Abstract
Erythroblastic synartesis is a rare form of acquired dyserythropoiesis, first described by Breton-Gorius et al in 1973. This syndrome is characterized by the presence of septate-like membrane junctions and “glove finger” invaginations between erythroblasts, which are very tightly linked together. This phenomenon, responsible for ineffective erythropoiesis, leads to an isolated severe anemia with reticulocytopenia. In the following report, we describe 3 new cases of erythroblastic synartesis associated with dysimmunity and monoclonal gammapathy. In all cases, the diagnosis was suggested by characteristic morphological appearance of bone marrow smears, and further confirmed by electron microscopy. Ultrastructural examination of abnormal erythroblast clusters showed that these cells were closely approximated with characteristic intercellular membrane junctions. The pathogenesis of the dyserythropoiesis was modeled in vitro using crossed erythroblast cultures and immunoelectron microscopy: when cultured in the presence of autologous serum, the erythroblasts from the patients displayed synartesis, whereas these disappeared when cultured in normal serum. Moreover, synartesis of normal erythroblasts were induced by the patient IgG fraction. Immunogold labeling showed that the monoclonal IgG were detected in, and restricted to, the synartesis. A discrete monoclonal plasmacytosis was also found in the patient bone marrow. The adhesion receptor CD36 appeared to be concentrated in the junctions, suggesting that it might be involved in the synartesis. These experiments indicated that a monoclonal serum immunoglobulin (IgG in the present cases) directed at erythroblast membrane antigen was responsible for the erythroblast abnormalities. Specific therapy of the underlying lymphoproliferation was followed by complete remission of the anemia in these cases.
HEMATOPOIETIC CELLS, unlike many other tissues that develop specialized intercellular junctions, remain loose and do not normally generate cytoplasmic interconnections with adjacent cells. In normal bone marrow, erythroid precursors are often observed to cluster around macrophages, forming the erythroblastic island: A privileged relationship exists between these three cell types, in which one cell nurtures the other. This phenomenon, in which an erythroblast takes up ferritin from the macrophage, has been termed rhopheocytosis. However, in areas of intimate association between a macrophage and erythroblasts, or between 2 erythroblasts, no morphologic evidence of surface specialization has been noted, and only ferritin has been seen in the interstitium.1 2
Acquired cases of dyserythropoiesis may have multiple causes and the erythroid lineage may exhibit varied morphological features on bone marrow examination. However, alterations at the sites of contacting membranes have rarely been reported. Abnormal linkages between erythroblasts have been observed in hypersiderotic marrows, and a common feature was that ferritin deposition was consistently located along the pathological junctions.3 Some specific morphological abnormalities were described in 1973 in a patient with an acquired dyserythropoietic anemia, where erythroblasts were tightly linked together.4 In that case, electron microscopy showed that these junctions were formed by interdigitating cell processes and that they were linked by regularly spaced septake-like structures that were devoid of ferritin deposition. This observation led to the description of a new syndrome called “erythroblastic synartesis,” from the Greek words “ςυν” (with) and “αρτηςις” (link), which is characterized by severe anemia with reticulocytopenia and marked dyserythropoiesis. Relatively few descriptions of this rare disorder have been published.4 5 Here, we report 3 new cases, with a detailed description of the morphological abnormalities leading to the diagnosis. We describe in vitro experiments, the results of which indicate that a monoclonal serum immunoglobulin is solely responsible for the erythroblastic abnormalities; in addition, a monoclonal plasmacytosis (<5% of total cells) was found in the bone marrow. Finally, and in support of the auto-immune origin of dyserythropoiesis, corticosteroid therapy or chemotherapy to induced complete remission of the underlying hemopathy was associated with complete recovery from the disease.
MATERIALS AND METHODS
Patients
Case 1.
A 24-year-old woman was admitted to hospital in September 1973 with severe aplastic, slightly microcytic anemia. The blood count showed a hemoglobin level of 6 g/dL, with normal white blood cell (WBC) and platelet counts and a normal serum iron level; the reticulocyte count was reduced. The patient had splenomegaly and small diffuse lymphadenopathy, and a lymphoproliferative disease was discovered (small lymphocytic lymphoma). Unfortunately, no record of serum electrophoresis and immunoelectrophoresis were found in this patient’s file. There was no lymphocytosis in the peripheral blood; direct Coombs test was positive for complement. The red blood cell (RBC) aplasia did not respond to initial therapy with chlorambucil. The hemoglobin level steadily decreased (3 g/dL within 1 month) and the patient continued to be RBC transfusion dependent.
Eighteen months later, corticosteroid was introduced. This resulted in a reduction in RBC transfusion requirement. The corticosteroid dose was gradually reduced. Anemia recurred in January 1975 and on this occasion did not respond to corticosteroids. Splenectomy was performed in May 1975. The spleen weighed 1.8 kg. Histological examination documented lymphocytic infiltration. Subsequently, the anemia improved. Multiagent chemotherapy was commenced to control the lymphoproliferative disorder. This induced only a partial response in the lymphadenopathy, but the hemoglobin level was stabilized. In 1983, the patient died of Richter syndrome.
Case 2.
A 70-year-old woman was admitted to hospital in November 1992 because of severe and progressive anemia. Nine years previously the patient had been hospitalized for Sjögren’s syndrome. Investigations on that occasion had showed a low complement C4 level and strongly positive antinuclear antibodies. Testing for the lupus anticoagulant and serum monoclonal immunoglobulin was negative and there had been no evidence of hemolysis. On this admission, the hemoglobin level was 5.5 g/dL with a reticulocyte count of 20.109/L. RBC indices confirmed a normocytic and normochromic anemia. Examination of the blood smears showed normal WBC and differential counts, and a normal platelet count. Serum iron was 35 mmol/L and serum ferritin 175 mg/L. Serum lactate-dehydrogenase was 667 (normal <330), bilirubin was 9.3 mg/dL, and haptoglobins were markedly reduced. The direct Coombs test was positive for complement. The lupus anticoagulant was present. Anti-cardiolipin antibody tests were also positive at 53 U/mL. Serum immuno-electrophoresis showed a monoclonal kappa IgG paraprotein at a concentration of 3 g/L. Oral corticosteroid therapy was started. This treatment resulted in progressive disappearance of the bone marrow erythroid dysplasia and to the disappearance of the anemia. However, the monoclonal immunoglobulin remained unchanged. When withdrawal of corticosteroid treatment was attempted, the anemia relapsed. Thus, the patient continues to receive corticosteroid therapy and maintains a normal hemoglobin level.
Case 3.
In July 1995, a 46-year-old woman was referred because of lymphadenopathy and lymphocytosis. WBC count was 9,200 × 106/L with 38% polymorphonuclear cells and 58% lymphocytes. The hemoglobin level was 13.2 g/dL. The platelet count was 317 × 109/L. Morphology and immunophenotyping were consistent with the diagnosis of chronic lymphocytic leukemia. Hypogammaglobulinemia was present (4.8 g/L) associated with a monoclonal IgG kappa gammapathy (1.7 g/L). By February 1996, the tumoral syndrome had progressed. The WBC was 18.7 × 109/L with 76% lymphocytes and the hemoglobin level had decreased to 7.9 g/dL. The direct Coombs test was negative. The patient was started on low-dose CHOP (chlorambucil, vincristine, and prednisone) chemotherapy regimen. Because of the occurrence ofStreptococcus pneumoniae pneumonia she also received prophylactic intravenous immunoglobulins for 6 months. In July 1997, after 6 cycles followed by 1 year of chlorambucil therapy, she was in clinical remission. The WBC was 4.5 × 109/L with 72% neutrophils and 16% lymphocytes and the hemoglobin level was 12.7 g/dL. The monoclonal IgG kappa was still present (2 g/L). Therapy was stopped. In December 1997, she relapsed with lymphadenopathy, splenomegaly, and severe anemia. The hemoglobin level was 6.9 g/L, the reticulocyte count was 42 × 109/L, the lactico-deshydrogenase (LDH) level was 265 IU/L (N < 190), and the direct Coombs test was negative. The monoclonal IgG kappa level was stable (2 g/L). Polymerase chain reaction (PCR) analysis for Parvovirus B19 was negative in both bone marrow and blood. Fludarabine therapy was associated with transient incomplete remission and the patient died in October 1998 with septic cellulitis.
Cell Preparations
Bone marrow.
Bone marrow from the patients was aspirated from the sternum and the smears were prepared with Romanovsky staining. Control human bone marrow was harvested from normal adult donors during hip surgery after obtaining informed consent and in accordance with the institutional guidelines of the Committee on Human Investigation.
Isolation of a Purified IgG Fraction
Serum IgG from patients 2 and 3 were purified by affinity chromatography over a Staphylococcus Protein A (SPA) (Pharmacia, Saint Quentin, France) column. Three milliliters of serum (collected in March 1993) were absorbed on an SPA column (6 mL of gel equilibrated in 0.1 mol/L phosphate buffer pH 7.5 and 0.15 mol/L NaCl). The unabsorbed fraction was collected and concentrated by ultrafiltration on PM 10 Amicon membrane (Amicon, Epernon, France) to a volume of 4 mL (protein concentration, 33 mg/mL). The IgG fractions were eluted from the SPA column with 0.1 mol/L glycine HCl buffer pH 2.8, and then quickly brought to neutrality with 1 mol/L Tris HCl buffer pH 7.5. The resulting eluates were concentrated by ultrafiltration as described above. Three milliliters of a purified IgG fraction (8 mg/mL) were thus obtained.
The fractions were analyzed by agarose gel electrophoresis (Hydragel; Sebia, Issy-les-Moulineaux, France), followed by immunoprecipitation with anti-gamma, anti-kappa, anti-lambda immune serum. The unabsorbed fractions were almost completely devoid of IgG, whereas the acid-eluted fractions corresponded to the purified IgG, included the monoclonal component (IgG kappa) in the same proportion as the original serum. By the same procedure, we obtained 2 fractions of similar composition and concentration from 3 mL of a pool of sera of 10 blood donors as starting material. These fractions were used as control.
Erythroblast Cultures
Bone marrow cells were separated on Ficoll (density = 1.077 g/mL) and light-density cells were isolated. Cells were plated at a final concentration of 10 × 104 cells/mL in 0.8% methylcellulose in Iscove’s modified Dubelcco’s medium containing 1% deionized bovine serum albumin (BSA), 10.4 mol/L 2-b mercaptoethanol (Terry Fox Lab, Vancouver, Canada) and 20% of either normal AB human serum, or the patient’s serum—entire or IgG depleted, or normal serum containing the patient’s IgG (2 g/L). Cultures were set up in presence of recombinant human (rhu)-erythropoietin (Janssen-Cilag, Issy-les-Moulineaux, France) 2 U/mL. Dishes were incubated at 37°C in air atmosphere supplemented with 5% CO2 and saturated with humidity. Erythroid colony burst–forming units erythroid (BFU-E) were harvested at day 14-15. The methyl cellulose was dissolved in an excess of culture medium. The cells were either cytospun or fixed for electron microscopy.
Electron Microscopy
Aspirated bone marrow samples and cultured cells were directly fixed on 2.5% glutaraldehyde in phosphate buffer 0.1 mol/L, pH 7.4 for 1 hour at 22°C, then washed 3 times in phosphate buffer. Alternatively, tannic acid was added into the fixative to enhance electron density in the extracellular space.
The samples were postfixed in osmic acid 1%, and embedded in Epon. Thin sections were stained with uranyl acetate and lead citrate and observed on a CM10 Philips electron microscope (Philips, Heindoven, The Netherlands).
Immunoelectron microscopy was performed on cells embedded in glycol-methacrylate. Thin sections were incubated on polyclonal antibodies directed against kappa, lamda, mu, and gamma chains purchased from Dakopatts (Glostrup, Denmark) and used at a 1/2,000 dilution, and antibody to CD36 as previously described6 followed by goat anti-rabbit immunoglobulins conjugated to 10 nm colloidal gold (Amersham, Les Ullys, France).
Flow Cytometry
The fixation of normal and pathological IgG on erythroblast and erythrocyte membranes was tested by flow cytometry (FACSalibur; Becton Dickinson, Mountain View, CA). Mean fluorescence intensity (MFI) was used to measure antibody binding.
Erythroblasts and erythrocytes (106 cells in 50 μL) were incubated with IgG (50 μL at 500 μg/mL of purified IgG) for 1 hour at room temperature. Cells were washed in phosphate-buffered saline (PBS) and incubated with a rabbit anti-human IgG labeled with phycoerythrein (50 μL, 1/20; Dako, Glostrup, Denmark) for 30 minutes at 4°C and washed again in PBS. The volume of sample was adjusted to 500 μL. About 10,000 cells were counted in each experiment.
Western Blot
Proteins of erythroblast stroma were separated by polyacrylamide gel electrophoresis (PAGE) in the presence of sodium dodecyl sulfate and lithium dodecyl sulfate and transferred onto nitrocellulose sheets, then incubated with purified IgG of a normal subject and of the 2 patients (nos. 2 and 3) after the technique described by Towbin et al.7 An anti-CD36 immune serum raised in the rabbit was used as a positive control. The reaction was detected with ECL detection kit (Amersham, Little Chalfont, UK).
RESULTS
Bone Marrow Morphology
Light microscopy.
Bone marrow aspirates from the 3 patients showed marked hypercellularity and erythroid hyperplasia (>70% for case nos. 1 and 2). Bone marrow smear from patient 3 showed diffuse lymphocytic infiltration (86%) and numerous erythroid islands (Fig1). The main abnormalities observed in the bone marrow smears of the 3 patients were the presence of numerous erythroid islands, with aggregated erythroblasts and frequent binuclear forms. Most often, adjacent cells shared the appearance of the same maturation stage, but some cells of apparently different maturity were also in close contact. A nonbasophilic clear area was observed at sites of close proximity between adjacent erythroblasts. This feature allowed a distinction to be made between this type of erythroid island and that of sideroblastic anemia. No macrophages were found in contact with these erythroblast clusters.
Bone marrow smear (patient 2): The erythroblasts are numerous and several of them are closely apposed to each other. At the intercellular junction, a clear nonbasophilic zone is evident.
Bone marrow smear (patient 2): The erythroblasts are numerous and several of them are closely apposed to each other. At the intercellular junction, a clear nonbasophilic zone is evident.
Apart from these abnormalities, the qualitative maturation of the erythroid cell line was normal with a balanced proportion of the different maturation stages, normal nuclear chromatin pattern, and hemoglobinization. Granulocytic and megakaryocytic cell lines displayed a normal morphology.
Electron microscopy.
Electron microscopy confirmed the diagnosis of erythroblastic synartesis in the 3 patients. Plasma membranes of adjacent erythroblasts were joined by closely interdigitating processes (Fig2). Cytochemical demonstration of the peroxidatic activity of hemoglobin (not shown) allowed identification of maturation stages of the erythroblasts, because its level of intensity relates to the hemoglobin content of each cell. This technique showed that erythroblasts of the same maturation stage, as well as those of different maturation stages, were linked. Ribosomes were absent at sites of linkage via interdigitating process, corresponding with the nonbasophilic areas observed by light microscopy (Fig3a). At the junction sites, coated pits and rhopheocytosis vesicles were absent, probably because a macrophage expansion would find it impossible to penetrate this zone of tightly linked plasma membrane. Ferritin granules were never observed within these junctions. Junctions between erythroblasts had a characteristic morphology: resembling gap junctions, they were formed by 2 closely placed membranes joined by periodical structures every 150 Å, giving rise to a zipper-like appearance (Fig 3b). Isolated normoblasts often displayed picnotic nuclei and/or binuclearity. The 2 external leaflets of the plasma membrane of adjacent erythroblasts were separated by a constant space of 145 Å in patient 1, and 180 Å in patients 2 and 3.
The electron microscopic aspect of the bone marrow erythroblasts from patient 1: Cells are joined by interdigitating processes of the plasma membrane (arrowheads). They delineate areas of cytoplasm which are deprived of ribosomes (N, nucleus; m, mitochondria). Original magnification: ×21,000.
The electron microscopic aspect of the bone marrow erythroblasts from patient 1: Cells are joined by interdigitating processes of the plasma membrane (arrowheads). They delineate areas of cytoplasm which are deprived of ribosomes (N, nucleus; m, mitochondria). Original magnification: ×21,000.
(a) High magnification of the cell junction between 2 erythroblasts: The electron dense tracer of the extracellular space, tannic acid, decorates the intercellular space. The cytoplasm density of the 2 cells (1 and 2) is unequal, showing that different maturation-stage erythroblasts can be linked together. N, nucleus. Original magnification ×41,400. (b) At a higher magnification, these cell junctions display a septate-like structure (arrowheads). Original magnification ×177,000.
(a) High magnification of the cell junction between 2 erythroblasts: The electron dense tracer of the extracellular space, tannic acid, decorates the intercellular space. The cytoplasm density of the 2 cells (1 and 2) is unequal, showing that different maturation-stage erythroblasts can be linked together. N, nucleus. Original magnification ×41,400. (b) At a higher magnification, these cell junctions display a septate-like structure (arrowheads). Original magnification ×177,000.
In Vitro Experiments
The erythroblastic synartesis could be reproduced in vitro: indeed, erythroblast cultures established with bone marrow progenitors from cases 2 and 3 displayed similar morphological abnormalities to those observed in vivo (Fig4a, see page 3686). Crossed culture experiments using the patient erythroblasts grown in the presence of their own serum or of a control serum, or using erythroblasts of a control subject grown in the presence of the patient sera (see Table1) were performed and gave the following results: the erythroblast abnormalities reproduced in culture in the presence of autologous serum were absent when the culture was performed with a control AB serum (Fig 4b). Electron microscopy confirmed these findings, showing that the same intercellular junctions were observed when the patient erythroblastic progenitors were cultured with autologous serum, but that they were absent when the culture was performed in the presence of a control serum. These abnormalities were not reproduced in vitro when the erythroblasts were grown in the patient sera that had been IgG depleted. This allowed us to conclude that the synartesis was caused by an IgG component. Further confirmation was given by the observation that cultured normal erythroblasts displayed authentic synartesis when grown in the presence of patient to serum. Finally, the IgG fractions from the patients were added to normal serum at a concentration of 2 g/L and were able to induce typical synartesis on control erythroblasts grown in this preparation (Fig 5).
Light microscopical aspect of the erythroblasts cultured from patient 2 bone marrow. (a) In the presence of autologous serum: The erythroblasts are closely apposed to each other with occasional nonbasophilic areas present at their junction, mimicking the morphology of the bone marrow smears. (b) In the presence of a control serum: The grown erythroblasts are scattered and individually disposed on this cytospun preparation and do not display the characteristic abnormalities of the disease.
Light microscopical aspect of the erythroblasts cultured from patient 2 bone marrow. (a) In the presence of autologous serum: The erythroblasts are closely apposed to each other with occasional nonbasophilic areas present at their junction, mimicking the morphology of the bone marrow smears. (b) In the presence of a control serum: The grown erythroblasts are scattered and individually disposed on this cytospun preparation and do not display the characteristic abnormalities of the disease.
Induction of Erythroblast Synartesis in Crossed Culture Experiments
| . | Control Erythroblasts . | Patient 2 Erythroblasts . | Patient 3 Erythroblasts . |
|---|---|---|---|
| Control serum | − | − | − |
| Patient 2 serum | + | + | |
| Patient 2 IgG | + | + | |
| Patient 3 serum | ND | + | |
| Patient 3 IgG | ND | + |
| . | Control Erythroblasts . | Patient 2 Erythroblasts . | Patient 3 Erythroblasts . |
|---|---|---|---|
| Control serum | − | − | − |
| Patient 2 serum | + | + | |
| Patient 2 IgG | + | + | |
| Patient 3 serum | ND | + | |
| Patient 3 IgG | ND | + |
Abbreviation: ND, not done.
Typical synartesis (arrows) can be induced between control erythroblasts, when grown in the presence of patient isolated IgG fraction (shown here, patient 2). Original magnification ×16,100.
Typical synartesis (arrows) can be induced between control erythroblasts, when grown in the presence of patient isolated IgG fraction (shown here, patient 2). Original magnification ×16,100.
Flow Cytometry
The results of the immunofluorescence test are indicated in Table2. Purified IgG of patient no. 2 was detected on the surface of erythroblasts (MFI: 52) whereas the binding of the same IgG preparation on erythrocytes was weak (MFI: 2.5). Background fluorescence measured after incubation with IgG purified from a normal serum shows an MFI of 2.2 whatever the tested bone marrow cells were.
Flow Cytometric Analysis of the Binding of Two IgG Preparations on Erythroblasts and Erythrocytes
| Cells . | Normal IgG . | Patient’s IgG (Case no. 2) . |
|---|---|---|
| Erythroblasts | 2.2 | 52 |
| Erythrocytes | 2.2 | 2.5 |
| Cells . | Normal IgG . | Patient’s IgG (Case no. 2) . |
|---|---|---|
| Erythroblasts | 2.2 | 52 |
| Erythrocytes | 2.2 | 2.5 |
The figures indicate the MFI.
Immuno-electron Microscopy (Table 3)
A positive immunogold labeling for immunoglobulin gamma chains was specifically detected along the synartesis, indicating that immunoglobulin could be the responsible agent for the synartesis. Moreover, the observation of a positive reaction only for the gamma chains and not for the mu chains suggested that the immunoglobulin located within the synartesis was an IgG. Finally, immunolabeling for the immunoglobulin light chains kappa and lambda showed that only kappa chains were detected in the synartesis, whereas lambda chain labeling was consistently negative. This observation was evidence for the monoclonal nature of the IgG. Knowing that both patients had a serum monoclonal IgG kappa, the IgG detected in a synartesis seemed to be identical to the one in their serum.
Detection of Immunogold Labeling for Immunoglobulin Light and Heavy Chains in the Erythroblastic Synartesis
| . | Patient 2 . | Patient 3 . |
|---|---|---|
| Gamma | + | + |
| Mu | − | − |
| Kappa | + | + |
| Lambda | − | − |
| . | Patient 2 . | Patient 3 . |
|---|---|---|
| Gamma | + | + |
| Mu | − | − |
| Kappa | + | + |
| Lambda | − | − |
Thus, immunogold labeling showed that the monoclonal IgG, gamma, and kappa chains were present all along these abnormal junctions (Fig6), but labeling was only rarely observed on the parts of the erythroblast membranes that were not physically involved in the junctions.
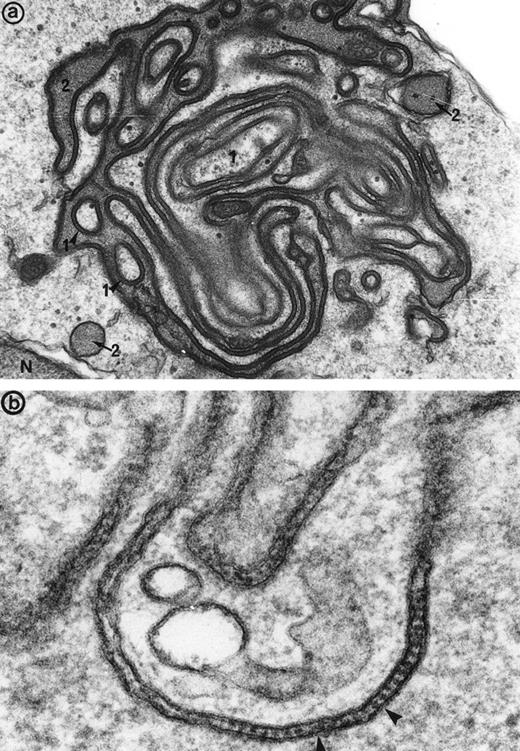
Immunogold labeling for gamma chain (and kappa chain, not shown) is positive along the linear junctions of erythroblasts (arrowheads) (a), as well as between glove finger invaginations (b and c), showing that the monoclonal IgG are indeed responsible for the synartesis (Original magnification: [a]: ×55,000; [b]: 55,000; [c]: 45,000).
Immunogold labeling for gamma chain (and kappa chain, not shown) is positive along the linear junctions of erythroblasts (arrowheads) (a), as well as between glove finger invaginations (b and c), showing that the monoclonal IgG are indeed responsible for the synartesis (Original magnification: [a]: ×55,000; [b]: 55,000; [c]: 45,000).
Some plasma cells were also found in the marrow and synthesized the same type of monoclonal immunoglobulin as the one found in the synartesis, such identified by the positivity of immunolabeling for gamma and kappa chains (Fig 7a) and negativity for lambda chains: indeed, plasma cells stained for the same light and heavy chain isotypes as the one found in the serum and synartesis. Immunolabeling for mu, delta, and lambda chains were consistently negative.
(a) Bone marrow plasma cells display immunolabeling for the monoclonal gamma chains in the numerous prominent cisternae of endoplasmic reticulum (er) in patient 2. m, mitochondria, N, nucleus. Original magnification ×30,000. (b) Bone marrow immunogold labeling for CD36: Gold particles outline the erythroblast junctions (arrowheads) where immunolabeling is more concentrated compared with the erythroblast plasma membrane (pm) not involved in the junction. Original magnification ×40,000.
(a) Bone marrow plasma cells display immunolabeling for the monoclonal gamma chains in the numerous prominent cisternae of endoplasmic reticulum (er) in patient 2. m, mitochondria, N, nucleus. Original magnification ×30,000. (b) Bone marrow immunogold labeling for CD36: Gold particles outline the erythroblast junctions (arrowheads) where immunolabeling is more concentrated compared with the erythroblast plasma membrane (pm) not involved in the junction. Original magnification ×40,000.
Finally, control labeling was performed on control normal erythroid islands and was consistently negative for both gamma and kappa chains.
When bone marrow sections were incubated for the immunodetection of the adhesion receptor CD36, which is a marker present in normal erythroblasts and absent from erythrocytes, some specific labeling was found in the junctions where it was definitely concentrated, compared with the erythroblast surface not involved in the junctions (Fig 7b).
Western Blot Analysis
No specific reaction between the blotted proteins and the purified IgG from patients 2 and 3 could be detected by this technique (results not shown), regardless of the detergent (sodium dodecyl sulfate [SDS] or lithium dodecyl sulfate [LiDoS]) used during PAGE. In parallel, a strong signal was obtained with the positive control.
DISCUSSION
This report describes 3 new cases of erythroblastic synartesis, whose diagnosis was made possible by morphological examination of bone marrow erythroblasts by light and electron microscopy. Very few cases of this disease have been described in the literature. This report presents a detailed clinical and morphological description of the disease. It is important that erythroblastic synartesis, which is probably an under-recognized disease, is not misdiagnosed as refractory anemia or as a malignant erythroblastic leukemia since a specific therapeutical approach should be undertaken. This report also elucidates the pathophysiology of the formation of abnormal junctions between the erythroblasts, and shows that this is due to serum IgG, and most probably to the monoclonal component IgG kappa, ie, a monoclonal immunoglobulin. Finally, successful therapeutic interventions are derived from these findings.
Observation of these 3 cases follows that of 2 previous cases reported in the literature.4,8-10 The peculiarity of this syndrome is the presence of specific junctions and interdigitating cytoplasmic processes between erythroblasts, which can be identified by electron microscopy and which do not exist either in the normal erythroblastic island or in dyserythropoiesis of other types.11-14 The existence of an abnormal physical link between erythroblasts may lead to impaired release of free RBCs and their intramedullary destruction.
The ultrastructure of these junctions displays a similar appearance to the gap junctions described in various normal and pathological human tissues, with intercellular particles joining the 2 cell membranes.15-17 Abnormal connections between erythroblasts involving the presence of ferritin molecules within the intercellular space have been described in hypersiderotic bone marrows from cases of acquired transient or permanent dyserythropoiesis.18-23Erythroblastic interdigitations and junctions are not a specific finding but only another nonspecific feature of dyserythropoiesis18,19,23 that can be found in various disorders, such as megaloblastic anemia, hereditary spherocytosis, thalassemia, and other dyserythropoietic conditions (sideroblastic anemia, refractory anemia transient dyserythropoiesis after bone marrow transplantation, paraneoplasis).14,20,22 24 The anatomical specificity of erythroblastic synartesis is the presence of a zipperlike structure between the abnormal junctions, in addition to the absence of detectable ferritin. Although ferritin granules could easily be detected by high magnification electron microscopic examination, ferritin was definitely absent from the intercellular junctions in the present 3 cases. Careful morphological examination and measurement of the ultrastructure and periodicity of the intra-junctional constituents showed that there were slight differences between the 3 patients with respect to the size of the particles punctuating the junction space. This may mean that the molecules sealing the 2 cell membranes together are of different nature in each of the patients.
An original finding in the 3 present cases is the association of the erythroid lineage disorder with immunologic humoral abnormalities. A positive Coombs test and a B-cell lymphoma were present in case 1. Sjögren syndrome with the presence of antinuclear factor, low complement fraction C4 level, a circulating anticoagulant, and a discrete monoclonal immunoglobulin were observed in case 2, and case 3 was associated with chronic lymphocytic leukemia. In addition, this study has led to some understanding of the pathophysiology of the anemia and its successful therapy. Experiments made in vitro on cultured erythroblasts from 2 of the 3 patients indicated that a serum factor was responsible for the erythroblast synartesis. Indeed, the erythroid ultrastructural abnormalities could be reproduced in vitro when patient marrows were grown in autologous serum, and were absent when grown with a control serum. However, the possibility that this may be the result of an abnormal antigen present on the patient erythroblasts and acting in conjunction with an abnormal serum factor could not be ruled out by the first set of experiments. In subsequent experiments, the observation that the patient serum could induce the characteristic ultrastructural abnormalities on normal control erythroblasts allowed us to conclude that an extracellular factor was the cause of the abnormal intercellular junctions. This factor was shown to be part of the patient IgG fraction; serum complement did not take part in the synartesis induction because isolated IgG fraction was able to induce synartesis on a control erythroblast culture. Moreover, monoclonal IgG kappa protein was concomittently detected in the erythroblast junctions.
This conclusion led to the introduction of corticosteroid therapy with a successful outcome. This was an important progression to understanding the pathophysiology and treatment of this disease, because the first patient described in the literature died of hemochromatosis after long-term transfusions.10 The death of patient 1 precluded performance of crossed culture experiments.
The fact that patient serum depleted of IgG could not reproduce in vitro the erythroblastic synartesis led us to implicate the role of the monoclonal immunoglobulin in the induction of abnormal inter-cellular junctions. The purification of the IgG and the further appearance of typical synartesis on control erythroblasts grown in their presence confirmed that immunoglobulin was the sole factor responsible for the dyserythropoiesis. It is notable that the erythroid intercellular junctions observed in the 3 present cases have a rather similar appearance (intercellular space and periodicity) whereas the equivalent structures in previously reported cases were larger.8 This could reflect the presence of immunoglobulins (of different nature and size) within the abnormal junctions.
Septate junctions are widespread in invertebrate tissues and have a highly distinctive morphology, but the junctional proteins are still poorly characterized.25 In this study, we attempted to determine nature of the antigen target located on the erythroblast membrane that was recognized by the antibody. Unfortunately, the identification of the target antigen by Western blot was negative. This could be explained by the fact that the epitope of the antigen recognized by the IgG is denatured by SDS and lithium dodecyl sulfate during Western blot analysis, whereas they are in a native conformation on erythroblast membrane during cytometric analysis. The positivity of flow cytometry experiment is an additive evidence for the recognition of an erythroblast antigen by a serum antibody. Flow cytometry shows that the candidate antigen is present on erythroblasts but absent from the RBC membrane. We have hypothetized that the target membrane antigen is an adhesive receptor, which would be activated by the fixation of the monoclonal IgG and then induce the junctions between erythroblasts: This is the case for CD36, which acts as a thrombospondin receptor,26 the function of which increases upon activation27 and thus is a good candidate antigen28 for the synartesis. Indeed, in favor of this hypothesis, immunolabeling for CD36 was more concentrated in the synartesis than on the free erythroblast surface, suggesting capping of this receptor. Western blot analysis did not allow us to obtain any specific signals with both patient sera. Unfortunately, the epitope recognized by the monoclonal antibodies seems to be quite sensitive to denaturation by SDS and LiDoS. The same observation was made for the erythroblast membrane CD36: Western blot analysis detected a positive signal when a polyclonal antibody to CD36 was used, but the reaction was negative with a monoclonal antibody (directed a single epitope). The fact that our patients did not have thrombocytopenia argues against the hypothesis of CD36 as the target antigen. However, the serum IgG might not react in vivo with platelet CD36 because of inadequate antibody/antigen ratio.
Other potential antigens are the integrins VLA4 and VLA5, the fibronectin receptors present on erythroblasts and absent from erythrocytes.29E-cadherin, a component of inter-endothelial cell adherent junctions, is also expressed by erythroblasts30 and the erythroblast-macrophage-protein (Emp), which mediates the attachment of the erythroblasts to the macrophage in the erythroblastic island31 and could be involved in this disorder. Finally, many components of the erythroblast membrane are still unknown and the responsible antigen could be one of them.
In conclusion, this report highlights characteristic features of a potentially under-recognized disorder. Crossed erythroblast culture experiments indicate that an abnormal monoclonal immunoglobulin, possibly directed against an adhesive receptor of the erythroblast membrane, is the causal factor of dyserythropoiesis. It is important that erythroblastic synartesis is recognized as such (and not misdiagnosed as a malignant erythroblastic leukemia or refractory anemia, which would imply repeated transfusions) and the patient offered a potentially more appropriate therapy with corticosteroids or effective treatment of an underlying lymphoid neoplasia.
ACKNOWLEDGMENT
The authors acknowledge Dr Jean-Philippe Rosa for helpful discussions; Prof. Henri Rochant for useful clinical informations concerning patient 1; Dr Paul-Henri Roméo for constant support; Josette Guichard, Martine Debbia, Pierre Gane, and Viviane Bony for their precious technical assistance, and Dorothée Ménage for secretarial help.
Supported by “Association pour la Recherche sur le Cancer” (ARC), and “Fondation pour la Recherche Médicale.”
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked“advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Elisabeth M. Cramer, MD, PhD, INSERM U.474, Hôpital Henri Mondor 94010 Créteil, France; e-mail:emcramer@im3.inserm.fr.





![Fig. 6. Immunogold labeling for gamma chain (and kappa chain, not shown) is positive along the linear junctions of erythroblasts (arrowheads) (a), as well as between glove finger invaginations (b and c), showing that the monoclonal IgG are indeed responsible for the synartesis (Original magnification: [a]: ×55,000; [b]: 55,000; [c]: 45,000).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/11/10.1182_blood.v94.11.3683/4/m_blod42323006y.jpeg?Expires=1763500402&Signature=A7d4xEkrmkHPJA7ZiK8r7zJt~NEciUn7sIh2oNxzRaKt~1L0h6QyDnyHxR~lOn5DKj7mHRLEmmtTiTlnFXbmZu8Md~m97lYUm4M4KoRt4f7WdWSHF-yzi2ppjUSh91uSBDSFWBebvOBwiST5RNvxo~AKRLElvYTMHxiFZZv-7W-2k3B0AlaoS3nFq9dy0~kU6NykhVoGqPW3XASdPvCs8CjJS5YCIl3LjGQJonJCFM5qRFtuE5U4JfM9hjEyrQUh5hqup2v5hxmcn6s7-E2RgR6WalMxtI60zm7d8ZA-DD9dXlAnwy7aAzm6SQprVyov8LJXFbxw4K8bPZ4hUVL73w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal