To the Editor:
Recent studies on the development of the hematopoietic system have led to substantial progress in describing the variety of hematopoietic cells within the mammalian embryo, but have resulted in the nonuniform and confusing usage of the term hematopoietic stem cell (HSC). In its conventional and well-accepted usage, the term (definitive) HSC describes those cells at the foundation of the adult hematopoietic hierarchy that, upon transplantation, give rise to long-term, multilineage, high-level hematopoietic engraftment of adult recipients. However, within a developmental framework, unique challenges are presented in naming the hematopoietic cells of the embryo, because lineage relationships between the embryo and adult have not been established. We propose here a terminology that reflects our current but limited understanding of the embryonic and developing definitive hematopoietic hierarchies.
Confusion in the terminology appears to stem from the implicit belief that the embryonic hematopoietic hierarchy is identical to that in the adult. Because the yolk sac is the first haematopoietic site during development, it was assumed for many years that the first HSCs emerge there. Yolk sac-derived HSCs were thought to give rise to the primitive erythroid lineage and then sequentially migrate, expand, and differentiate into the definitive hematopoietic lineages in the fetal liver, spleen, and bone marrow. This convenient dogma suggested that the process of adult HSC differentiation might be applied to embryonic blood development. But how can HSCs differentiate into the first yolk sac erythrocytes (7.5 day postcoitum [dpc]) within 1 day after mesoderm formation (6.5 dpc), when it is assumed that in the adult it takes weeks to months for the HSCs to generate mature red blood cells? The most convincing evidence indicating that the embryonic hematopoietic hierarchy is constructed differently from that of the adult is that a variety of lymphoid and hematopoietic progenitors are detected in the embryo before the first definitive HSCs emerge.1 The first HSCs capable of giving rise to complete hematopoietic engraftment of adult recipients appear in the embryo only by the end of 10 dpc within the aorta-gonad-mesonephoros (AGM) region.2,3 These observations in the mouse embryo indicate that such a concept of the yolk sac origins of adult HSCs might not necessarily be correct and indicate that, as with other embryonic tissues, the rudiment of the hematopoietic system is not simply a small model of the adult tissue. Indeed, the molecular genetic program supports this notion; the definitive blood system requires some gene products, eg, β 1 integrin or AML1, that are not necessary for normal yolk sac hematopoiesis.4-6
So, how does it happen that embryonic blood cells differentiate in the absence of definitive HSCs? One idea is that, during development, primitive erythrocytes, hematopoietic progenitors, and definitive HSCs are generated sequentially from the same cohort of multipotent founder cells that are laid down almost immediately after mesoderm formation. Developing within the embryo, these founder cells acquire properties of definitive HSCs that may include expression of homing receptor(s), high proliferative potential, etc. Another possible scenario, and the currently favored hypothesis, is that the embryonic and the definitive hematopoietic hierarchies are borne independently within the embryo. Indeed, the avian yolk sac forms an endemic embryonic hierarchy that does not contribute to the adult hematopoietic system.7Recent experiments suggest that the chick allantois is another site capable of independent hematopoietic cell production.8 In the early mouse embryo, hematopoietic progenitors can be generated both within the embryo body and the yolk sac well before completion of the circulatory system.9 Assuming that hematopoietic progenitors migrate exclusively via the circulation, these data indicate the existence of multiple independent hematopoietic sites, one of which may be the true source of the definitive/adult hematopoietic system.
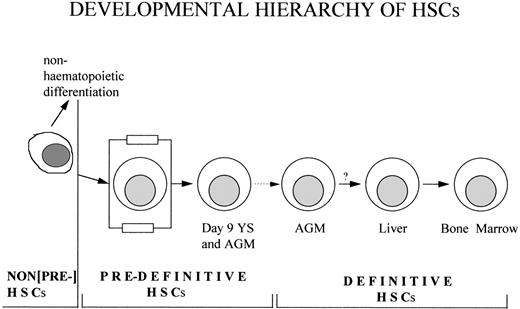
In this complex emerging hematopoietic hierarchy, when can a cell be termed a definitive HSC? Yoder et al10 have found that, if day-9 yolk sac and AGM cells are transplanted into newborn mice, they become capable of long-term multilineage repopulation. These findings indicate that, before being able to function within the adult bone marrow microenvironment, day-9 embryonic cells must go through an additional step of maturation. In normal development, this occurs within the embryonic microenvironment, most likely by late 10 dpc. In the newborn, a favorable fetal microenvironment likely can be provided to transplanted embryonic cells by the still hematopoietically active liver. It was concluded by Yoder et al10 that the day-9 embryo contains definitive lymphohematopoietic stem cells. Their rational appears to be that the progeny of cells transplanted into newborns can be found later within the definitive hematopoietic microenvironment of the adult mouse. However, following this logic, embryonic stem (ES) cells can also be termed as definitive HSCs, because, upon blastocyst injection, their progeny can contribute to the hematopoietic system of the adult mouse. Hence, we propose that the term definitive HSC be restricted to describe a cell characterized by its own ability to differentiate and expand on definitive, adult, hematopoietic territories. Those cells that require preliminary maturation, whether within a blastocyst, the midgestation embryo, the fetus, or the newborn animal, are not definitive HSCs. We propose that cells such as those identified by Yoder et al10 and those previously described in the transplacental assay by Weissman et al11 that cannot on their own repopulate adult mice, but do develop into definitive HSCs, should be called predefinitive HSCs. Strictly speaking, it needs to be elucidated in further experiments if the differentiation potential of these cells is restricted to the hematopoietic lineage. It also needs to be established if such a lineage continuity takes place in the developing embryo. As shown in Fig 1, predefinitive HSCs must originate from some early embryonic ancestor cells in which the differentiation potential is broader than hematopoietic.
Schematic representation of the cells and terms within the emerging hematopoietic hierarchy. Non–pre-HSCs are those cells that have potential for the hematopoietic lineage as well as other lineages. Predefinitive HSCs are those cells that have only a hematopoietic fate. Definitive HSCs are defined as those cells possessing their own ability to differentiate and expand on definitive, adult, hematopoietic territories. As noted by the dotted arrow, the lineage relationship between day-9 predefinitive HSCs and definitive HSCs has not been proven experimentally. The question mark between the AGM and fetal liver denotes the following. Although AGM-derived HSCs can directly repopulate adult bone marrow in transplantation experiments, experimental evidence showing embryonic migration of these cells through fetal liver before colonization of the bone marrow is 1acking.
Schematic representation of the cells and terms within the emerging hematopoietic hierarchy. Non–pre-HSCs are those cells that have potential for the hematopoietic lineage as well as other lineages. Predefinitive HSCs are those cells that have only a hematopoietic fate. Definitive HSCs are defined as those cells possessing their own ability to differentiate and expand on definitive, adult, hematopoietic territories. As noted by the dotted arrow, the lineage relationship between day-9 predefinitive HSCs and definitive HSCs has not been proven experimentally. The question mark between the AGM and fetal liver denotes the following. Although AGM-derived HSCs can directly repopulate adult bone marrow in transplantation experiments, experimental evidence showing embryonic migration of these cells through fetal liver before colonization of the bone marrow is 1acking.
The nature of the early embryonic ancestor cells and the process of their multistage development into adult bone marrow definitive HSCs is still at a rudimentary level of understanding. Thus, we propose the terminology shown in Fig 1, which we believe will be useful for future experimental analysis and will broaden our understanding of the development of definitive HSCs within the mammalian embryo.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal