Abstract
We used a first-generation adenovirus vector (AVC3FIX5) to assess whether human factor IX could be expressed and detected in the rhesus macaque, which we have shown does not make high-titer antibodies to human factor IX protein. Three animals received 1 × 1010to 1 × 1011 plaque-forming units per kilogram by intravenous injection. Human factor IX was present within 24 hours of vector administration and peaked 4 days later at 4,000 ng/mL in the high-dose recipient, and lower levels were seen in the intermediate-dose recipient. No human factor IX was detected in the low-dose recipient's plasma. Serum cytokine analysis and early hypoferremia suggested a dose-dependent acute-phase response to the vector. Human factor IX was detectable in rhesus plasma for 2 to 3 weeks for the high- and intermediate-dose recipients, but disappeared concomitant with high-titer antihuman factor IX antibody development. There was substantial, dose-dependent, dose-limiting liver toxicity that was manifest as elevated serum transaminase levels, hyperbilirubinemia, hypoalbuminemia, and prolongation of clotting times. Of particular interest was prolongation of the thrombin clotting time, an indicator of decreased fibrinogen or fibrinogen dysfunction. All evidence of liver toxicity resolved except for persistent hypofibrinogenemia in the high-dose recipient, indicating possible permanent liver damage. Our data suggest a narrow therapeutic window for first-generation adenovirus-mediated gene transfer. The development of antihuman factor IX antibodies and abnormalities of fibrinogen in the rhesus macaque is of concern for application of adenovirus (or other viral) vectors to hemophilia gene therapy.
WE HAVE PREVIOUSLY shown that the rhesus macaque factor IX cDNA is highly conserved compared with the human factor IX cDNA and the protein is greater than 97% identical to human factor IX, the highest known homology between human and nonhuman factor IX proteins.1 Repeated intravenous injections of human factor IX protein in rhesus macaques resulted either in no antibody formation or transient, low-titer, nonneutralizing antibodies that disappeared despite further administration of protein. These findings suggested that the rhesus macaque might be a suitable nonhuman primate animal model for preclinical testing of human factor IX gene transfer.
Hemophilia, especially hemophilia B (factor IX deficiency), has been considered an ideal candidate for gene therapy, because relatively low amounts of protein expression would be sufficient for amelioration of severe disease.2,3 Adenovirus vectors have been studied as potential vectors for hemophilia A and B gene therapy.4-13Obvious advantages of adenoviral vectors are efficient in vivo gene transfer (particularly to hepatocytes) by simple intravenous injection and the ability to produce high-titer vector stocks for animal studies. Potential disadvantages of adenovirus vectors include immunogenicity of the virus, which may cause T-cell–mediated elimination of transduced cells, thereby limiting transgene expression,14-17 and concomitant humoral immunity against the vector, which may preclude repeated administration.9,10,13,17 A related problem is the potential immune adjuvant effect of the adenovirus, which may serve to induce antibodies against the transgene of interest (eg, factor IX). This is especially important, because antibodies against factor IX, for instance, would preclude its use in hemophilia B and would subject the patient to the risk of anaphylaxis and nephrotic/nephritic syndromes with further exposure to factor IX protein concentrates.18,19 This would be particularly problematic, because alternatives to factor IX therapy (recombinant human factor VIIa, prothrombin complex concentrates, etc) are less predictable in efficacy and, in the case of recombinant factor VIIa, available (until recently) in the United States only as an investigational drug.20
We asked whether human factor IX could be expressed in rhesus macaques using a first generation E1, E3-deleted adenovirus vector to mediate in vivo gene transfer of the human factor IX gene. Our studies focused on factor IX expression, the immune response to human factor IX, and toxicity of the vector in vivo. Dose-dependent expression of human factor IX was observed in the 3 animals studied, but significant coagulopathy and liver toxicity was associated with the administration of the vectors. These findings are especially important for the evaluation of adenovirus vectors for hemophilia gene therapy.
MATERIALS AND METHODS
Animals.
Rhesus macaques weighing 7 to 10 kg (age range, 6 years, 2 months to 7 years, 3 months) were maintained at a primate research facility at the National Institutes of Health (Rockville, MD). Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Heart, Lung, and Blood Institute. The animals used for these experiments were the subjects of our previous report on intravenous injection of human factor IX protein in rhesus macaques.1
Adenovirus vector.
Adenovirus vector AVC3FIX5 was constructed as described previously,21 and high-titer preparations were prepared by infection of HEK293 cells (ATCC, Manassas, VA) at high multiplicity of infection (MOI) and freeze-thaw of cells with cytopathic effect 40 hours after infection, followed by ultracentrifugation of crude viral lysate on cesium chloride. Cesium-chloride banded virus was dialyzed extensively against 10 mmol/L Tris (pH 7.5), 2 mmol/L MgCl2, and 10% glycerol before freezing at −80°C. Titers of 4 × 1011 plaque-forming units (pfu)/mL were documented on HEK293 cells by plaque titering under soft-agar overlays. Limiting dilution of adenovirus vector on HEK293 cells was performed and TCID50(tissue-culture infectious dose, 50%) titers of 2 × 1012 TCID50/mL were demonstrated using Reed-Munch proportional distance factors. The vector preparation was tested for replication competent adenovirus (RCA) by MA Bioservices (Rockville, MD) and found to be negative at less than 1 RCA per 107 vector TCID50 by testing for cytopathic effect on human A549 cells at limiting dilutions.
Enzyme-linked immunosorbent assay (ELISA) for human factor IX antigen.
An ELISA for human factor IX, described as reported previously,1 was used to measure human factor IX in rhesus macaques after injections of the AVC3FIX5 vector or human factor IX protein (Mononine; Centeon, Kankakee, IL).
Anti-AVC3FIX5 antibody titration.
Ninety-six–well polystyrene plates were coated with 3 × 107 pfu of AVC3FIX5 in 100 μL 0.1 mol/L sodium carbonate buffer (pH 8.8) per well overnight at 4°C and then blocked with 6% bovine serum albumin (BSA)/phosphate-buffered saline (PBS)/0.05% Tween 20 for 1 hour at 37°C. Dilutions of rhesus serum were incubated at 37°C for 1 hour and washed, and rhesus antibodies were detected by incubating a 1:10,000 dilution of rabbit antirhesus IgG conjugated to horseradish peroxidase (Sigma, St Louis, MO), washing with PBS/0.05% Tween 20, and color developed with 1 mg/mL o-phenylenediamine in 0.1 mol/L sodium citrate (pH 4.5) with 2 μL H2O2 per 10 mL. Color development was stopped with 1 mol/L HCl and then measured with a Thermomax plate reader (Molecular Devices Corp, Sunnyvale, CA) at 492 nm, and the optical density was compared with that of equal dilutions of preadenovirus serum. Antiadenovirus titers were defined as the greatest dilution at which the absorbance at 492 nm exceeded the identical dilution of baseline serum by greater than 0.05 absorbance units.
Antihuman factor IX antibody titration.
Ninety-six–well polystyrene plates were coated with 0.1 μg human factor IX (Mononine) in 100 μL 0.1 mol/L sodium carbonate buffer (pH 8.8) per well overnight at 4°C and then blocked with 6% BSA/PBS/0.05% Tween 20 for 1 hour at 37°C. Various dilutions of rhesus serum were incubated at 37°C for 1 hour and washed, and antibodies were detected and quantified as described above for antiadenovirus antibodies.
Coagulation assays.
Prothrombin times were performed with a fibrometer by mixing 0.1 mL citrated plasma with 0.2 mL thromboplastin reagent (Organon Technica, Durham, NC) at 37°C. Activated partial thromboplastin times (aPTT) were performed with 0.1 mL of citrated plasma mixed with 0.1 mL of factor-sensitive lipid (FSL) partial thromboplastin reagent (Sigma) and mixed with 0.1 mL of 20 mmol/L CaCl2 to start the coagulation process. Thrombin clotting time (TCT) assays were performed by mixing 150 μL of 0.4 U/mL thrombin (Boehringer Mannheim, Indianapolis, IN) diluted in Owren's buffer (Sigma) with an equal volume of citrated plasma. Baseline TCT on normal pooled human or rhesus plasma were approximately 15 seconds in this assay.
Fibrinogen ELISA.
Ninety-six–well ELISA plates were coated with 100 μL/well of 1:1,000 dilution of goat antihuman fibrinogen (Sigma) in 0.1 mol/L sodium carbonate buffer (pH 8.8) overnight at 4°C and then blocked with 6% BSA/PBS/0.05% Tween 20 for 1 hour at 37°C. Rhesus plasma (50 μL/well) was then incubated for 1 hour at 37°C and then washed with PBS/0.05% Tween 20. Fibrinogen was detected by subsequent incubation with mouse monoclonal antihuman fibrinogen antibodies FG-21 and 85D4 (Sigma) diluted 1:10,000 in 6% BSA/PBS/Tween 20 and then with goat antimouse IgG conjugated to horseradish peroxidase (Sigma) diluted 1:1,000 in 6% BSA/PBS/0.05% Tween 20. Color was developed witho-phenylenediamine/hydrogen peroxide and stopped with 1 mol/L hydrochloric acid, and fibrinogen levels were quantified by comparison with dilution of baseline citrated plasma from the same animal. Under these conditions, the ELISA for fibrinogen gave approximately 30% cross-reactivity between rhesus and human fibrinogen in citrated plasma.
Fibrinogen immunoelectrophoresis.
Immunoelectorphoresis was performed on 4 μL plasma samples using precast sodium barbital agarose gels (Kallestadt, Chaska, MN) at 200 V for 45 minutes. After electrophoresis, goat antihuman fibrinogen antibody (Sigma) diluted 1:1,000 was placed in slots parallel to the direction of electorphoresis and precipitation was permitted to take place overnight at 4°C. After 3 days of washing with normal saline, precipitin arcs were stained with naphthol blue-black dye (Sigma) and then destained with 5% acetic acid/0.5% glycerol.
D-dimer assay.
Semiquantitative D-dimer latex agglutination testing was performed on citrated plasma as per the manufacturer's directions (Sigma).
Complete blood counts (CBC) and serum chemistry determination.
CBC were performed on EDTA-anticoagulated blood on a Cell Dyne 3500 analyzer (Abbott Laboratories, Abbott Park, IL), and serum chemistry evaluations were performed on serum samples using an Ektachem chemistry analyzer (Kodak, Rochester, NY).
Adenovirus cultures.
Heparinized blood and body fluids were collected at various time points after adenovirus vector administration, and adenovirus vector was cultured by plating various dilutions of material on HEK293 cells to determine TCID50 levels of the vector in the body fluid. Blood samples were diluted 1:100 in Dulbecco's modified Eagle's medium (DMEM)-H/10% fetal bovine serum supplemented with 2 mmol/L glutamine and antibiotics and plated directly. Urine and fecal samples were diluted 1:100 (wt:vol) in PBS and filtered through 0.2-μm filters before plating on HEK293 cells to determine the TCID50 levels. Sterile cotton swabs passed through the oropharynx of each animal were then placed in 10 mL PBS and the PBS was filtered before plating on HEK293 cells.
Human factor IX falloff assays.
Human factor IX (Mononine; Centeon) was reconstituted at 100 U/mL, and 25 U/kg body weight was injected intravenously and citrated plasma was collected at T = 0 hours, 0.5 hours, 1 hour, 2 hours, 4 hours, 8 hours, 12 hours, 24 hours, and 2 weeks after injection for analysis of human factor IX antigen and/or antihuman factor IX antibodies. Human factor IX antigen levels were compared with previous falloff studies in the same animals published previously.1
Bethesda inhibitor assay.
Bethesda inhibitor assays were performed as per the method of Kasper et al.22 Samples of citrated plasma diluted in Owren's buffer (Sigma) were incubated with either normal human plasma or normal rhesus plasma for 2 hours at 37°C, and then residual factor IX activity was determined with a fibrometer using human factor IX-deficient human plasma (Sigma). One Bethesda unit was defined as the reciprocal of the dilution of test plasma at which 50% of factor IX activity is inhibited. The sensitivity of the assay was 1 Bethesda inhibitor assay unit (BIAU) per milliliter.
Interleukin-6 (IL-6) cytokine assay.
Serum IL-6 was assayed with a human IL-6 immunoassay (Biosource, Camarillo, CA) as per the manufacturer's instructions. Cross-reactivity with rhesus IL-6 derives from its 98% homology to human IL-6 protein.23
RESULTS
Dose-dependent expression of human factor IX after injection of AVC3FIX5 vector.
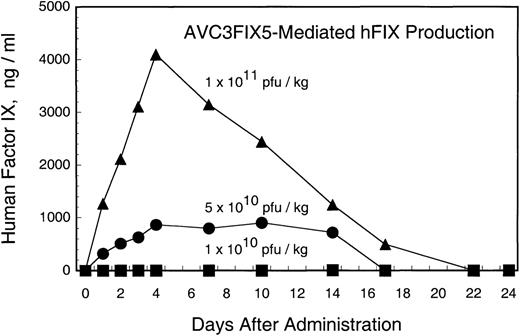
Adenovirus vector AVC3FIX5 is a first generation design vector that uses the cytomegalovirus (CMV) promoter to drive the expression of a human factor IX cDNA.21 Three rhesus macaques were injected with 8 × 1010, 4 × 1011, or 8 × 1011 pfu of AVC3FIX5 in 10 mL of PBS by intravenous injection under sedation. All 3 animals displayed no distress other than mild tachypnea shortly after the injection in the high- and low-dose recipients. Figure 1 shows human factor IX antigen levels in 3 rhesus macaques after the administration of different doses of AVC3FIX5. The recipient of the highest dose (RQ1305) developed peak levels of factor IX that were 80% of normal human levels (∼4,000 ng/mL) at day 4, followed by a gradual decrease to undetectable levels by 3 weeks after vector administration. The intermediate-dose recipient (84269) demonstrated peak human factor IX levels of 18% of normal (∼900 ng/mL), which remained approximately at this level for 2 weeks before becoming undetectable at 3 weeks. The low-dose recipient (RQ1234) failed to demonstrate detectable (>30 ng/mL) human factor IX at any time point after vector administration. Thus, adenovirus vector administration resulted in dose-dependent expression of human factor IX in the rhesus macaque, with a threshold of greater than 8 × 1010 pfu (1 × 1010 pfu/kg) for detectable factor IX expression.
Human factor IX in rhesus plasma after administration of AVC3FIX5 vector (normal human factor IX plasma level, 5,000 ng/mL). Each point is the average of ELISA results performed in duplicate. (▪) Low-dose; (•) intermediate-dose; (▴) high-dose.
Human factor IX in rhesus plasma after administration of AVC3FIX5 vector (normal human factor IX plasma level, 5,000 ng/mL). Each point is the average of ELISA results performed in duplicate. (▪) Low-dose; (•) intermediate-dose; (▴) high-dose.
Hepatotoxicity of adenovirus vector.
All 3 animals demonstrated hepatotoxicity that was dose-dependent. Serum AST was elevated (Fig 2) compared with baseline levels as soon as 6 hours after vector injection and increased to peak levels 2 to 19 times baseline from 6 hours after administration (low-dose and intermediate-dose recipients) to 7 days after administration (high-dose recipient). The intermediate- and high-dose recipients demonstrated elevation of serum alkaline phosphatase of 3 to 8 times normal and elevation of bilirubin as high as 15 times baseline at day 10 for the high-dose recipient after vector administration (Fig 2). The high-dose recipient displayed lethargy and poor appetite at the time of peak transaminasemia and required intravenous and subcutaneous fluid support and transfusion with rhesus donor plasma. Serum albumin levels decreased from a baseline of 3.6 g/dL to a nadir of 2.0 g/dL for the high-dose recipient and 2.6 g/dL for the intermediate-dose recipient (Fig 2). The low-dose recipient did not exhibit elevation of alkaline phosphatase levels or total bilirubin or any significant perturbation of albumin levels.
Liver function tests after administration of AVC3FIX5 vector. (▪) Low-dose; (•) intermediate-dose; (▴) high-dose.
Liver function tests after administration of AVC3FIX5 vector. (▪) Low-dose; (•) intermediate-dose; (▴) high-dose.
Blood urea nitrogen (BUN) and creatinine levels remained within normal limits for all time points tested after vector administration (data not shown).
Coagulation abnormalities after AVC3FIX5 administration.
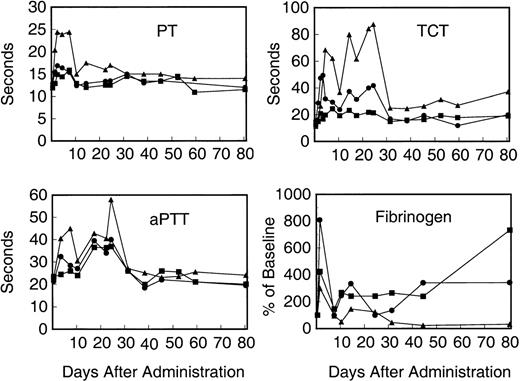
The high-dose recipient demonstrated a marked prolongation of the prothrombin time that doubled from its baseline value of 12 seconds to 25 seconds within 48 hours of vector administration. This high level decreased to a plateau of 15 to 17 seconds at day 10, but did not normalize until day 31 (Fig 3). The intermediate-dose recipient displayed a prompt increase in the prothrombin time from a baseline of 12 seconds to 17 seconds within 48 hours of vector administration, which returned to baseline levels by 10 days after vector administration. The low-dose recipient displayed a 2-second increase in the prothrombin time from baseline and, like the intermediate-dose recipient, returned to baseline by day 10. The prolongations of the prothrombin times in the 3 monkeys were concomitant with the elevated liver transaminases and total bilirubin, suggesting liver toxicity.
Coagulation studies of recipients of AVC3FIX5 vector. (Top left panel) Prothrombin time (PT); (bottom left panel) aPTT; (top right panel) TCT; (bottom right panel) plasma fibrinogen levels normalized to the percentage of baseline value. (▪) Low-dose; (•) intermediate-dose; (▴) high-dose.
Coagulation studies of recipients of AVC3FIX5 vector. (Top left panel) Prothrombin time (PT); (bottom left panel) aPTT; (top right panel) TCT; (bottom right panel) plasma fibrinogen levels normalized to the percentage of baseline value. (▪) Low-dose; (•) intermediate-dose; (▴) high-dose.
The aPTT were also elevated in a dose-dependent fashion, as seen in Fig3. Because this was seen in the low-dose recipient, which had no demonstrable (<30 ng/mL) human factor IX antigen circulating in plasma, the possibility of human factor IX interfering with the rhesus coagulation cascade in vitro to produce a prolonged aPTT is unlikely. We measured the aPTT of pooled normal rhesus plasma spiked with 50, 100, 500, or 5,000 ng/mL of purified human factor IX (Mononine) and saw no change in the aPTT from baseline (data not shown). Thus, interference with rhesus factor IX or the aPTT by human factor IX is effectively ruled out as an explanation for the prolonged aPTT values.
A particularly striking finding was the biphasic prolongation of the TCT seen in the intermediate- and high-dose animals after vector administration, which was suggestive of hypofibrinogenemia and/or dysfibrinogenemia (Fig 3). The high-dose recipient displayed peak TCT values of greater than 90 seconds at the highest point (22 days after vector administration) as compared with a baseline of 12 seconds. In fact, the high-dose recipient demonstrated a partial recovery of the TCT before a marked elevation to peak levels subsequently, suggesting a second, delayed insult or injury resulting in disturbances of fibrinogen. The first peak coincides with the markers of liver toxicity; however, the second, more abnormal peak occurs after these markers of liver injury have largely normalized. A similar, although less pronounced biphasic pattern is also seen for the intermediate-dose animal, whereas the low-dose recipient's TCT was only minimally changed after vector administration. Fibrin D-dimer assays were negative at days 1, 2, 3, 4, 10, and 14, except for the intermediate- and high-dose animals, which were weakly positive with undiluted day-4 plasma (but negative at 1:2 or greater dilutions of day-4 plasma).
Effect of adenovirus vector on rhesus fibrinogen levels.
An ELISA for human fibrinogen with 30% cross-reactivity for rhesus fibrinogen was developed and used to measure fibrinogen antigen levels in the animals after adenovirus vector administration. Figure 3 shows plasma fibrinogen antigen levels normalized to pretreatment baseline levels in the 3 rhesus monkeys. Fibrinogen protein levels vary widely and reach peak values 3 to 8 times baseline in the period immediately after vector administration. The high-dose recipient eventually reaches a steady-state fibrinogen level approximately 20% of normal, consistent with the persistently elevated TCT shown in Fig 3, whereas the low- and intermediate-dose recipients demonstrate increased fibrinogen antigen levels well after normalization of their TCTs.
Antibodies to human factor IX.
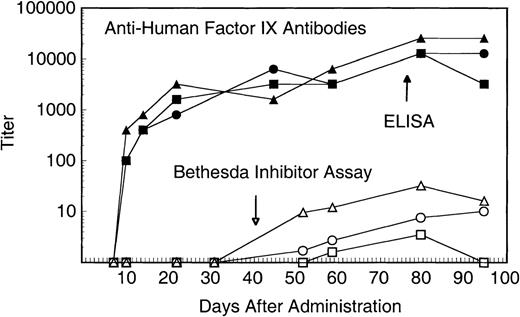
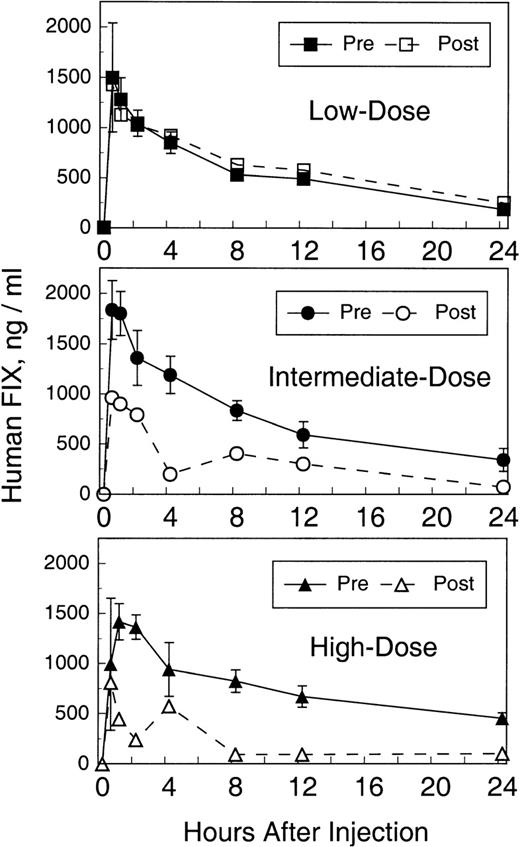
We previously demonstrated that these animals produce transient, low-titer, or no antibodies to intravenously administered human factor IX protein. As can be seen in Fig 4, high-titer (>1:10,000) IgG antibodies were produced in all 3 animals within a few days of vector injection. The relatively rapid IgG response suggests an anamnestic response to human factor IX to which these animals had previously been exposed.1 Antihuman factor IX Bethesda inhibitor assays were also performed to assess antibodies that inhibit factor IX coagulant activity. As seen in Fig 4, antibodies that inhibit human factor IX activity were demonstrated in all 3 animals 8 weeks after vector administration. The Bethesda inhibitor titers varied according to the dose of the vector administered. Factor IX falloff studies were performed 80 days after vector administration to assess the pharmacokinetics of human factor IX protein in the presence of high-titer antibodies. As can be seen in Fig 5, no significant difference in recovery and half-life (8.4 hours) is seen for the low-dose recipient compared with all previous falloff studies. In contrast, the intermediate- and high-dose recipients demonstrate a blunted recovery peak (∼50% of expected) followed by a rapid falloff (1.3 and 1.8 hours, respectively) and a second, delayed peak 4 to 8 hours later, suggesting a biphasic pattern of distribution/redistribution of human factor IX protein.
Antihuman factor IX antibodies after administration of AVC3FIX5 vector. Solid symbols represent titer of rhesus antihuman factor IX IgG determined by microtiter plate immunoassay (ELISA). Open symbols represent antihuman factor IX neutralizing antibodies determined by a Bethesda inhibitor assay. (▪) Low-dose; (•) intermediate-dose; (▴) high-dose.
Antihuman factor IX antibodies after administration of AVC3FIX5 vector. Solid symbols represent titer of rhesus antihuman factor IX IgG determined by microtiter plate immunoassay (ELISA). Open symbols represent antihuman factor IX neutralizing antibodies determined by a Bethesda inhibitor assay. (▪) Low-dose; (•) intermediate-dose; (▴) high-dose.
Human factor IX falloff studies, before and after AVC3FIX5 administration. Twenty-five units per kilogram of Mononine (Centeon) were injected intravenously and human factor IX plasma antigen levels were determined by ELISA at the time points shown. Preadenovirus falloff values are represented with solid symbols and are the average of 4 pre-AVC3FIX falloff studies (±1 standard deviation) as described previously by Lozier et al.1 Postadenovirus falloff values are represented by open symbols and each value is the average of ELISA results in duplicate for 1 falloff study at day 80. (▪) Low-dose, pre-AVC3FIX5; (•) intermediate-dose, pre-AVC3FIX5; (▴) high-dose, pre-AVC3FIX5; (□) low-dose, post-AVC3FIX5; (○) intermediate-dose, post-AVC3FIX5; (▵) high-dose, post-AVC3FIX5.
Human factor IX falloff studies, before and after AVC3FIX5 administration. Twenty-five units per kilogram of Mononine (Centeon) were injected intravenously and human factor IX plasma antigen levels were determined by ELISA at the time points shown. Preadenovirus falloff values are represented with solid symbols and are the average of 4 pre-AVC3FIX falloff studies (±1 standard deviation) as described previously by Lozier et al.1 Postadenovirus falloff values are represented by open symbols and each value is the average of ELISA results in duplicate for 1 falloff study at day 80. (▪) Low-dose, pre-AVC3FIX5; (•) intermediate-dose, pre-AVC3FIX5; (▴) high-dose, pre-AVC3FIX5; (□) low-dose, post-AVC3FIX5; (○) intermediate-dose, post-AVC3FIX5; (▵) high-dose, post-AVC3FIX5.
No evidence for rhesus antirhesus factor IX antibodies was seen in Bethesda inhibitor assays (sensitive to ∼1 BIAU) performed on citrated plasma. Plasma was heat-inactivated at 56°C for 30 minutes to destroy any residual rhesus factor IX that might mask antirhesus factor IX antibodies; no inhibition of rhesus factor IX activity was seen in heat-inactivated samples.
Indicators of acute-phase response.
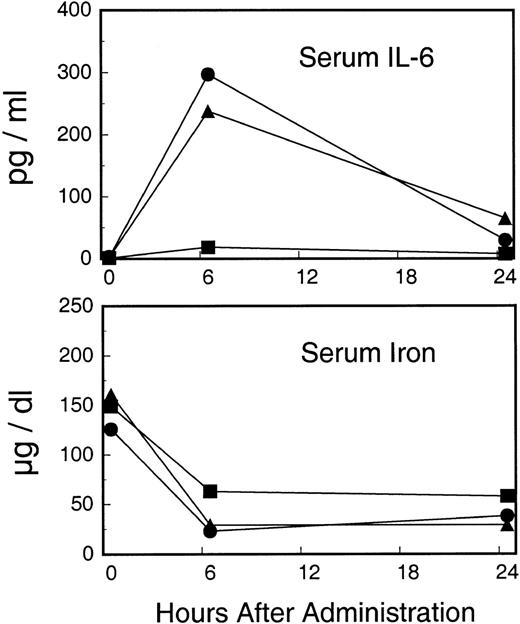
Serum IL-6 and serum iron were measured in the first 24 hours after administration of AVC3FIX5. As seen in Fig6, IL-6 levels increased sharply in the intermediate- and high-dose recipients, whereas little or no response was seen in the low-dose recipient. Conversely, serum iron levels decreased in all 3 animals in a dose-dependent manner (Fig 6).
Early acute-phase indicators after administration of AVC3FIX5. Upper panel depicts serum IL-6 cytokine levels. Bottom panel depicts serum iron levels. (▪) Low-dose; (•) intermediate-dose; (▴) high-dose.
Early acute-phase indicators after administration of AVC3FIX5. Upper panel depicts serum IL-6 cytokine levels. Bottom panel depicts serum iron levels. (▪) Low-dose; (•) intermediate-dose; (▴) high-dose.
Vector distribution in bloodstream and secretions and antibody production.
Adenovirus vector levels were assayed in the bloodstream of each animal at various time points after injection, starting with a time point immediately after the injection (T = 5 minutes). The vector could be detected immediately after administration for all 3 animals and at 24 hours in the blood of the intermediate- and high-dose recipients but not in the low-dose animal. No virus could be detected in any animals' blood at 48 hours. These data indicate a volume of distribution ranging from 10 to 14 L (Vd = AVC3FIX5 injected/AVC3FIX5 at T0), indicating extremely rapid clearance of the vector from the bloodstream, perhaps in the reticuloendothelial system of the liver, spleen, and/or lungs. No virus was detected in urine, feces, or oral swabs at any time point tested up to 2 weeks after injection. Vector clearance was followed by vigorous antibody production. Figure 7 shows high-titer (>1:10,000) antiadenovirus IgG antibodies by 14 days that persisted for as long as it was measured (>90 days).
Rhesus serum anti-AVC3FIX5 IgG titers after AVC3FIX5 administration. (▪) Low-dose; (•) intermediate-dose; (▴) high-dose.
Rhesus serum anti-AVC3FIX5 IgG titers after AVC3FIX5 administration. (▪) Low-dose; (•) intermediate-dose; (▴) high-dose.
DISCUSSION
Administration of our first generation, E1-deleted, E3-deleted, human factor IX adenovirus vector to rhesus macaques at doses of 5 × 1010 pfu/kg to 1 × 1011 pfu/kg led to therapeutic levels of human factor IX for up to 3 weeks, indicating the utility of the rhesus macaque as a nonhuman primate animal model for testing safety and (short-term) efficacy of human factor IX vectors. Expression was dose-dependent and at the highest dose led to essentially curative (∼80% of normal) human factor IX plasma protein levels in the recipient. Although the low-dose recipient had active AVC3FIX5 vector in the bloodstream immediately after injection and would be expected to express approximately 200 to 400 ng/mL of human factor IX in its plasma by comparison with the intermediate- and high-dose animals, no plasma human factor IX could be detected. This is consistent with a threshold effect for adenovirus transduction, previously observed in hemophilia A canines and mice subjected to adenovirus-factor VIII vector transduction and in mice and primates receiving adenovirus-erythropoietin vectors at similar doses.8,24 The mechanism for this phenomenon is unclear. It is possible that the acute-phase response may activate transcription factor NFκB to facilitate CMV promoter-driven expression in the intermediate- and high-dose recipients, but not in the low-dose recipient.25The rapid increase in IL-6 (the chief mediator of the early acute-phase response)26-28 in the intermediate- and high-dose recipients (but not seen in the low-dose recipient) and the dose-dependent decrease in serum iron (an inverse indicator of the acute-phase response)28 29 supports this hypothesis (Fig6).
The apparent loss of factor IX expression in the intermediate-and high-dose recipients may be explained largely on the basis of the high-titer, antihuman factor IX antibodies that were evident as soon as 10 days after treatment, which has been seen in other animal species after adenovirus-factor IX vector administration in vivo.9,10,12,13 High-titer antibodies to human factor IX were not necessarily expected, because these animals previously failed to develop high-titer antibodies when injected repeatedly with human factor IX protein.1 In addition to antihuman factor IX antibodies, silencing of the CMV promoter in adenovirus vectors may be considered as a reason for diminished transgene expression, especially in the liver, as demonstrated previously.25 30 Furthermore, loss of nonintegrating, episomal adenovirus vector might be accelerated by hepatocyte turnover/regeneration after liver injury, particularly in our high-dose recipient.
Administration of the adenovirus vector was associated with substantial, dose-limiting liver pathology, which is likely due to direct toxicity to hepatocytes.27 There is only a 10-fold difference in vector dose over which the response ranges from no detectable human factor IX in plasma to curative levels of factor IX associated with life-threatening liver toxicity. Such a narrow therapeutic window may be considered problematic for the design of any study of adenovirus vectors for use in humans with hemophilia or other diseases in which liver expression of a particular transgene is desired. The endosomolytic properties of external adenovirus fiber proteins can be toxic to cells and might cause significant liver toxicity in vivo, because approximately 80% of injected adenovirus vector goes to the liver.5 Expression of adenovirus genes (eg, E2 and E4) present in our vector also might have contributed to the liver toxicity that we found in our animals. For example, it has recently been demonstrated that adenovirus vectors containing the E4 region can induce cell cycle dysregulation, leading to growth arrest and cell death.31 In this regard, results of gene transfer in mice using a gutless adenovirus vector expressing an α-1-antitrypsin gene suggest that liver toxicity can largely be circumvented by elimination of all adenovirus genes in the vector.32 33 The chief limitation to such vectors is the difficulty in preparing large quantities of helper virus-free vector stocks.
It is possible that plasma factor IX levels in the low-dose recipient were below the sensitivity of the ELISA, which in our hands is approximately 30 ng/mL of human factor IX protein in a background of rhesus plasma.1 Levels below this amount (<1% of normal human levels) are not likely to be therapeutic for patients with hemophilia B. Antibodies to human factor IX in the low-dose recipient serve to indicate that there must have been at least some low-level human factor IX expression (enough to immunize) despite the lack of measurable protein in plasma.
It is interesting that antibodies to human factor IX are of similar titer in all 3 animals by microtiter plate immunoassay (Fig 4) but have different effects on factor IX pharmacokinetics and factor IX activity assays. High- and intermediate-dose recipients' antibodies alter the pharmacokinetics of infused human factor IX protein, but the low-dose recipient's antibodies do not (Fig 5). This may be due to recognition of different epitopes or perhaps differences in the antibody subclass/isotype produced in the low-dose recipient. In the low- and intermediate-dose animals, the aPTT normalized after resolution of liver toxicity, despite the persistence of antihuman factor IX titers of 1:10,000 or more (Figs 3 and 4). Although the high-dose animal's aPTT remained slightly elevated (Fig 3), this is more likely to be due to liver damage and/or fibrinogen abnormalities in light of the abnormal PT, TCT, and fibrinogen studies (Fig 3).
Transient canine anticanine factor VIII antibodies have been seen in hemophilia A canines receiving canine factor VIII adenovirus vectors (Sheila Connelly, Genetics Therapy Inc [Gaithersburg, MD], personal communication, March 18, 1999) and transient canine anticanine factor IX antibodies have been described in hemophilia B canines receiving intramuscular AAV-canine factor IX vectors,34 which suggests that some viruses may serve as adjuvants for antibody formation and temporary loss of self-tolerance when used for in vivo gene transfer. In our limited series, there was no apparent loss of self-tolerance as reported in these studies or in studies of erythropoietin gene transfer in mice.35 Our model differs from other hemophilic animal models in that endogenous rhesus factor IX is continuously present after vector administration. Endogenous rhesus factor IX might serve to prevent antirhesus factor IX antibodies (by promoting immune tolerance) or might mask low-titer antibodies. Antirhesus factor IX activity antibodies were not detected in any of our animals by an aPTT-based Bethesda inhibitor assay sensitive to approximately 1 BIAU/mL. It is quite possible that there were rhesus antirhesus factor IX antibodies that we could not detect on the basis of a low titer or inability to neutralize factor IX activity in vitro. However, antibodies that do not neutralize factor IX activity or significantly alter factor IX metabolism/clearance are probably of little importance.
The induction of high-titer, antihuman factor IX antibodies after vector administration suggests that adenovirus might serve as an immune adjuvant, ie, a danger signal when presented simultaneously with a potential immunogen.36 In this regard, it is noteworthy that adenovirus vectors expressing β-galactosidase can provoke cell-mediated immunity (against transduced muscle fibers), but adeno-associated viruses expressing β-galactosidase do not; this correlates with the ability of adenovirus vectors to transduce antigen-presenting dendritic cells.37 Administration of our vector also caused antiadenovirus antibodies, as expected (Fig 7); such antibodies are one of the chief limiting features of adenovirus vectors and have prevented repeated administration of adenovirus vectors in vivo.9,10,12 13
The coagulopathy that followed adenovirus administration was associated with a dose-dependent prolongation of the prothrombin time, the aPTT, and, most dramatically, the TCT. Because the TCT is a direct indicator of the quantity and function of plasma fibrinogen, it is likely that the coagulopathy seen after administration of the adenovirus vector is a consequence of fibrinogen dysfunction and, in the case of the high-dose recipient, hypofibrinogenemia as a consequence of severe liver toxicity (Figs 2 and 3). The prolonged prothrombin time and aPTT may be indirectly due to abnormal fibrin aggregation from an abnormal/dysfunctional fibrinogen or low levels of fibrinogen, as noted above. We investigated whether there was an abnormal fibrinogen arc on immunoelectrophoresis that would indicate dysfibrinogenemia. None of our subjects had abnormal fibrinogen mobility or additional arcs on fibrinogen immunoelectrophoresis (data not shown); however, this is a rather insensitive test due to the minimal electrophoretic mobility of fibrinogen.38 The trace levels of fibrin D-dimers after vector administration (seen only at one time point) makes disseminated intravascular coagulation an unlikely explanation of the persistently abnormal TCT.
Liver toxicity is not an unexpected finding after gene transfer with high doses of adenoviruses; however, the coagulopathy seen after adenovirus-mediated gene transfer has not been previously studied in detail, although possible hypofibrinogenemia (based on prolongation of the TCT) was alluded to in an early report of adenovirus-mediated gene transfer in hemophilia B canines.10 The latter phenomenon should be carefully evaluated during future development of gene transfer vectors to be used in patients with hemophilia A or B.
ACKNOWLEDGMENT
The authors thank James Higginbotham for plaque-purifying the AVC3FIX5 vector before injection into the rhesus macaques and Earl West for analysis of serum chemistry and CBC on these animals. In addition, we thank Jim Meade for helpful discussions on factor IX Bethesda inhibitor assays.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Richard A. Morgan, PhD, Bldg 10, Room 10C103, 10 Center Dr, Bethesda, MD 20892-1851; e-mail:rmorgan@nhgri.nih.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal