Lactoferrin (LF), a human serum protein, strongly inhibited the adherence of Plasmodium falciparum–infected erythrocytes (PE) to immobilized chondroitin sulfate A (CSA)–conjugated albumin at a concentration of 100 μg/mL and blocked the PE binding to CD36-expressing Chinese hamster ovary (CHO) cells, as well as immobilized CD36 at concentrations of 5 μg/mL and 100 μg/mL, respectively. Biotinylated LF bound to CD36 in a saturable manner, and such binding was inhibited by unlabeled LF and the anti-CD36 monoclonal antibody, 8A6, suggesting specificity of binding. Additionally, LF inhibited PE binding to immobilized thrombospondin (TSP) at a concentration of 100 μg/mL, and specific binding of LF to TSP was confirmed using biotinylated LF. LF inhibited PE binding to C32 amelanotic melanoma cells in a dose-dependent manner. A peptide of LF, Arg-Asn-Met Arg-Lys-Val Arg-Gly-Pro-Pro-Val-Ser-Cys (amino acid residues 25-37 of LF), which has been suggested to contribute to LF binding to various materials, including CSA, inhibited PE binding to immobilized CSA-conjugated albumin, immobilized CD36, CD36-expressing CHO cells, immobilized TSP, and C32 amelanotic melanoma cells, as well as LF itself. These results suggest that LF peptide may provide the basis for developing agents that are able to inhibit CSA-, CD36-, and TSP-mediated cytoadherence of PE.
HUMAN MALARIA is caused by four species of parasites (Plasmodium falciparum, P vivax, P ovale, andP malariae). Five hundred million people are infected with malaria each year, resulting in 1.5 to 2.7 million deaths (mainly children under the age of 5 and a number of pregnant women) annually.1P falciparum is responsible for most human deaths. P falciparum–parasitized erythrocytes (PE) bind to postcapillary endothelial cells (EC) of the brain and other organs.2 Several EC receptors, including CD36,3intercellular cell adhesion molecule-1 (ICAM-1),4thrombospondin (TSP),5 chondroitin sulfate A (CSA),6,7 vascular cell adhesion molecule-1 (VCAM-1), E-selectin,8 and CD31,9 have been reported to contribute to the cytoadherence of PE. One of the first steps in developing antiadhesive agents is to find substances that can block cytoadherence. For instance, it was reported that a peptide, Arg-Gly-Asp (RGD), first identified in fibronectin, has the potential to reduce experimental metastasis (ie, adhesion of cancer cells to endothelial cells) in vivo.10 We previously reported that infusion of a synthetic peptide, His-Pro-Leu-Gln-Lys-Thr-Tyr (HPLQKTY), based on a sequence found in an erythrocyte-membrane protein (band 3), into monkeys infected with P falciparum malaria resulted in the appearance of PE (bearing mature stage parasites) in the peripheral blood, suggesting an antisequestering effect.11
The human serum protein, lactoferrin (LF), binds to cell-surface chondroitin sulfate12 and has been shown to inhibit sporozoite invasion of hepatocytes.13 We examined the effects of LF on PE adherence, first focusing on CSA as a receptor and extending this to other PE receptors, ie, CD36 and TSP. The N-terminus peptidic sequence of LF was suggested to be involved in LF binding to CSA and inhibition of sporozoite invasion. Therefore, we also examined the inhibitory effects of an N-terminus peptide of LF, Arg-Asn-Met-Arg-Lys-Val-Arg-Gly-Pro-Pro-Val-Ser-Cys (RNMRKVRGPPVSC; amino acid residues 25-37 of LF),14 on CSA-, CD36-, and TSP-mediated PE binding.
MATERIALS AND METHODS
Materials.
LF (from human milk), transferrin (TF; human), CSA (from bovine trachea; molecular weight of 20 to 30 kD), mouse anti-CSA monoclonal antibody (MAb), and bovine serum albumin (BSA; fraction V) were obtained from Sigma (St Louis, MO). The biotinylation reagent, biotin-BMCC, and alkaline phosphatase–conjugated streptavidin were obtained from Pierce (Rockford, IL). A peptide, RNMRKVRGPPVSC (LF peptide), was obtained from Research Genetics (Huntsville, AL). Other peptides (Lys-Pro-Ser-Glu-Lys-Ile-Gln-Val-Leu-Lys-Asn-Leu-Lys-Arg-Asn-Tyr [KPSE peptide; amino acid residues 394-409 of CD36] and Phe-Ala-Ser-Pro-Val-Glu-Asn-Pro-Asp-Asn-Tyr-Gly-Phe [FASP peptide; amino acid residues 300-312 of CD36]) were obtained from Coast Scientific (San Diego, CA). CSA-conjugated BSA (CSA-BSA) was prepared to facilitate immobilization as described previously.15Briefly, a mixture of CSA (final concentration 80 mg/mL), BSA (final concentration 13.6 mg/mL), and sodium cyanoborohydride (final concentration 20 mg/mL) in 0.2 mol/L potassium phosphate (pH 8.0) were incubated at 37°C for 96 hours. The mixture was dialyzed against 1 L of distilled H2O (dH2O) before use. Conjugation of CSA to BSA was confirmed by checkerboard titration with anti-CSA antibody in an enzyme-linked immunosorbant assay, because unconjugated CSA will not bind to plastic. CSA-BSA conjugate or BSA (2 mg/mL) was serially diluted in dH2O in precleaned Costar vinyl microtiter plates (Corning, Acton, MA) at 50 μL/well and allowed to bind at 4°C overnight. Plates were blocked with 2% BSA in 10 mmol/L Tris-buffered saline (TBS; pH 7.4) at 100 μL/well at room temperature for 1 hour. After rinsing with TBS, anti-CSA MAb diluted 1:200 in TBS was added at 50 μL/well at room temperature for 3 hours. Wells were rinsed with TBS, and alkaline phosphatase–conjugated antimouse IgM (1:500 dilution in TBS) was added at 50 μL/well at room temperature for 1 hour. Wells were washed with TBS and color developed with p-nitrophenyl phosphate reagent (Sigma) and read at 405 nm on a BioRad 450 microplate reader (BioRad, Hercules, CA). The anti-CSA MAb did not bind to BSA-coated wells. The concentration of BSA in CSA-BSA solution was determined by Protein Assay (BioRad) using BSA as a standard, and the concentrations of CSA-BSA were expressed as the concentration of BSA (μg BSA/mL). The Protein Assay was not interfered with by the presence of CSA in solution. The amount of CSA was quantified according to the method of Enobakhare et al.16 To immobilize CSA-BSA, 50 μL of CSA-BSA solution (2 mg BSA/mL) was added to wells of the microtiter plate and incubated at 37°C for 4 hours. Free CSA was completely removed by several washings with dH2O. The amount of BSA and CSA of immobilized CSA-BSA was determined as described above. It was determined that 12 molecules (average) of CSA bound to 1 molecule of BSA by assuming the molecular weights of CSA and BSA are 25 kD and 67 kD, respectively. (Molecular weight of CSA ranges from 20 to 30 kD, according to the manufacturer.) Chinese hamster ovary (CHO) cells transfected with genes coding for CD36 (CD36-CHO cells)17 were grown in RPMI 1640/10% fetal calf serum. The C32 amelanotic melanoma cell line (American Type Culture Collection No. CRL 1585; ATCC, Manassas, VA) was maintained in culture as described earlier.18 The CD36-CHO cells and C32 amelanotic melanoma cells were deposited on multiwell glass slides (Cel-line Associates, Inc, Newfield, NJ) for cytoadherence assays as described previously.19 Partially purified CD36 (CD36 fraction) was obtained from human platelets essentially by the method of Tandon et al.20 However, the last purification step using Ultrogel AcA-44 (LKB, Rockville, MD) was omitted. TSP was purified as described previously.21 Anti-CD36 MAbs, FA6, and 8A6 were kindly provided by Dr John Barnwell (New York University Medical Center, New York, NY) and Dr Lena Edelman (Institut Pasteur, Paris, France), respectively.
Parasites.
All P falciparum lines (FCR-3 from The Gambia, Brazil; and the CSA–preferring line FAF-EA8CHO5, designated CS2,22 derived from the Brazilian Ituxi isolate) were cultured in O+ human erythrocytes as described previously.23 Cultures of FCR-3 and CS2 were synchronized at the mature stage by gelatin flotation.24
Immobilization of proteins on slides.
CSA-BSA was diluted to 5 μg BSA/mL in dH2O, and 3 μL was spotted on plastic slides (Nunc, Inc, Naperville, IL). After drying at room temperature, the surface of the CSA-BSA–bound spot was blocked with 0.1% BSA in phosphate-buffered saline (PBS) at room temperature for 2 hours. In the case of CD36, 5 mL of CD36 solution containing 0.1% Triton X-100 (Sigma) (initial concentration 268 μg protein/mL) was incubated with 0.5 g of SM-2 beads (Bio-Rad) at room temperature for 2 hours and dialyzed against 500 mL of distilled water to remove most of the detergent. Five microliters of the dialyzed solution was mounted onto multiwell glass slides, and incubated at 4°C overnight. To immobilize TSP, a TSP solution (50 μg/mL in 50 mmol/L Bis-Tris, 100 mmol/L NaCl, 25 mmol/L calcium lactate, 1 mmol/L MnCl2, pH 7.4 [BTC]) was spotted on plastic slides and incubated at 4°C overnight. CD36- and TSP-immobilized spots were blocked with 0.5% BSA in PBS at room temperature for 1 hour.
Cytoadherence assay.
Cytoadherence assays were performed as described previously.19,25 The spots of fixed target cells or immobilized protein were pretreated with 5 μL of inhibitor (LF, LF peptide, and control proteins/peptides) in 10 mmol/L PBS (pH 7.0) at room temperature for 2 hours. After removal of inhibitor solution, the slides were placed in individual compartments of a partitioned plastic box containing 7 mL Bis-Tris buffer (50 mmol/L Bis-Tris, 100 mmol/L NaCl, 2 mmol/L CaCl2, pH 6.6 for CSA and CD36, BTC for TSP). One to two hundred microliters of packed erythrocytes (5 to 10% parasitemia) was added to each compartment of the plastic box. After incubating under a heat lamp for 90 minutes with gentle rocking, the erythrocyte suspension was carefully aspirated. The slides were rinsed by adding 7 mL of the buffer and rocking for 5 minutes, after which time the buffer was aspirated. The slides were washed three times, fixed, and stained as described previously.19 The number of bound erythrocytes/target cells or square millimeters area of immobilized protein was counted under a light microscope.
LF binding to immobilized CD36 and TSP.
Ten microliters of ascites fluid of the anti-CD36 MAb, FA6, was diluted 1:100 in distilled water and dried on microtiter wells. To immobilize CD36 protein on the FA6-bound wells, the solution of CD36 (5.36 μg protein/mL) was added to the wells and incubated at 4°C overnight. The wells were blocked with 100 μL of 1% casein at room temperature for 1 hour. In the case of TSP, TSP was immobilized by incubating the wells with 5 μL of 50 μg/mL TSP at 4°C overnight. The wells were blocked with 100 μL of 1% casein at room temperature for 1 hour. Twenty microliters of biotinylated LF (prepared according to the manufacturer’s [Pierce] instructions using biotin-BMCC) was added to the wells and incubated at room temperature for 2 hours. Unbound biotinylated LF was removed by several washes. The amount of bound biotinylated LF to the immobilized CD36 or TSP was determined using alkaline phosphatase–conjugated streptavidin (50 μL of 1:1,000 dilution). Sigma 104 phosphatase substrate (Sigma) was used for color development, and the plate was read at 405 nm.
RESULTS
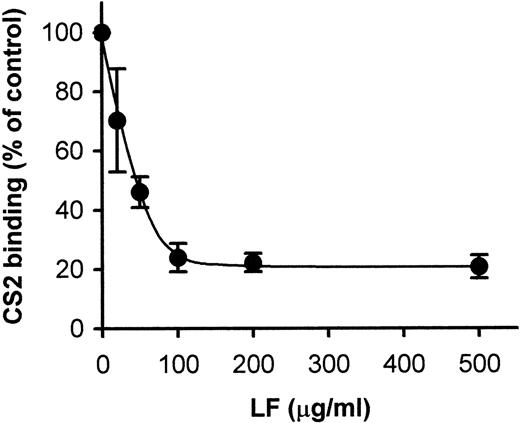
P falciparum PEs have been shown to bind to cell-surface CSA, and it has been suggested that this binding is implicated in the development of malarial infections of the placenta.26Recently, it was reported that LF binds to cell-surface CSA12; so first, we examined the effect of LF on the binding of CS2 (CSA-preferring P falciparum line)-PE to CSA-BSA immobilized on plastic slides. Binding of PE to CSA was inhibited in a dose-dependent manner when immobilized CSA-BSA was pretreated with LF-containing solutions; 70% inhibition was obtained at a concentration of 100 μg/mL (Fig 1). We used human TF as a negative control, because LF and TF have 59% homology in their primary amino acid sequence.14Pretreatment of immobilized CSA-BSA with TF was without effect at a concentration of 1 mg/mL. TF was without effect in all cytoadherence assays (described below), even at concentrations in which LF exerted a maximum inhibitory effect (data not shown).
Inhibition of CS2-PE binding to immobilized CSA-BSA by LF. Spots of CSA-BSA immobilized on plastic slides were pretreated with indicated concentrations of LF. After removal of the LF solution, the slides were subjected to the cytoadherence assay. Each point represents the mean ± standard deviation (SD) of triplicate determinations. The average of the number of CS2 bound to immobilized CSA-BSA in the absence of LF was 298.8 PEs/mm2.
Inhibition of CS2-PE binding to immobilized CSA-BSA by LF. Spots of CSA-BSA immobilized on plastic slides were pretreated with indicated concentrations of LF. After removal of the LF solution, the slides were subjected to the cytoadherence assay. Each point represents the mean ± standard deviation (SD) of triplicate determinations. The average of the number of CS2 bound to immobilized CSA-BSA in the absence of LF was 298.8 PEs/mm2.
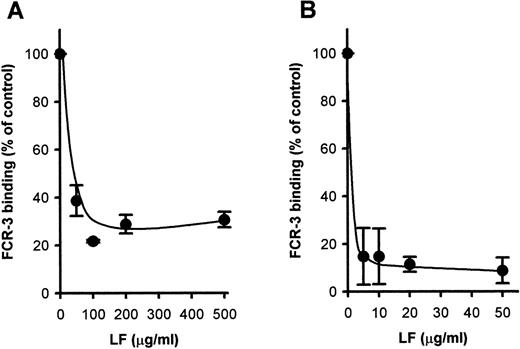
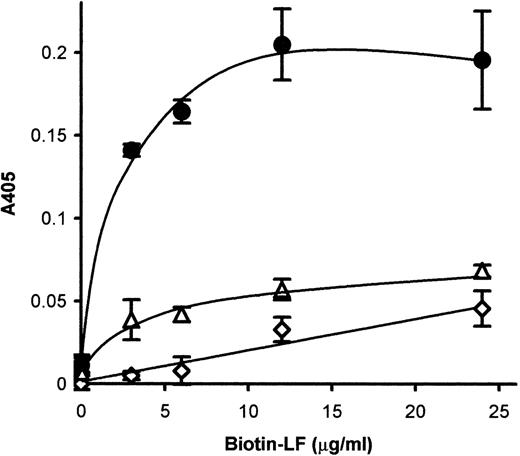
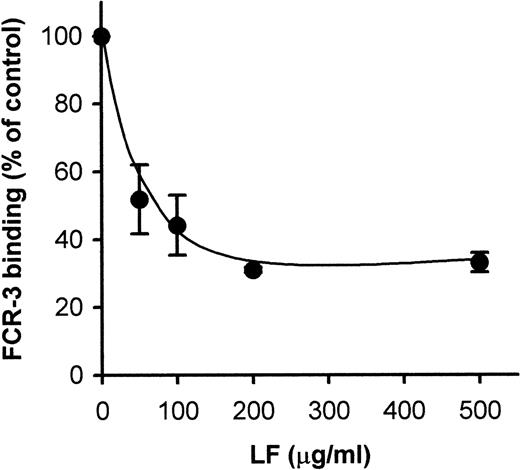
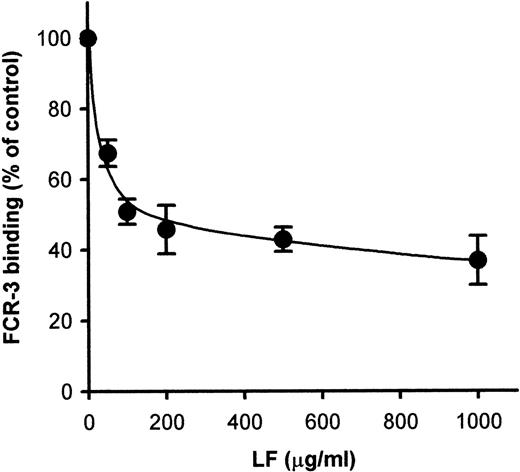
Because CD36-mediated cytoadherence has been claimed to be implicated in cerebral malaria, we next attempted to see whether LF would also inhibit PE binding to CD36. Pretreatment of immobilized CD36 with LF inhibited the binding of FCR-3 (CD36-preferring P falciparumline)–PE to immobilized CD36 in a dose-dependent manner (Fig2A). Maximal inhibition (∼70% inhibition) was obtained at 100 μg/mL. The FCR-3–PE binding to CD36-expressing CHO cells was also inhibited (∼90%) by pretreatment with LF (Fig 2B) at a concentration of 5 μg/mL. These results indicated that LF had the ability to bind to CD36 protein. To confirm this, the binding of biotinylated LF to CD36 was examined as described in Materials and Methods. MoAb FA6–immobilized microtiter plates were used to specifically immobilize CD36 protein on the wells. The binding of biotinylated LF to immobilized CD36 was observed and reached a plateau at a concentration of 15 μg/mL (Fig3). Binding was considerably lower when FA6 or CD36 was omitted, showing the specificity of binding. Furthermore, binding was inhibited by pretreatment of immobilized CD36 by LF (Fig4A) or the anti-CD36 monoclonal antibody, 8A6 (Fig 4B), but not by TF or an irrelevant monoclonal antibody (data not shown). This, again, showed the specificity of the LF binding and confirmed that the observed inhibitory activity of LF was caused by its binding to CD36. The PE binding of FCR-3 to immobilized TSP was also inhibited by pretreatment of TSP with LF in a dose-dependent manner (Fig 5), reaching 30% of the control at a concentration of 200 μg/mL. Binding of biotinylated LF to immobilized TSP was also observed (Fig 6A), and such binding was inhibited by the presence of unlabeled LF (Fig 6B), but not by TF (data not shown), showing that the inhibitory activity of LF was a result of its binding to TSP. The effect of LF as an antiadhesive for PE binding to the amelanotic melanoma cell, C32 was also examined: binding was inhibited by pretreatment of C32 cells with LF (Fig7). Fifty percent inhibition was obtained at a concentration of 200 μg/mL; higher concentrations were without further effect. Therefore, we next examined inhibitory effect of LF peptide (RNMRKVRGPPVSC) on PE binding to target cells or immobilized protein used above. Surprisingly, LF peptide inhibited PE binding in a dose-dependent manner to all targets we tested (Table1). Maximum inhibition of PE binding to immobilized CSA-BSA, immobilized CD36, CD36-CHO cells, immobilized TSP, and C32 amelanotic melanoma cells by LF peptide was 20.7%, 34.9%, 49.0%, 20.1%, and 39.5% of control at concentrations of 500, 1,000, 100, 200, and 100 μg/mL, respectively. Two irrelevant peptides, FASP and KPSE peptide, were without effect even at a concentration of 1 mg/mL.
Inhibition of FCR-3-PE binding to (A) immobilized CD36 and (B) CD36-CHO cells. The immobilized CD36 or CD36-CHO cells on glass slides were pretreated with LF at the indicated concentrations. After removal of the LF solution, the slides were subjected to the cytoadherence assay. Each point represents the mean ± SD of triplicate determinations. The averages of the number of FCR-3 bound to CD36-CHO cell and immobilized CD36 in the absence of LF were 3.85 PEs/CHO cell and 1,415.9 PEs/mm2, respectively.
Inhibition of FCR-3-PE binding to (A) immobilized CD36 and (B) CD36-CHO cells. The immobilized CD36 or CD36-CHO cells on glass slides were pretreated with LF at the indicated concentrations. After removal of the LF solution, the slides were subjected to the cytoadherence assay. Each point represents the mean ± SD of triplicate determinations. The averages of the number of FCR-3 bound to CD36-CHO cell and immobilized CD36 in the absence of LF were 3.85 PEs/CHO cell and 1,415.9 PEs/mm2, respectively.
The binding of biotinylated LF to immobilized CD36. CD36 was immobilized to microtiter wells via FA6 anti-CD36 antibody. The biotinylated-LF binding to the immobilized CD36 was examined (•). The same assays were conducted, omitting immobilization of FA6 (◊) or CD36 (▵). Each point represents the mean ± SD of triplicate determinations. A405, absorbance at 405 nm.
The binding of biotinylated LF to immobilized CD36. CD36 was immobilized to microtiter wells via FA6 anti-CD36 antibody. The biotinylated-LF binding to the immobilized CD36 was examined (•). The same assays were conducted, omitting immobilization of FA6 (◊) or CD36 (▵). Each point represents the mean ± SD of triplicate determinations. A405, absorbance at 405 nm.
Inhibition of biotinylated-LF binding to immobilized CD36 by (A) unlabeled LF or (B) anti-CD36 antibody, 8A6. The CD36-immobilized wells were pretreated with (A) unlabeled LF or 8A6 at the indicated concentration. After removal of pretreatment solution, the microtiter plates were subjected to biotinylated-LF-binding assay. Absorbance at 405 nm (A405) obtained in the absence of FA6 was subtracted from data, and the results are expressed as a percentage of the binding in the absence of inhibitor. Each point represents the mean ± SD of triplicate determinations. The averages of A405 obtained by biotinylated LF binding to CD36 in the absence of unlabeled LF were (A) 0.126 and (B) 0.115, respectively.
Inhibition of biotinylated-LF binding to immobilized CD36 by (A) unlabeled LF or (B) anti-CD36 antibody, 8A6. The CD36-immobilized wells were pretreated with (A) unlabeled LF or 8A6 at the indicated concentration. After removal of pretreatment solution, the microtiter plates were subjected to biotinylated-LF-binding assay. Absorbance at 405 nm (A405) obtained in the absence of FA6 was subtracted from data, and the results are expressed as a percentage of the binding in the absence of inhibitor. Each point represents the mean ± SD of triplicate determinations. The averages of A405 obtained by biotinylated LF binding to CD36 in the absence of unlabeled LF were (A) 0.126 and (B) 0.115, respectively.
Inhibition of FCR-3–PE binding to immobilized TSP by LF. The spots of TSP immobilized on plastic slides were pretreated with the indicated concentrations of LF. After removal of the LF solution, the slides were subjected to the cytoadherence assay. Each point represents the mean ± SD of triplicate determinations. The average of the number of FCR-3 bound to immobilized TSP in the absence of LF was 1,757.5 PEs/mm2.
Inhibition of FCR-3–PE binding to immobilized TSP by LF. The spots of TSP immobilized on plastic slides were pretreated with the indicated concentrations of LF. After removal of the LF solution, the slides were subjected to the cytoadherence assay. Each point represents the mean ± SD of triplicate determinations. The average of the number of FCR-3 bound to immobilized TSP in the absence of LF was 1,757.5 PEs/mm2.
(A) The binding of biotinylated LF to immobilized TSP. TSP was immobilized to microtiter wells. The biotinylated-LF binding to the immobilized TSP (•) was examined. The same assay was conducted omitting TSP (▵). Each point represents the mean ± SD of triplicate determinations. (B) Inhibition of biotinylated-LF binding to immobilized TSP by unlabeled LF. Biotinylated LF binding to immobilized TSP was examined in the presence of the indicated concentrations of unlabeled LF. Absorbance at 405 nm obtained in the absence of TSP was subtracted, and the results are expressed as a percentage of the binding in the absence of inhibitor. The average A405 obtained by biotinylated LF binding to TSP in the absence of unlabeled LF was 0.26. Each point represents the mean ± SD of triplicate determinations.
(A) The binding of biotinylated LF to immobilized TSP. TSP was immobilized to microtiter wells. The biotinylated-LF binding to the immobilized TSP (•) was examined. The same assay was conducted omitting TSP (▵). Each point represents the mean ± SD of triplicate determinations. (B) Inhibition of biotinylated-LF binding to immobilized TSP by unlabeled LF. Biotinylated LF binding to immobilized TSP was examined in the presence of the indicated concentrations of unlabeled LF. Absorbance at 405 nm obtained in the absence of TSP was subtracted, and the results are expressed as a percentage of the binding in the absence of inhibitor. The average A405 obtained by biotinylated LF binding to TSP in the absence of unlabeled LF was 0.26. Each point represents the mean ± SD of triplicate determinations.
Inhibition of FCR-3–infected cell binding to C32 cells. The C32 cells were pretreated with LF at the indicated concentrations. After removal of the LF solution, the slides were subjected to the cytoadherence assay. Each point represents the mean ± SD of triplicate determinations. The average number of FCR-3 bound to C32 amelanotic melanoma cells in the absence of LF was 6.66 PEs/melanoma cell.
Inhibition of FCR-3–infected cell binding to C32 cells. The C32 cells were pretreated with LF at the indicated concentrations. After removal of the LF solution, the slides were subjected to the cytoadherence assay. Each point represents the mean ± SD of triplicate determinations. The average number of FCR-3 bound to C32 amelanotic melanoma cells in the absence of LF was 6.66 PEs/melanoma cell.
Effect of Peptides on PE Binding to Immobilized Proteins and Cells
| Peptides . | Concentration (μg/mL) . | PE Binding (% of control) . | ||||
|---|---|---|---|---|---|---|
| Targets . | ||||||
| CSA-BSA . | CD36 . | CD36-CHO Cell . | TSP . | C32 Cell . | ||
| None | — | 100 | 100 | 100 | 100 | 100 |
| LF peptide | 10 | 76.1 ± 11.3 | ND | ND | 75.2 ± 13.5 | ND |
| 20 | 42.6 ± 1.2 | ND | ND | 68.0 ± 8.7 | ND | |
| 50 | 41.2 ± 20.9 | ND | 77.3 ± 10.7 | 30.8 ± 2.6 | 68.1 ± 28.9 | |
| 100 | 38.0 ± 14.4 | 85.8 ± 32.7 | 49.0 ± 5.0 | 38.2 ± 4.4 | 39.5 ± 14.6 | |
| 200 | ND | 79.6 ± 26.4 | ND | 20.1 ± 6.7 | ND | |
| 500 | 20.7 ± 1.9 | 58.0 ± 5.6 | 47.8 ± 8.1 | ND | 43.7 ± 8.8 | |
| 1,000 | 21.9 ± 10.1 | 34.9 ± 20.3 | 50.6 ± 10.4 | ND | 37.2 ± 10.9 | |
| FASP peptide | 1,000 | 93.2 ± 16.9 | 107.6 ± 13.3 | 107.6 ± 30.0 | 98.4 ± 2.3 | 93.7 ± 12.4 |
| KPSE peptide | 1,000 | 90.0 ± 18.7 | 106.6 ± 10.1 | 98.2 ± 14.7 | 95.5 ± 10.7 | 108.3 ± 27.4 |
| Peptides . | Concentration (μg/mL) . | PE Binding (% of control) . | ||||
|---|---|---|---|---|---|---|
| Targets . | ||||||
| CSA-BSA . | CD36 . | CD36-CHO Cell . | TSP . | C32 Cell . | ||
| None | — | 100 | 100 | 100 | 100 | 100 |
| LF peptide | 10 | 76.1 ± 11.3 | ND | ND | 75.2 ± 13.5 | ND |
| 20 | 42.6 ± 1.2 | ND | ND | 68.0 ± 8.7 | ND | |
| 50 | 41.2 ± 20.9 | ND | 77.3 ± 10.7 | 30.8 ± 2.6 | 68.1 ± 28.9 | |
| 100 | 38.0 ± 14.4 | 85.8 ± 32.7 | 49.0 ± 5.0 | 38.2 ± 4.4 | 39.5 ± 14.6 | |
| 200 | ND | 79.6 ± 26.4 | ND | 20.1 ± 6.7 | ND | |
| 500 | 20.7 ± 1.9 | 58.0 ± 5.6 | 47.8 ± 8.1 | ND | 43.7 ± 8.8 | |
| 1,000 | 21.9 ± 10.1 | 34.9 ± 20.3 | 50.6 ± 10.4 | ND | 37.2 ± 10.9 | |
| FASP peptide | 1,000 | 93.2 ± 16.9 | 107.6 ± 13.3 | 107.6 ± 30.0 | 98.4 ± 2.3 | 93.7 ± 12.4 |
| KPSE peptide | 1,000 | 90.0 ± 18.7 | 106.6 ± 10.1 | 98.2 ± 14.7 | 95.5 ± 10.7 | 108.3 ± 27.4 |
Spots of immobilized proteins and cells were pretreated with the indicated concentrations of peptide solutions, and the pretreatment solutions were removed before cytoadherence assay. Cytoadherence assays were conducted as described under Materials and Methods and in the legends of Figs 1, 2, 5, and 7. Each value represents the mean ± SD of triplicate determinations.
Abbreviation: ND, not determined.
DISCUSSION
LF, an iron-binding glycoprotein with molecular weight of 80 kD, exists in milk, other body fluids, serum, and specific granules of neutrophils. Several roles for LF have been suggested, such as bactericidal and immunomodulatory effects.27 It was also reported that LF inhibited the invasion of P falciparumsporozoites into host hepatocytes.13 Cytoadherence of PE to postcapillary EC is believed to relate to pathogenesis and death in falciparum malaria. As a possible antiadhesion protein, we focused on LF, because it was reported that LF bound to CSA,12 a receptor for PE. Herein we have shown that LF inhibited PE binding to immobilized CSA (Fig 1), immobilized CD36, CD36-CHO cells (Fig 2), immobilized TSP (Fig 5), and C32 amelanotic melanoma cells (Fig 7), and the effective concentrations of LF ranged from 5 to 100 μg/mL. These concentrations are much higher than the physiological level of LF in serum (0.2 μg/mL).28 However, because LF is released from neutrophils activated by tumor necrosis factor (TNF)-α29and this cytokine is increased in serum during a malarial infection,30 it is possible that higher concentrations of LF do occur during infection. Increased concentrations of LF (0.9 μg/mL) were found in the serum of patients with sepsis.28Furthermore, other investigators have observed 12 μg/mL LF in serum of relapsing chronic myeloid leukemia.31 Possibly in the microenvironment, where infected cells are sequestered, the concentration of LF released from neutrophils can be sufficient to partially inhibit cytoadherence.
We showed that the inhibitory effects of LF on PE binding were a result of specific binding of LF to CD36 and TSP. Recently, we found that the PE binding site on TSP is the RGD sequence–containing motif in the type 3 repeat region; LF also bound to the type 3 repeat region (S. Eda, unpublished results). This region of TSP has been reported to be involved in the binding of many cell types, including human endothelial cells, smooth muscle cells, C32 amelanotic melanoma cells, U937 monocytic cell line, etc, to TSP.32 This raises the possibility that LF inhibits not only PE binding, but also the binding of many cell types via the type 3 repeat region of TSP. In the case of CD36, Asch et al reported that the amino acid sequences 8-21 and 97-110 were responsible for PE binding.33 Later, Serghides et al claimed that the amino acid sequence 243-333 was responsible for PE binding.34 The LF binding site on CD36 is presently unknown; however, it is possible that LF binds to the CD36 binding site for the PE, or to adjacent sites. It will be of interest to examine this possibility, because PE-binding sites on CD36 have been located to overlapping or adjacent binding sites for various CD36 ligands, such as TSP (amino acid residues 87-9933 and 93-12035 of CD36), oxidized low density lipoprotein (LDL; residues 155-183 of CD36),36 and fatty acid (residues 127-279 of CD36).37
LF binds to a wide range of materials (such as lysozyme, lipopolysaccharide (LPS), oxidized LDL, DNA, etc) and cell surface receptors (intestinal brush border, phytohemagglutinin-activated lymphocyte, monocyte/macrophage, hepatocyte, platelet, etc).27 Our present results add CD36 and TSP to the list of LF-binding materials. This feature of LF may be favorable to block PE binding in the malarial patient, because as mentioned above many EC receptors are thought to contribute to PE binding. Indeed, the binding of PE to C32 cells (which express CD36, ICAM-1, CSA, and TSP5,38,39) was blocked by LF in a dose-dependent manner (Fig 7). This result was supported by the fact that the binding of FCR-3 to immortalized human brain endothelial cells, BB19 (which express CD36, ICAM-1, VCAM-1, and E-selectin40), was also inhibited by LF in a static microtiter cytoadherent assay, which we have recently developed (J.G. Prudhomme, unpublished results).
These results suggested that it is possible and important to find an active peptide from LF for the future chemical design and synthesis of antiadhesion agents. We focused on amino acid residues 25-37 of LF (RNMRKVRGPPVSC,14 termed LF peptide in this paper), because this region was suggested to bind to CSA41 and to inhibit sporozoite invasion of the hepatocyte.13 Moreover, this region has been suggested to contribute to a multifunctional property of LF, such as LF binding to LF receptors on hemagglutinin-activated lymphocytes42 and platelets,43 remnant receptor,44 and lipopolysaccharide.45 Synthetic LF peptide inhibited PE binding to immobilized CSA-BSA, CD36 and TSP, and C32 amelanotic melanoma cells, as well as LF protein per se. FASP and KPSE peptide did not inhibit PE binding, showing the specificity of the inhibitory effect of LF peptide. Furthermore, the inability of KPSE peptide to inhibit PE binding indicates the inhibitory effect of LF peptide on PE binding was not simply because of the cationic feature (4 basic amino acids out of 13 amino acids) of LF peptide, because KPSE peptide also contains a net 4 positive charges in 15 amino acids (5 basic amino acids and 1 acidic amino acid).
Several surface proteins on PE, such as PfEMP-1, sequestrin, and band 3–related adhesin, were implicated in PE cytoadherence.2In the case of PfEMP-1 and the band 3–related adhesin, the region(s) that contribute to PE binding to their receptor have been identified, and antiadhesive peptides have been developed. Baruch et al reported that the fusion protein (rC1-2) that lies in cysteine-rich interdomain region of PfEMP1 mediated binding to CD36, but not to TSP nor CSA.46 Amino acid residues 1-179 of rC1-2 were able to inhibit PE binding to CD36, but not to CSA under both static and flow conditions.47 We have found that the peptidic sequence HPLQKTY, a exofacial loop 3 region of band 3, is involved in TSP-mediated PE binding, and the synthetic peptide inhibited PE binding to immobilized TSP, but not to CD36.48 It should be noted that the combination of the relatively small size (tridecapeptide) and the multi-inhibitory effects of the LF peptide could be of considerable advantage as a lead compound for developing potent antiadhesive agents. Further studies on the binding sites of CD36 and TSP for LF may provide valuable information on the molecular mechanisms of the inhibitory action of LF peptide, as well as the possible effects of the peptide on the functions of CD36 and/or TSP.
Supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (AI21251).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Irwin W. Sherman, PhD, Department of Biology, University of California, Riverside, CA 92521; e-mail: sherman@mail.ucr.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal