To the Editor:
After the Shc gene was identified, biological function studies have focused on the oncogenic potentials of Shc proteins, especially their role in the Ras-dependent mitogen-activated protein kinase activation.1,2 Jucker et al3 reported inBlood that Shc proteins are constitutively tyrosine-phosphorylated in primary acute myelogenous leukemia (AML) cells but not in primary cell cultures or normal tissues.3,4 The presence of constitutively phosphorylated Shc proteins found in the AML peripheral blood (PB) cells, but not in normal PB cells, led to the suggestion that the Ras pathway may be constitutively activated in AML.3 However, it should be noted that in these studies Shc phosphorylation was compared in PB cells of AML and PB cells of healthy donors. One potential problem with their conclusion is that AML cells are immature myeloid cells while normal PB cells are end-stage mature cells. Hence, the Shc phosphorylation difference between these two groups of cells might reflect difference in the level of cell maturation rather than a difference between leukemic and normal myeloid cells. To distinguish between Shc phosphorylation in these two groups of cells, one must compare Shc phosphorylation in AML cells and in normal bone marrow (BM) cells that are at a comparable level of maturation.
We evaluated Shc protein expression and phosphorylation in normal hematopoietic progenitor cells and leukemia hematopoietic cells. Five normal individuals and 10 AML patients participated in this study. Three leukemic cell lines (HL-60, K562, and KG-1) were also studied. BM and PB specimens were collected before any treatment from the 10 patients with newly diagnosed AML. BM aspirates were obtained from the five normal donors. Standard Ficoll density centrifugation was performed to collect mononuclear cells (MNCs). MNCs from BM specimens of two patients with AML and five normal donors were subjected to CD34 separation by MACS separation columns (Miltenyi Biotec Inc, Auburn, CA). Cell pellets of PB and BM were lysed in cell lysis buffer. The cell lysates were diluted to 1 mg/mL total cell protein measured by the Bio-Rad Protein Assay. Polyclonal anti-human Shc antibody (5 μg) (Upstate Biotechnology Inc, Lake Placid, NY) was added. The immunocomplex was captured by Protein A/G Plus agarose beads. Total cell lysates or immunoprecipitates were subjected to Western blotting. Blots were probed with antiphosphotyrosine (4G10) (0.5 μg/mL) (Upstate Biotechnology Inc). Antiphosphotyrosine blots were stripped and reprobed with anti-Shc anti-serum (3 μg/mL).
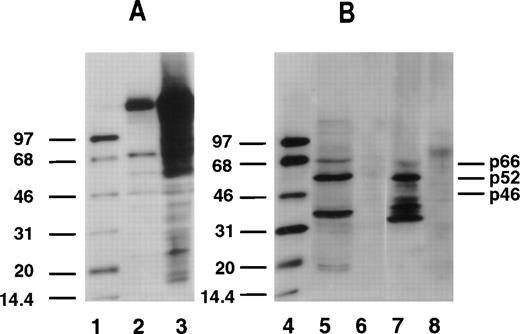
Our data show that among the 10 AML patients reported here, tyrosine-phosphorylated Shc proteins were expressed constitutively in PB cells of all 10 AML patients. Phosphorylated p66Shc, p52Shc, and p46Shc was found in 8, 10, and 7 of 10 AML PB samples, respectively. Shc tyrosine phosphorylation was examined in CD34+ and CD34− cells separated from BM aspirates of two AML patients. The expression of Shc proteins was found in both subsets. However, tyrosine-phosphorylated Shc proteins were expressed only in CD34+ cells and not in the CD34− leukemia cells. Among five normal BM specimens, tyrosine-phosphorylated Shc proteins were expressed exclusively in the CD34+ cell compartment, but not in CD34−cells or in the unseparated cells. Phosphorylated p66Shc, p52Shc, and p46Shc was found in 2, 5, and 5 of 5 normal CD34+ samples, respectively. With respect to tyrosine-phosphorylated Shc proteins, no significant difference was observed between CD34+ leukemia cells and CD34+normal cells (Fig 1). Phosphorylation of p66Shc, p52Shc, and p46Shc on tyrosine was detected in K562 and KG-1 cells, but not in HL-60 cells.
Tyrosine phosphorylation of Shc proteins in normal CD34+ progenitor cells and leukemic CD34+cells. Total cell lysates (20 μg protein) were subjected to Western blot analysis. Phosphotyrosine was identified by antiphosphotyrosine antibody (4G10). Representative blots are shown. The positions of p66Shc, p52Shc, and p46Shc are indicated on the right. Molecular-weight marker bands are indicated on the left. Lanes 1 and 4, enhanced chemiluminescence protein molecular-weight markers; lane 2, Nonstimulated A431 cell lysate; lane 3, epidermal growth factor–stimulated A431 cell lysate; lane 5, CD34+ cells from normal BM; lane 6, CD34− cells from normal BM; lane 7, CD34+cells from AML patient BM; lane 8, CD34− cells from AML patient BM.
Tyrosine phosphorylation of Shc proteins in normal CD34+ progenitor cells and leukemic CD34+cells. Total cell lysates (20 μg protein) were subjected to Western blot analysis. Phosphotyrosine was identified by antiphosphotyrosine antibody (4G10). Representative blots are shown. The positions of p66Shc, p52Shc, and p46Shc are indicated on the right. Molecular-weight marker bands are indicated on the left. Lanes 1 and 4, enhanced chemiluminescence protein molecular-weight markers; lane 2, Nonstimulated A431 cell lysate; lane 3, epidermal growth factor–stimulated A431 cell lysate; lane 5, CD34+ cells from normal BM; lane 6, CD34− cells from normal BM; lane 7, CD34+cells from AML patient BM; lane 8, CD34− cells from AML patient BM.
Our study shows that the presence of constitutively tyrosine-phosphorylated Shc proteins does not distinguish between normal progenitor cells and AML cells, suggesting that phosphorylation of Shc proteins might be associated more with cell maturity than with malignant transformation.
ACKNOWLEDGMENT
Supported by National Cancer Institute Grant No. 1-PO-1 CA75606-01.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal