To the Editor:
In September 1994 we reported the results of a randomized trial comparing 2 regimens to condition patients with chronic myeloid leukemia (CML) in chronic phase (CP) for allogeneic marrow transplantation.1 Patients were treated between October 1988 and November 1992 and received marrow from HLA-identical siblings. Sixty-nine patients were treated with 120 mg/kg of cyclophosphamide and 6 daily exposures each of 200 cGy of total body irradiation (TBI), and 73 patients received 16 mg/kg of oral busulfan over 4 days followed by 120 mg/kg of cyclophosphamide (BU-CY). The 2 regimens had similar survival and relapse probabilities. The BU-CY regimen was better tolerated than the CY-TBI regimen. The median duration of follow-up in this study is now more than 7 years, and we report these outcomes updated to July 1, 1998.
Patients treated with the CY-TBI regimen (N = 69).
In the initial report, 29 patients had developed chronic graft-versus-host disease (GVHD) and 6 of these had died of causes not associated with relapse. Since then, 2 more of these patients have died of causes not associated with relapse and 2 additional patients (both of whom survive) have developed clinical extensive chronic GVHD.
In the initial report, 15 patients had cytogenetic relapse and none of these had died. The relapse was transient in 5 instances and these patients still survive in continuing remission without treatment. Of the 10 patients with persistent relapse, 3 have died in relapse, 4 are in cytogenetic remission after treatment with interferon (IFN) (polymerase chain reaction [PCR] status is positive in 2, negative in 1, and unknown in 1), and 3 are in continuing cytogenetic relapse despite IFN therapy. Since publication of the initial report, 6 patients have developed cytogenetic relapse between 2 and 6 years after transplant; 2 of these patients have died in relapse and 4 are being treated but remain in relapse.
In the initial report, 14 patients had died (4 during the first 100 days). Since then, 8 more patients have died; 5 (between 2 and 9 years after transplant) after relapse, 1 of myocardial infarction at 4 years, and 2 of chronic GVHD disease at 5 years.
Patients treated with the BU-CY regimen (N = 73).
In the initial report, 30 patients had developed chronic GVHD and 4 of these had died of causes not associated with relapse. Since then, none of these patients has died and 1 more patient (who survives) has developed clinical extensive chronic GVHD.
In the initial report, 10 patients had cytogenetic relapse and none of these had died. The relapse was transient in 3 instances and these patients still survive in continuing cytogenetic (and PCR) remission without treatment. Of the 7 patients with persistent relapse, 3 had died at the time of the original report and 1 additional patient has died at 7 years after transplant and 3 patients are in cytogenetic (and PCR) remission (1 after treatment with interferon). Since publication of the initial report, 6 patients have developed cytogenetic relapse between 2½ and 8½ years after transplant, and all of these patients survive. Two patients were recently diagnosed and remain in relapse untreated, 2 are in cytogenetic (and PCR) remission after donor lymphocyte infusion (DLI), and 2 are in cytogenetic remission (1 PCR-negative and 1 PCR-positive) after IFN treatment.
In the initial report, 15 patients had died (3 during the first 100 days). Since then, 2 more patients have died, 1 after relapse (at 7 years after transplant) and 1 of colon carcinoma at 8½ years after transplant.
Survival estimates.
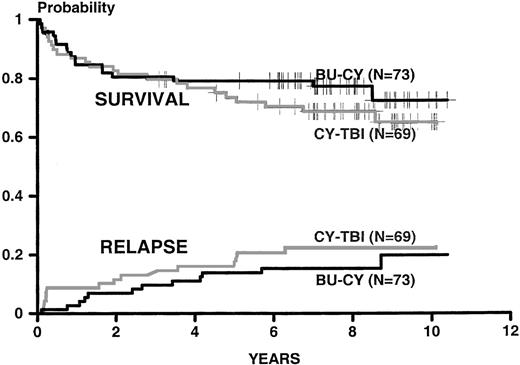
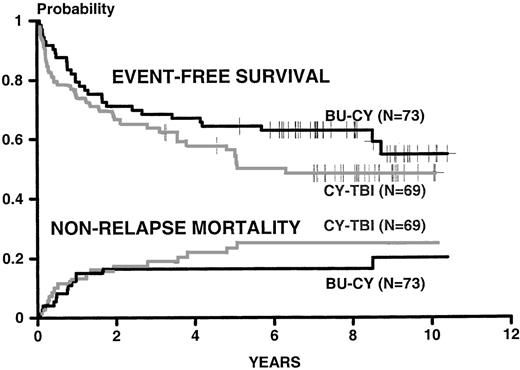
Figure 1 shows the 9 year Kaplan-Meier probabilities of survival (0.73 for BU-CY and 0.65 for CY-TBI,P = not significant [NS]) and the cumulative incidences of relapse (0.19 for BU-CY and 0.22 for CY-TBI, P = NS). Figure2 shows the Kaplan-Meier probabilities of event-free survival (0.55 for BU-CY and 0.48 for CY-TBI,P = NS) and the cumulative incidences of nonrelapse mortality (0.20 for BU-CY and 0.25 for CY-TBI, P = NS). Table1 describes the current status of survivors in each study group.
Kaplan-Meier estimates of survival and cumulative incidence of relapse for patients conditioned for HLA-identical marrow transplantation with the BU-CY and CY-TBI regimens. Endpoints were calculated as in the original report.1 Results are updated to July 1999.
Kaplan-Meier estimates of survival and cumulative incidence of relapse for patients conditioned for HLA-identical marrow transplantation with the BU-CY and CY-TBI regimens. Endpoints were calculated as in the original report.1 Results are updated to July 1999.
Kaplan-Meier estimates of surviving event-free (relapse and death were identified as events) and of dying from causes other than relapse for patients conditioned for HLA-identical marrow transplantation with the BU-CY and CY-TBI regimens. Endpoints were calculated as in the original report.1 Results are updated to July 1999.
Kaplan-Meier estimates of surviving event-free (relapse and death were identified as events) and of dying from causes other than relapse for patients conditioned for HLA-identical marrow transplantation with the BU-CY and CY-TBI regimens. Endpoints were calculated as in the original report.1 Results are updated to July 1999.
Current Status of Survivors
| . | Study Groups . | |
|---|---|---|
| BU-CY (N = 73) . | CY-TBI (N = 69) . | |
| Alive—No history of relapse | 48 | 37 |
| No history of chronic GVHD | ||
| Karnofsky score = 100% | 18 | 14 |
| Karnofsky score 80-100% | 3 | 4 |
| Karnofsky score less than 80% | 1 | 2 |
| History of chronic GVHD | ||
| Off all medications. Karnofsky score = 100% | 13 | 12 |
| Karnofsky score 80-100% | 12 | 5 |
| Karnofsky score less than 80% | 1 | 0 |
| Alive with history of relapse | 8 | 10 |
| In remission after treatment | 6 | 4 |
| Total no. of survivors | 56 | 47 |
| . | Study Groups . | |
|---|---|---|
| BU-CY (N = 73) . | CY-TBI (N = 69) . | |
| Alive—No history of relapse | 48 | 37 |
| No history of chronic GVHD | ||
| Karnofsky score = 100% | 18 | 14 |
| Karnofsky score 80-100% | 3 | 4 |
| Karnofsky score less than 80% | 1 | 2 |
| History of chronic GVHD | ||
| Off all medications. Karnofsky score = 100% | 13 | 12 |
| Karnofsky score 80-100% | 12 | 5 |
| Karnofsky score less than 80% | 1 | 0 |
| Alive with history of relapse | 8 | 10 |
| In remission after treatment | 6 | 4 |
| Total no. of survivors | 56 | 47 |
Discussion.
Since publication of the original report, there have been 2 other reports of randomized studies comparing the BU-CY and TBI regimens in patients with CML in CP.2,3 The study by the Nordic Bone Marrow Transplantation group2 included patients with advanced stages of acute and chronic leukemia, and only 28% had CML in CP. Patients treated with busulfan had more early toxicity, which resulted in increased transplant-related mortality and worse survival in patients with advanced disease, but this was not the case for patients with early disease. A recent update of this study4 concluded that late toxic effects (including chronic GVHD and obstructive bronchiolitis) were more common after treatment with busulfan compared with TBI, but the report did not discriminate between patients with early or advanced disease with respect to these toxicities. Transplant-related mortality and survival were worse in patients with advanced disease who received busulfan. In patients with early disease there was no difference in outcome with the 2 conditioning regimens. The study from the French Society of Bone Marrow Grafts3 was limited to patients with CML in CP. It was concluded that BU is an acceptable alternative to TBI for patients with CML in first chronic phase receiving bone marrow transplantation from HLA-identical sibling donors. Both regimens caused similar transplant-related mortality. The antileukemic efficacy of the BU-CY regimen was either similar to or possibly better than that of the CY-TBI regimen.
The results from our updated analysis with a median follow-up of 7.7 years support our original conclusions. For patients with CML in CP, BU-CY is better tolerated than CY-TBI as a preparative regimen for marrow transplantation from an HLA-identical sibling. The 2 regimens have comparable efficacy against CML and similar risks of mortality from causes other than relapse.