Abstract
Multiple myeloma is associated with unbalanced bone remodeling causing lytic bone lesions. Interleukin-11 (IL-11) promotes osteoclast formation and inhibits osteoblast activity and may, thus, be one factor involved in cancer-induced bone destruction. We have previously shown that myeloma cells produce hepatocyte growth factor (HGF). We now report that HGF induces IL-11 secretion from human osteoblast-like cells and from the osteosarcoma cell lines Saos-2 and HOS. In coculture experiments, both the myeloma cell line JJN-3 and primary myeloma cells from 3 patients induced IL-11 secretion from osteoblasts, whereas no induction was observed with the non-HGF producing myeloma cell line OH-2. Enhanced IL-11 induction was observed with physical contact between osteoblasts and myeloma cells as compared with experiments in which contact was prohibited by tissue inserts. Anti-HGF serum strongly reduced the myeloma cell-induced IL-11 secretion. Furthermore, we show that JJN-3 cells express HGF on the cell-surface. Removal of surface-bound HGF on JJN-3 cells reduced IL-11 production induced in cocultures. Transforming growth factor β1 and IL-1 potentiated the effect of HGF on IL-11 secretion, whereas an additive effect was observed with tumor necrosis factor. Thus, myeloma-derived HGF can influence the bone marrow environment both as a soluble and a surface-bound factor. Furthermore, HGF emerges as a possible factor involved in myeloma bone disease by its ability to induce IL-11.
MULTIPLE MYELOMA is a neoplasm affecting terminally differentiated B lymphocytes (ie, plasma cells). The malignant plasma cells are generally localized to the bone marrow in close vicinity to bone trabeculae. A characteristic feature of multiple myeloma is localized osteolytic bone destruction or diffuse osteopenia. This is caused by an increase in osteoclastic bone resorption and, in more advanced stages, a decrease in osteoblastic bone formation.1-3 The lesions are found adjacent to nests of myeloma cells suggesting involvement of myeloma-derived factors. Earlier, we have shown that primary myeloma cells and most myeloma cell lines secrete hepatocyte growth factor (HGF)4,5 and that high serum HGF is associated with poor prognosis in myeloma patients.6 Because osteoblasts and osteoclasts express the HGF high-affinity receptor encoded by the proto-oncogene c-met,7 it is possible that HGF is involved in the uncoupled bone remodeling process in myeloma bone disease.
Osteoblasts are not only involved in bone formation, but also in bone resorption by producing factors necessary for recruitment and activation of osteoclasts. Interleukin-11 (IL-11) is one such factor produced by osteoblasts,8 which in vitro stimulates osteoclastogenesis9,10 and suppresses bone formation.11 Furthermore, IL-11 was recently shown to stimulate TRANCE (TNF [tumor necrosis factor]-related activation-induced cytokine) expression in mouse primary osteoblasts.12 TRANCE, a membrane-bound protein also known as osteoclast differentiation factor or RANK-ligand, activates mature osteoclasts in vitro13 and short-term administration of soluble TRANCE into normal adult mice results in osteoclast activation and systemic hypercalcemia.14 A number of agents have previously been shown to stimulate IL-11 secretion from osteoblasts, including transforming growth factor β (TGF-β), parathyroid hormone (PTH), PTH-related peptide, IL-1, TNF, prostaglandin E2, and 1,25-dihydroxyvitamin D3 [1,25(OH)2D3].8,10Klein15 has detected IL-11 in the culture supernatants of bone marrow cells from some myeloma patients, suggesting that IL-11 may be involved in myeloma bone disease. On the other hand, there is no evidence for production of IL-11 by myeloma cells. Here, we report that soluble HGF as well as HGF bound to the surface of myeloma cells stimulate IL-11 secretion from primary human osteoblast-like (hOB) cells as well as from osteosarcoma cell lines.
MATERIALS AND METHODS
Reagents.
HGF was purified from conditioned medium derived from the myeloma cell line JJN-3 as previously described in detail.5 Neutralizing anti-human HGF rabbit serum was prepared in our laboratory by repeated immunization with HGF. A 1:1,000 dilution of this serum totally inhibited the activity of 50 ng/mL of HGF in the Mv-1-Lu bioassay,16 although preimmune serum from the same rabbit had no effect. The mouse monoclonal HGF-antibody, denoted 2B5, was established in our laboratory as described.5 The 18D11, an isotype-matched mouse antibody against human CD14 established in our laboratory, was used as a control antibody.
Purification of primary myeloma cells.
Bone marrow samples were obtained from myeloma patients as part of the diagnostic procedure. Leftover material was used for research after informed consent was obtained. Bone marrow plasma cells were isolated and purified as previously reported.17 Briefly, mononuclear cells were isolated by density gradient centrifugation and plasma cell purification was done by positive immunomagnetic selection by using the plasma cell specific antibody B-B4 (Diaclone Research, Besancon, France). This purification method gives samples of myeloma cells that are more than 98% pure, as determined by morphologic evaluation of May-Grünwald-Giemsa-stained cytospin smears.
Cell cultures.
The isolation of hOB cells was essentially performed as described by Robey and Termine.18 Trabecular bone specimens were obtained from osteoartritic patients without known malignant disease undergoing replacement knee or hip surgery. Soft connective tissue and periosteal and cortical bone were removed and the remaining trabecular bone was minced and extensively washed with phosphate-buffered saline (PBS) to remove bone marrow cells. The bone fragments, approximately 3 mm3, were then digested at 37°C with 1 mg/mL type II bacterial collagenase (Sigma Chemical Co, St Louis, MO) in Dulbecco’s modified Eagle’s medium (DMEM)/F12 (GIBCO, Paisly, UK). After 2 hours, the released cells were discarded and the remaining bone fragments were extensively washed with DMEM/F12 containing 10% fetal calf serum (FCS). The fragments were seeded into 25 cm2tissue culture flasks (Costar, Cambridge, MA) and incubated at 37°C in a humidified atmosphere with 5% CO2. The culture medium was DMEM/F12 supplemented with 10% FCS, 100 IU/mL of penicillin, 100 μg/mL of streptomycin, 1 mmol/L pyruvate, and 2 mmol/L glutamine. The medium was changed weekly until a confluent cell monolayer was obtained (4 to 6 weeks). Confluent cells were detached with trypsin (0.25%) and EDTA (0.02%), subcultured once or twice and then seeded for the experiments described below. Bone cultures from 4 different donors were used for the experiments in this study. As determined by an alkaline phosphatase (ALP) staining kit from Sigma (85L-2), 50% to 70% of the cells in the bone cultures stained positive for ALP and, thus, represent well-differentiated osteoblasts. This is in accordance with the original report describing the isolation and characterization of hOB cells.18
The human osteosarcoma cell lines HOS TE85 and Saos-2 were obtained from the American Type Culture Collection (Rockville, MD). The human myeloma cell line JJN-3 was a gift from Jennifer Ball (Department of Immunology, University of Birmingham, UK). These cell lines were routinely grown in RPMI 1640 (GIBCO) supplemented with 10% FCS, 2 mmol/L glutamine, and 40 μg/mL gentamycin. The OH-2 cell line was established in our laboratory19 and was routinely grown in RPMI 1640, 10% human A+ serum (The Bloodbank, University Hospital, Trondheim, Norway), 1 ng/mL IL-6 (Sandoz, Basel, Switzerland), 2 mmol/L glutamine, and 40 μg/mL gentamycin.
Preparation of conditioned medium.
Saos-2 and HOS cells were seeded in 25 cm2 tissue culture flasks at a concentration of 3 × 105 cells per flask in RPMI 1640 with 10% FCS. hOB cells were seeded into 25 cm2 tissue culture flasks at a concentration of 105 cells per flask in DMEM/F12 with 10% FCS. After 24 hours, the medium was replaced with 3 mL of fresh medium with 2% FCS and various concentrations of HGF. After another 48 hours, the supernatants were harvested, centrifuged, aliquoted, and frozen at −20°C until analysis. For analysis of the IL-11 secretion in coculture system, the osteoblasts were seeded in 24-well plates (Costar) at 2.5 × 104 cells per well (hOB cells) or 5 × 104 cells per well (Saos-2) in medium with 10% FCS. After 24 hours, the medium was replaced with 1 mL of fresh medium with 2% FCS, and 105 JJN-3, OH-2 or primary myeloma cells were added directly to the culture or placed in the upper chamber of tissue inserts (Transwell; Costar, catalog no. 3413). Rabbit HGF antiserum or preserum was added simultaneously with myeloma cells. After 12 or 48 hours of coculture, the supernatants were collected, centrifuged, aliquoted, and frozen at −20°C until IL-11 determination. Similarly, costimulation with HGF and either TGF-β1 (R&D Systems, Minneapolis, MN), IL-1α (Glaxo, Geneva, Switzerland), or TNF-α (kindly provided by Genentech, South San Francisco, CA) was performed in 24-well plates with 5 × 104 Saos-2 cells per well. In experiments with heparin-washed JJN-3 cells, the cells were incubated with 10 μg/mL heparin in PBS for 1 hour. The cells were washed in PBS containing 0.1% bovine serum albumin (BSA) 4 times and then used for experiments. In other experiments, OH-2 cells were incubated with 1 μg/mL HGF for 4 hours, washed 6 times in culture medium, and then used for experiments.
IL-11 determination.
IL-11 was measured in duplicate by enzyme-linked immunosorbent assay (ELISA) from R&D Systems. The sensitivity of this assay is about 20 pg/mL.
Reverse transcription-polymerase chain reaction (RT-PCR).
Total RNA was isolated from Saos-2 and HOS cells by cell lysis in 1% NP-40, nuclei were removed by centrifugation, and nucleic acids were extracted by phenol and chloroform. cDNA was synthesized with the use of oligo dT primer and Moloney murine leukemia virus reverse transcriptase (Promega Corp, Madison, WI). PCR was performed with the IL-11 specific primers synthesized in our laboratory with a Bechman DNA SM synthesizer. Primer sequences were as follows: 5′- GCCTGGTCCTGGTCGTG-3′ and 5′-GTTGTGGTCCCCGTCAGC-3′. The expression of β actin mRNA was used as an internal standard.
Detection of cell surface-bound HGF by flow cytometry.
106 OH-2 cells, incubated with or without HGF, and 106 JJN-3 cells were incubated with unconjugated 2B5 (anti-HGF) or 18D11 (control antibody) for 1 hour at 4°C before washing and subsequent staining was with fluorescin isothiocyanate-conjugated goat antimouse IgG (Becton Dickinson, Mountain View, CA). Before final analysis with a FACScan flow cytometer (Becton Dickinson), the cells were washed 3 times in PBS containing 0.1% BSA. Five thousand cells from each sample were analyzed. Dead cells and debris were gated out on the basis of the forward and side scatter signals.
RESULTS
Upregulation of IL-11 secretion by HGF-stimulated osteoblasts.
Figure 1A shows the amount of IL-11 obtained in media from Saos-2 cells incubated with or without HGF for 48 hours. Saos-2 cells constitutively produced IL-11 (982 pg/mL per 3 × 105 cells). Upon HGF-stimulation, the secretion of IL-11 increased in a dose-dependent manner to a maximum 15-fold increase with 50 ng/mL HGF. About 10 ng/mL HGF gave a half-maximal increase. Similar results were obtained with HOS cells (Fig 1B). HOS cells constitutively produced IL-11 (334 pg/mL). Again, about 10 ng/mL HGF led to half-maximal stimulation of IL-11 secretion and a maximum 4-fold increase was seen after stimulation with 50 ng/mL HGF. The HGF-induced effect on IL-11 secretion from hOB cells is shown in Fig1C. hOB cells constitutively produced IL-11 (40 pg/mL per 1 × 105 cells). Again, HGF induced IL-11 in a dose-dependent manner.
Dose response of HGF on secretion of IL-11 from osteoblasts. (A) Saos-2 cells, (B) HOS cells, and (C) hOB cells were incubated with 0 to 100 ng/mL HGF for 48 hours and IL-11 in conditioned medium was measured in duplicates by ELISA. Cell line experiments were repeated at least 3 times, and similar results were obtained with hOB cells from 4 different donors. Data are means ± SD from 1 representative experiment of 3.
Dose response of HGF on secretion of IL-11 from osteoblasts. (A) Saos-2 cells, (B) HOS cells, and (C) hOB cells were incubated with 0 to 100 ng/mL HGF for 48 hours and IL-11 in conditioned medium was measured in duplicates by ELISA. Cell line experiments were repeated at least 3 times, and similar results were obtained with hOB cells from 4 different donors. Data are means ± SD from 1 representative experiment of 3.
We also examined the relative IL-11 mRNA levels after HGF treatment in these cell lines by RT-PCR (data not shown). The levels of IL-11 mRNA in Saos-2 and HOS increased in parallel with the increase in protein levels indicating that the increase in IL-11 is at least partly caused by increased levels of IL-11 mRNA in the cells.
Detection of myeloma cell surface-bound HGF.
Previously, we have shown that JJN-3 cells secrete HGF into the cell supernatant.5 In contrast, the OH-2 cells have lost their ability to produce HGF during ex vivo culturing. HGF has been shown to bind to cell surface proteoglycans20 and, therefore, we examined JJN-3 cells for cell surface HGF. A major JJN-3 cell fraction expressed cell surface-bound HGF as seen in the flow cytometry histogram presented in Fig 2A. Washing the cells in heparin reduced the cell surface-bound HGF to background levels, indicating that the JJN-3 cells growing in cell culture were coated with noncovalently bound HGF. HGF was not detected on the cell surface of OH-2 cells (Fig 2B). However, after incubating OH-2 cells with 1 μg/mL HGF and subsequently washing the cells extensively, we were able to detect cell surface-bound HGF indicating that OH-2 cells have the potential of binding exogenous HGF. Furthermore, surface-bound HGF was washed off the cells by heparin, as was the case with the JJN-3 cells (data not shown).
Surface-bound HGF detected by flow cytometry. (A) Histogram of JJN-3 cells stained with the mouse monoclonal anti-HGF antibody 2B5 and fluorescein isothiocyanate (FITC) anti-mouse IgG before and after washing cells with heparin. The background staining obtained with the irrelevant antibody 18D11 and FITC anti-mouse IgG is also indicated. (B) Histogram of OH-2 cells before and after incubating cells with 1 μg/mL HGF followed by extensive cell washing before labeling with 2B5.
Surface-bound HGF detected by flow cytometry. (A) Histogram of JJN-3 cells stained with the mouse monoclonal anti-HGF antibody 2B5 and fluorescein isothiocyanate (FITC) anti-mouse IgG before and after washing cells with heparin. The background staining obtained with the irrelevant antibody 18D11 and FITC anti-mouse IgG is also indicated. (B) Histogram of OH-2 cells before and after incubating cells with 1 μg/mL HGF followed by extensive cell washing before labeling with 2B5.
IL-11 secretion in coculture experiments.
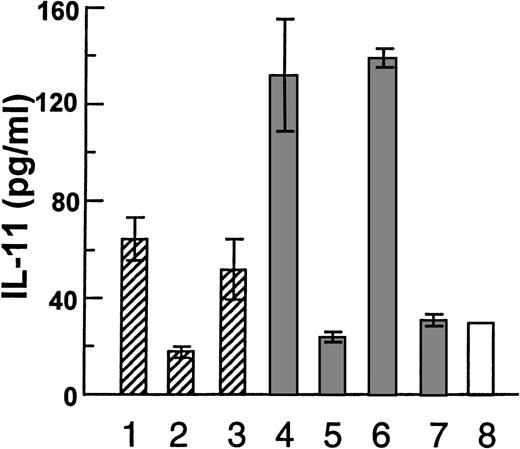
After having established that HGF is able to induce IL-11 secretion from osteoblasts, we examined the effects of myeloma cell-derived HGF in a coculture system. When the myeloma cell lines JJN-3 or OH-2 were cultured alone, no IL-11 was detected in the supernatants. Then, we incubated JJN-3 cells or OH-2 cells in tissue inserts, thus, prohibiting direct physical contact with hOB cells growing in the lower chamber (Fig 3). The tissue inserts are freely permeable to soluble HGF (data not shown). When the cells were separated by a tissue insert, the JJN-3 cells induced a 1.8-fold increase in IL-11 secretion as compared with control wells without myeloma cells. This increase was abolished by anti-HGF serum. In coculture experiments with the possibility of direct physical contact between the 2 cell types, JJN-3 cells induced a 5-fold increase in IL-11 secretion. Again, anti-HGF prevented the increase in IL-11 secretion. In contrast, the non-HGF producing OH-2 cells induced no increase in IL-11 secretion from hOB cells. Similar coculture experiments were performed with Saos-2 cells (Fig 4). With physical contact the JJN-3 cells gave a 13-fold increase in IL-11 secretion as compared with unstimulated Saos-2 cells. This increase was reversible in the presence of anti-HGF serum, as shown in Fig 4A (bar 2 v bars 3 and 4). Again, OH-2 cells were unable to upregulate the production of IL-11 (Fig 4A, bars 7-9). When Saos-2 cells and JJN-3 cells were separated by tissue inserts, we found only a 4.5-fold increase in IL-11 (Fig 4A, bars 1 v 9).
Coculture experiments with myeloma cell lines and hOB cells, with the following coculture conditions: (1-3) JJN-3 in upper chamber of tissue inserts and hOB in the lower chamber, (1) with no addition, (2) in the presence of 1:1000 of rabbit anti-HGF serum, or (3) preserum. (4-6) Coculture with physical contact between JJN-3 and hOB, (4) with no addition, (5) or in the presence of 1:1000 of rabbit anti-HGF serum, and (6) preserum. (7) Coculture with physical contact between OH-2 cells and hOB and (8) hOB incubated in the absence of other cells. Supernatants were harvested after 48 hours and IL-11 was measured in duplicates by ELISA. Data are means ± SD from 1 representative experiment of 3.
Coculture experiments with myeloma cell lines and hOB cells, with the following coculture conditions: (1-3) JJN-3 in upper chamber of tissue inserts and hOB in the lower chamber, (1) with no addition, (2) in the presence of 1:1000 of rabbit anti-HGF serum, or (3) preserum. (4-6) Coculture with physical contact between JJN-3 and hOB, (4) with no addition, (5) or in the presence of 1:1000 of rabbit anti-HGF serum, and (6) preserum. (7) Coculture with physical contact between OH-2 cells and hOB and (8) hOB incubated in the absence of other cells. Supernatants were harvested after 48 hours and IL-11 was measured in duplicates by ELISA. Data are means ± SD from 1 representative experiment of 3.
(A) Coculture experiments with myeloma cell lines and Saos-2 cells under the following conditions: (1) JJN-3 in the upper chamber of tissue inserts and Saos-2 cells in the lower. (2-6) Physical contact between JJN-3 and Saos-2 cells, (2) with no addition, (3) in the presence of 1:1000 or (4) 1:2000 rabbit anti-HGF serum, and (5) in the presence of 1:1000, or (6) 1:2000 dilutions of preserum. (7) OH-2 cells in the upper chamber of tissue inserts and Saos-2 in the lower. (8) Physical contact between OH-2 and Saos-2 cells.(9) Saos-2 cells alone without myeloma cells. Supernatants were harvested after 48 hours. (B) The right panel shows the following coculture conditions with physical contact: (1) Saos-2 alone, (2) coculture of JJN-3 and Saos-2 cells, (3) coculture of heparin-washed JJN-3 and Saos-2 cells, (4) coculture of OH-2 and Saos-2 cells, and (5) OH-2 cells incubated with 1 μg/mL HGF followed by extensive washing before coculture with Saos-2 cells. Supernatants were harvested after 12 hours. In all experiments, IL-11 was measured in duplicates by ELISA. Data are means ± SD from 1 representative experiment of 3.
(A) Coculture experiments with myeloma cell lines and Saos-2 cells under the following conditions: (1) JJN-3 in the upper chamber of tissue inserts and Saos-2 cells in the lower. (2-6) Physical contact between JJN-3 and Saos-2 cells, (2) with no addition, (3) in the presence of 1:1000 or (4) 1:2000 rabbit anti-HGF serum, and (5) in the presence of 1:1000, or (6) 1:2000 dilutions of preserum. (7) OH-2 cells in the upper chamber of tissue inserts and Saos-2 in the lower. (8) Physical contact between OH-2 and Saos-2 cells.(9) Saos-2 cells alone without myeloma cells. Supernatants were harvested after 48 hours. (B) The right panel shows the following coculture conditions with physical contact: (1) Saos-2 alone, (2) coculture of JJN-3 and Saos-2 cells, (3) coculture of heparin-washed JJN-3 and Saos-2 cells, (4) coculture of OH-2 and Saos-2 cells, and (5) OH-2 cells incubated with 1 μg/mL HGF followed by extensive washing before coculture with Saos-2 cells. Supernatants were harvested after 12 hours. In all experiments, IL-11 was measured in duplicates by ELISA. Data are means ± SD from 1 representative experiment of 3.
When cell surface-bound HGF was removed by washing JJN-3 cells with heparin, the IL-11 secretion after 12 hours of coculture was half that observed with JJN-3 cells still coated with HGF (Fig 4B, bars 2v 3). After 24 hours, however, there was no detectable difference (data not shown) probably caused by de novo synthesis of HGF. Furthermore, when we incubated OH-2 cells with HGF, extensively washed the cells, and incubated them with Saos-2 cells, the amount of IL-11 secreted was significantly higher in the cocultures than in the mono-cultures of Saos-2 cells (Fig 4B, bars 4 and 5). Taken together, these results indicate that also OH-2 cells have the potential of presenting cell surface HGF to osteoblasts and other cell types.
Primary myeloma cells induce osteoblastic IL-11 secretion.
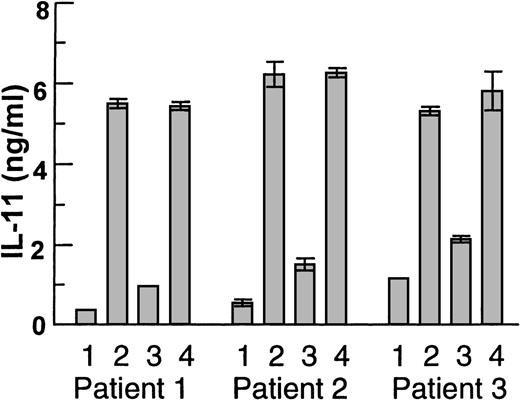
Highly purified primary myeloma cells from 3 patients were then seeded on Saos-2 cells (Fig 5). Again, cells from all patients induced IL-11 secretion and this increase was inhibited by anti-HGF serum, whereas no inhibition was observed with preserum.
Coculture experiments with physical contact between Saos-2 cells and myeloma cells (>98% pure) from 3 patients. (1) Saos-2 cells alone, (2) myeloma cells and Saos-2 cells, (3) myeloma cells and Saos-2 cells in the presence of anti-HGF serum 1:1000, or (4) preserum 1:1000. Supernatants were harvested after 48 hours. IL-11 was measured in duplicates by ELISA and data are means ± SD.
Coculture experiments with physical contact between Saos-2 cells and myeloma cells (>98% pure) from 3 patients. (1) Saos-2 cells alone, (2) myeloma cells and Saos-2 cells, (3) myeloma cells and Saos-2 cells in the presence of anti-HGF serum 1:1000, or (4) preserum 1:1000. Supernatants were harvested after 48 hours. IL-11 was measured in duplicates by ELISA and data are means ± SD.
Modulation of the HGF-effect on IL-11 secretion by other factors.
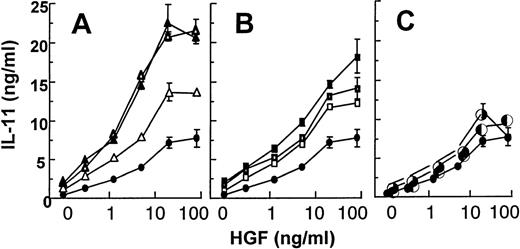
TGF-β, IL-1, and TNF are all factors being produced by myeloma cells or stromal cells in the myeloma bone marrow.21,22Furthermore, these cytokines have been shown to induce osteoblastic IL-11 secretion.8 10 When Saos-2 cells were stimulated with one of these factors alone, we observed a 2- to 3-fold increase in the IL-11 secretion above background levels. However, when we stimulated Saos-2 cells with HGF together with each of these factors, we observed a marked potentiation of the HGF-effect on IL-11 secretion together with TGF-β and IL-1, whereas an additive effect was observed with TNF (Fig 6).
Effect of HGF together with TGF-β1 (A), IL-1 (B), and TNF- (C). Saos-2 cells were stimulated with HGF (0 to 80 ng/mL) alone (•) or with HGF and TGF-β1 (▵; 10 pg/mL, ☞; 100 pg/mL, ▴; 1,000 pg/mL), IL-1 (□; 10 pg/mL, ➢; 100 pg/mL, ▪; 1,000 pg/mL), and TNF- (◐; 1 ng/mL, ✺; 10 ng/mL). Supernatants were harvested after 48 hours. IL-11 was measured in duplicates by ELISA. The data are expressed as means of duplicates ± SD from 1 representative experiment out of 3.
Effect of HGF together with TGF-β1 (A), IL-1 (B), and TNF- (C). Saos-2 cells were stimulated with HGF (0 to 80 ng/mL) alone (•) or with HGF and TGF-β1 (▵; 10 pg/mL, ☞; 100 pg/mL, ▴; 1,000 pg/mL), IL-1 (□; 10 pg/mL, ➢; 100 pg/mL, ▪; 1,000 pg/mL), and TNF- (◐; 1 ng/mL, ✺; 10 ng/mL). Supernatants were harvested after 48 hours. IL-11 was measured in duplicates by ELISA. The data are expressed as means of duplicates ± SD from 1 representative experiment out of 3.
DISCUSSION
The main finding in this study is that myeloma-derived HGF is able to induce osteoblastic IL-11 secretion, and this suggests for the first time a specific biologic role for HGF in multiple myeloma.
First, we show that soluble HGF alone induces IL-11 secretion from osteoblasts. Girasole et al9 have shown that IL-11 dose dependently stimulated osteoclast-like multinucleated cell formation in cocultures of mouse osteoblasts and bone marrow cells. Importantly, they reported that monoclonal anti-IL-11 antibody inhibited osteoclast formation induced by 1,25(OH)2D3, PTH, IL-1, and TNF. It has also been shown that HGF possesses osteotropic effects such as stimulating proliferation of murine osteoclastic cell precursors23 and promoting murine osteoclastic bone resorption.24 However, in both cases the effects of HGF were only observed in coculture systems involving either calvaria-derived stromal cells (MC3T3-G2/PA6) or osteoblastic cells (UMR 106). In the absence of coculture systems, HGF showed no effect on osteoclastic cell formation23 and, on the contrary, inhibited bone resorption.24 This clearly suggests that the effect of HGF is mediated by other factors. Together with our findings, it is tempting to speculate that the osteoclast-stimulating effect observed with HGF in the presence of osteoblasts and stromal cells is at least partly mediated by an induction of IL-11 secretion. However, as IL-11 was not capable of inducing osteoclastogenesis from bone marrow cells unless calvarial cells were present,9 it is likely that the effect of IL-11 is mediated by an additional factor. This factor may be TRANCE, because IL-11 induces expression of this membrane-bound protein, which acts as a direct and essential signal for osteoclast differentiation and activation.12-14
The second important finding presented here is that myeloma cells can express cell surface-bound HGF. In other cell systems, it has been shown that HGF binds to low-affinity heparan sulfate proteoglycan receptors on the cell surface and that heparin displaces proteoglycan-bound HGF from the cell surface.20 Recently, we have shown that heparin given intravenously gives a rapid increase in serum levels of HGF.25 This increase is probably caused by a displacement of reservoirs of preformed proteoglycan-bound HGF by soluble heparin. The fact that HGF on the cell surface was displaced by heparin suggests that HGF is at least partly bound to proteoglycans on JJN-3 cells as well.
Third, by comparing the levels of IL-11 in experiments with tissue inserts and with physical contact between myeloma cells and osteoblasts, it is clear that secretion of IL-11 is markedly increased by cell-to-cell contact. This observation is in parallel with an earlier study in which increased secretion of IL-6 was observed in experiments with physical contact between osteosarcoma cells and the myeloma cell line XG1.26 We provide several lines of evidence that show that HGF is the essential mediator of myeloma cell-induced IL-11 secretion in both types of cocultures. First, anti-HGF serum inhibited IL-11 induction in all experiments including experiments with primary myeloma cells. Second, non-HGF producing OH-2 cells were not able to induce IL-11 unless coated with exogenous HGF. Third, when we removed HGF from the cell surface of JJN-3 cells by heparin, a decrease in IL-11 secretion was detected. This shows that myeloma cell-derived HGF can influence the bone marrow environment both as a soluble factor and as a surface-bound factor.
The last finding in our report is that TGF-β1 and IL-1 potentiated the effect of HGF on IL-11 secretion, whereas an additive effect was observed with TNF. TGF-β, IL-1, and TNF are all cytokines that have been shown to be produced either by the myeloma cells themselves or by stromal cells in the myeloma bone marrow.21,22 Also, TGF-β is stored in a latent form in the bone matrix.27The strong increase in IL-11 secretion observed when we stimulated Saos-2 cells with both HGF and TGF-β, IL-1, or TNF opens up for the possibility that HGF may act in concert with other bone-modulating factors in the myeloma bone marrow to promote bone destruction.
In principle, hOB cell cultures should most closely reflect the osteoblastic cell population present in vivo. However, primary cell cultures are a heterogeneous mixture of cells, which may include other cell lineages such as fibroblasts, and there is a risk that cells isolated on different occasions may give inconsistent results. The fact that we obtained similar results with osteoblasts from 4 different donors and from 2 cell lines strengthen the biologic relevance of our data.
In conclusion, our in vitro data presented here suggest a paracrine pathway by which myeloma cells may affect bone homeostasis. Thus, a myeloma-derived factor, HGF, is presented to osteoblasts leading to an increase in IL-11 secretion, which, in turn, may stimulate osteoclast recruitment and inhibit osteoblastic bone formation. Because myeloma cells grow in close contact to osteoblasts in the bone marrow environment, this induction sequence is potentially operative also in vivo.
Supported by the Norwegian Cancer Society, Blix Legat, and the Cancer Fund, Trondheim, Norway.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Øyvind Hjertner, MD, Institute of Cancer Research and Molecular Biology, Norwegian University of Science and Technology, Medisinsk Teknisk Senter, N-7005 Trondheim, Norway; e-mail:oyvind.hjertner@medisin.ntnu.no.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal