNA positive patients was as follows: 1b, 12 patients; 2a/c, 2 patients; 3a, 3 patients; mixed, 4 (1b+3a, 3 patients and 1a+1b, 1 patient) and serum HCV-RNA ranged from <0.2 to 20 × 106 Eq/mL (median, 1.5 × 106). Overall, clearance of HCV-RNA occurred in 34% of coinfected patients versus 9% of patients in the HCV-infected group (P = .26)
Liver biopsy was performed in all 13 patients who presented replication of both viruses, in 2 of the 5 with HBV replication alone, in 6 of the 8 with HCV replication alone, and in 2 of the subjects who had cleared both viruses. The median interval between stopping chemotherapy and the liver biopsy was 9 years (range, 3 to 14). The histological findings of these 4 groups are reported in Table 2.
IFN therapy was administered to 12 of the 13 patients with replication of both viruses, to 3 of the 8 with replication of HCV alone, and to 1 of the 5 with replication of HBV alone. At the end of treatment (Fig 4) of the 12 treated patients with replication of both viruses, 1 showed an anti-HBV and anti-HCV response that persisted 1 year later. Another 4 patients had a sustained anti-HCV response, whereas the HBV DNA serum levels were unaffected by treatment, the mean levels being 241 pg/mL before and 284 pg/mL 12 months after treatment. In these 4 patients, ALT was constantly less than 1.5 × UNL. A sixth patient had an anti-HBV, but not anti-HCV response and showed ALT normalization. The serum HCV-RNA level was < 0.2 × 106 Eq/mL before treatment and 0.3 × 106 after the clearance of HBeAg.
Furthermore, 2 of the 3 treated patients with HCV replication alone cleared HCV-RNA and normalized ALT. The response to treatment was sustained at 1 year follow-up. The 1 treated patient with HBV replication alone showed no response to therapy (Fig 4).
The overall anti-HCV response was 47%. The responder patients had the following HCV genotypes: 1b, 3 patients; 3a, 2 patients, mixed (1a+1b and 1b+3a), 2 patients; their basal HCV viremia ranged from < 0.2 to 6.4 × 106 Eq/mL; median, 0.2 × 106Eq/mL.
Side effects of IFN therapy.
Treatment was, in general, well-tolerated by the patients. A transient influenza-like syndrome was observed in all treated children during the first weeks of treatment. Only 1 patient, an 8-year old girl, had to interrupt treatment after 5 months because of an episode of a febrile convulsion. She had had a biochemical remission, but was still viremic at the discontinuation of treatment. No patients showed evidence of autoimmune disorders during treatment with IFN.
DISCUSSION
The acquisition of hepatitis viral infection by children treated for a neoplastic disease represents a worrisome complication both for the possible sequelae later in life and for the psychological implications for the patients and their families, who are no sooner rid of a life-threatening condition, than they find themselves facing a new and potentially severe chronic illness. In our study population of children with oncologic disease, the rate of HBV infection during chemotherapy was 24% and that of HCV infection was 13%, although some HCV patients who had cleared serum HCV RNA during chemotherapy and did not develop elevated ALT or serum antibodies to HCV might have been missed.6 The prevalence of HCV infection in our series is similar to those reported by others,6,7 whereas that of HBV is higher, which presumably reflects the high endemicity of HBV infection in Southern Italy.19 It appeared that most HCV infections were acquired via transfusion of blood or blood products, whereas HBV infection was also acquired through other routes.
In recent published studies of children with a previous neoplasia who had been infected by HBV and/or HCV,5-7 the disease showed a benign course with no evidence of decompensation or of HCC over a period of at least 10 years. However, despite the mild course of the disease, histological evidence of cirrhosis was observed in 21% of patients with HBV and HCV coinfection.7 In patients without oncologic disease, HCV-HBV coinfection may be associated with a more severe course of liver disease20,21 and to a higher risk of fulminant hepatitis22,23 than the single HBV or HCV infections. Furthermore, HBV integration in the host genome at an early stage may predispose to the development of HCC.24 Thus, the risk of HCC is higher in patients infected early on in life and is even more elevated in patients with dual HBV-HCV infections.25
Our study showed variations in both the clinical presentation and the course of HBV and/or HCV hepatitis when these viruses were acquired during chemotherapy. In a substantial number of patients, the infection showed no evidence of an acute disease and no subfulminant events occurred. In the patients with a dual infection, the interval between the 2 peaks of ALT elevation, when present, suggested sequential infection by the 2 agents, rather than coinfection.
In accordance with other studies,5-7 we observed during a long-term follow-up that for the majority of our patients, even those with dual viremia, the disease ran a mild chronic course. None of the patients developed severe fibrosis or cirrhosis during the follow-up. The degree of histological lesions observed in our patients was similar to those reported by others in HBV- and/or HCV-infected children without oncologic disease.2 26
All of our cancer survivor children who acquired HBV alone appeared unable to muster an effective antiviral immune response to HBV and showed tolerance to the virus. In these subjects, the course of HBV infection after stopping chemotherapy seemed to identify 2 different cohorts of patients. The first showed a progressive restoration of the immune response and spontaneously cleared HBV viremia (HBeAg clearance rate, 28%). One of these patients also cleared the infection (HBsAg clearance rate, 2%). In white children without oncologic disease with chronic hepatitis B observed for a similar period of time, the reported prevalence of HBeAg and HBsAg clearances is 84% and 3%, respectively.2 The difference in the HBeAg seroconversion most likely reflects the state of immunotolerance of our patients to HBV.
The second group of patients (72%) showed a persistent infection with high viremia and a low histological and biochemical activity. The latter features are associated with a low rate of spontaneous seroconversion and arrest of viral replication.16
The long-term evaluation of pretreatment data seems to be important so as to select patients with predictive factors of favorable response to IFN. Indeed, if we consider all patients with HBV replication on the basis of an intention-to-treat analysis, the therapeutic efficacy of IFN treatment is 18%, whereas by optimizing the selection of patients to treat,16 the observed response rate to IFN is 35%. A similar prevalence has been reported in nonselected, white children without oncologic disease.10,11,16 In addition to pretreatment ALT, the baseline HBV DNA level has been confirmed as a predictive factor of response to IFN.10 16
The acquisition of HCV infection by children with oncologic disease during chemotherapy resulted in a HCV carrier state with minimal or mild chronic histological damage. Approximately one third of these patients showed no biochemical injury, but spontaneous recovery from the infection was a rare event. The HCV genotype determination in both HCV and HBV-HCV–infected patients showed a prevalence of type 1b, reflecting the general distribution in our geographical area.27 The low grade of histological injury was correlated to the low levels of HCV viremia. No differences were found in the genotype distribution or the HCV viral load between patients with normal versus elevated ALT. None of the patients in this group responded to IFN; the small number of patients treated does not, however, allow any therapeutic considerations to be made. In HCV-infected children without oncologic disease, the response to IFN has been reported between 25% to 43%.12,13 The intrinsic HCV genome resistance coupled with the immunotolerant state of our patients might explain not only the mild form of hepatic disease, but also the lack of response to IFN.
One of the most interesting aspects of our study emerges from the observations of the HBV-HCV coinfected patients who showed a higher number of spontaneous or IFN-induced virological events compared with the groups of patients infected by either virus alone. This suggests that mutual interference may occur between HBV and HCV.28
During chemotherapy, 1 patient apparently recovered from both HBV and HCV infections. Moreover, after the discontinuation of chemotherapy, a spontaneous recovery from HBV infection was significantly higher in coinfected patients than in the HBV-infected. In our study, the annual HBsAg seroconversion rate observed in coinfected patients (2.1%) was not only higher than that observed in HBV-infected patients (0.2%), but also than that reported in HBV-infected children without oncologic disease (0.6%).29 A high clearance rate of HBsAg (29%) in ex-oncological HBV-HCV coinfected children has also been reported by Cesaro et al.7 Furthermore, coinfected patients tended to show a higher prevalence of spontaneous seroconversion to anti-HBe.
These data suggest that HCV, when acquired as coinfection or as a superinfection in chronic HBV carriers, seems to interfere with HBV and may either attenuate its clinical presentation30 or suppress the HBV replicative state.31
Interestingly, however, HBV may also influence the HCV replicative state.32 As a spontaneous termination of HCV replication is rare in children as it is in adults, it was interesting to observe the greater tendency to clear serum HCV-RNA in patients with a dual HBV-HCV infection compared with those with HCV infection alone.
The response to IFN treatment against HCV infection was different for coinfected and singly infected patients. Although the small number of patients treated did not allow a statistical significance to be reached and caution should be applied in interpreting the data, it is noteworthy that a sustained anti-HCV response to IFN occurred in 47% of treated patients with dual infection as opposed to none of the patients with HCV infection alone. The high response rate to HCV in coinfected patients did not appear to be related to the more susceptible genotypes of HCV or to lower viremia levels. Theoretically, the difference in the anti-HCV response between coinfected and HCV-infected patients might be due, at least in part, to the higher IFN dosage received by coinfected patients, (5 v 3 MU/mq per dose). However, the fact that the response was sustained in all responder patients and that the response rate to HCV for coinfected patients is higher than that reported in the literature for patients infected by HCV alone in our area33 seems to suggest that other factors, namely the presence of HBV in the replicative or in the integrated state might have influenced the IFN-induced arrest of HCV viremia.
It has been reported that the suppression of HCV replication induced by IFN in HBV-HCV coinfected patients induced a reactivation of HBV chronic hepatitis.34 Thus, the IFN-induced abrogation of the putative suppressive effect of HCV on HBV might aggravate the HBV disease. However, we observed no worsening of HBV replication or of ALT levels in the 4 patients who showed an IFN-induced seroclearance of HCV-RNA, but not of HBV DNA.
It was interesting to observe in our series that 1 patient had a sustained favorable response to both HBV and HCV infection after IFN treatment. This event has rarely been reported in the literature.35
The mechanism of the HCV-HBV mutual interference has not yet been clarified and may involve an interplay of multiple cellular and immunological factors, as well as the replicating processes of both viruses.28 We may hypothesize that the impairment of the immune response of patients at the time when they acquired both viruses reduced the risk of a fatal outcome and caused a chronic indolent course of the liver disease, with many patients (59%) losing either 1 or both viruses over time because of mutual viral interference.
In conclusion, hepatitis B and/or C infections acquired by children during treatment for malignant disorders appear to have a mild course. Long-term surveillance of these patients is warranted to verify whether the recovery of the immune system after discontinuation of chemotherapy may favor a spontaneous arrest of the replication of 1 or both viruses. In patients who remain carriers of the viruses after 5 to 10 years, IFN treatment may be indicated, as it does not appear to be harmful and may be beneficial. The introduction of nucleoside analogues, ribavirin, and lamivudine for HCV- and HBV-infected patients, respectively, may offer new therapeutic options both for naive and nonresponder patients.36,37 Ribavirin seems to increase the response rate to IFN, whereas lamivudine may not only be better tolerated than IFN, but also may be more effective because it is an antiviral and not an immunomodulator drug. Although the importance of viral serum clearance in the natural history of HBV and HCV infection has yet to be established, we believe that any reasonable effort to eradicate the infections in these children must be pursued because their long-term outcome is still obscure.
ACKNOWLEDGMENT
The authors thank Geltrude Fiorillo for her valuable technical assistance.
Supported in part by a grant from Ministero dell'Università e della Ricerca Scientifica e Tecnologica, Italy, and in part, by a grant from the Second University of Naples, Naples, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Professor Riccardo Utili, MD, Istituto di Terapia Medica, 2° Università di Napoli, Via Cotugno,1–(c/o Ospedale Gesù e Maria), 80135 Napoli, Italy; e-mail: utili@unina.it.