Abstract
A central question in hematopoiesis is how cell-cycling behavior changes during the emergence of the differentiated state. To further understand what genetic regulators might couple proliferation status to differentiation, we studied the expression of the cell-cycle inhibitors p21 and p27 during the in vitro differentiation of normal CD34+ blast cells along the myeloid lineage. We find p27 but not p21 to be expressed in freshly harvested resting CD34+ cells. Thereafter, p21 levels peak concurrent with cellular proliferation and then decline in expression as cells undergo terminal differentiation. In contrast, p27 levels are fairly constant but the subcellular localization of p27 changes from nuclear expression to predominantly cytoplasmic expression and finally to perinuclear localization at progressive stages of differentiation. This report discusses the implications of these findings.
A CENTRAL FEATURE of differentiation is the transition from quiescent progenitor cells to a pool of proliferating precursor cells, giving rise to nonproliferating, differentiated progeny. The emergence of terminally differentiated cells in several lineages is coupled to a transition out of the mitotic pool. Genetic regulators responsible for the coupling of proliferation status to the emergence of the differentiated phenotype are under investigation. Previous studies from our laboratory showed that the p21 (WAF1) cell-cycle inhibitor is upregulated during myeloid differentiation in cell line models.1
p21 and other inhibitors of G1-phase cyclin-dependent kinases such as p27 could conceivably contribute to downmodulation of the proliferating potential of differentiating progeny. p21 and p27 share a similar domain involved in cdk and cyclin binding, and both have been shown to mediate growth arrest when overexpressed,2,3 contribute to restriction point G1 arrest,4-6 and are upregulated in myeloid differentiation models.1,7,8 However, the roles of p21 and p27 during myelopoiesis may be distinct rather than overlapping. p21 has been shown either to promote or inhibit cell cycle progression on a stoichiometric basis,9,10 operating as a cycling-cdk assembly factor at low concentrations. p27 levels have been shown to decrease and p21 levels to increase in lymphocytes stimulated to exit from quiescence11 and on stimulation of factor depleted Mo7e cells with stem cell factor (SCF) and granulocyte-macrophage colony-stimulating factor (GM-CSF).12 Moreover, p21 knockout mice are defective in steel-factor–driven myelopoiesis.12 Experiments in other systems suggest that terminal differentiation might involve a predominant role for p21; keratinocytes from p21-knockout mice but not p27-knockout mice exhibit a significant decrease in differentiation markers.13
As an initial step in establishing the role of these proteins during differentiation of normal hematopoietic precursor cells, we have analyzed the expression of these cdk inhibitors (cdki’s) in normal umbilical cord CD34+ cells stimulated to differentiate along the myeloid lineage. Morphologic and cell cycle profiles of cells expressing high levels of p21 and p27 were determined. Image analysis of immunostained cytospins and fluorescence-activated cell sorter (FACS) analysis showed that p21 expression is absent in freshly harvested CD34+ cells and increases from 2 to 6 days after exposure to SCF, granulocyte-colony-stimulating factor (G-CSF), and interleukin-3 (IL-3) p21 levels peak before terminal differentiation and fall within 11 days of exposure to cytokines. p27 is expressed both in freshly harvested and in differentiated cells, but its subcellular distribution is altered during differentiation; this suggests that p27 may serve different functions at different stages of myeloid differentiation. Neither the expression of p21 nor p27 is restricted to G0/G1 phase, as demonstrated by dual flow determination of cdki expression and cell-cycle partitioning. These results suggest that p21 and p27 have distinct roles during the myeloid differentiation process. The changing expression and subcellular patterns of these cdki’s during myelopoiesis indicate a complex function for p21 and p27 throughout differentiation, rather than a limited role mediating growth arrest in the final stages of terminal differentiation.
MATERIALS AND METHODS
Cells.
CD34+ cells were prepared as previously described.14 In brief, human umbilical cord blood (CB) samples were obtained in accordance with institutional guidelines, and low density mononuclear cells were isolated by Ficoll-Paque (1.077 g/mL) density gradient centrifugation. CB mononuclear cells were washed twice in phosphate buffered saline (PBS) and resuspended in PBS + 0.6% anticoagulant citrate dextrose (ACD) for magnetic labeling and separation. Cells were incubated with blocking reagent (human IgG) and QBEND/10 anti-CD34 antibody for 15 minutes at 4°C, then washed in PBS/ACD-A followed by incubation with a secondary antibody-magnetic microbead conjugate for an additional 15 minutes at 4°C. The percentage of CD34+ cells generally exceeded 95% as determined by flow cytometric analysis. The CD34+ cells were cultured in serum-free StemPro media (Life Technologies, Gaithersburg, MD) and supplemented with 50 ng/mL kit ligand (R&D Systems, Minneapolis, MN), 100 ng/mL IL-3 (R & D Systems), and 30 ng/mL G-CSF (Neupogen; Amgen, Inc, Thousand Oaks, CA). When indicated, CD34+ cells were prepared by negative selection by using Stem Sep columns (StemCell Technologies, Inc, Vancouver, Canada) and by using a CD34+ enrichment cocktail per the manufacturer’s protocol. Use of negative selection to enrich CD34+ cells yielded purity of roughly 70% CD34+ cells by flow cytometric analysis. Neutrophils were harvested after spinning plasma, removing residual erythrocytes with hypotonic washes, followed by Ficoll-Paque (1.077 g/mL) density gradient centrifugation for 30 minutes at 400 g and collection of the pellet in PBS. Lymphocytes were purified and washed from ficoll interface. Purity of all cells (generally >95%) was verified by Wright-Giemsa staining.
Flow cytometry.
Cells harvested at various time points adjusted to 1 × 106 per mL were fixed with 1% paraformaldehyde and kept in 75% ethanol at −20°C. Before staining, cells were permeabilized with 0.25% Triton-X 100 and 40 μg/mL digitonin for 5 minutes, followed by washing. Cells were then incubated with monoclonal antihuman p21 or antihuman p27 (Neomarkers, Freemont, CA) at a concentration of 1:50 for 1 hour on ice, followed by incubation with biotinylated horse antimouse antibody (Vector Laboratories, Burlingame, CA) at 1:200 for 40 minutes on ice and extraavidin-fluorescein isothiocyanate (FITC) conjugate at 1:100 (Sigma, St Louis, MO) for 30 minutes on ice. After antibody staining, the cells were prepared for DNA staining by addition of RNAse and propidium iodide and incubated on ice according to standard techniques. Additional antibodies used for surface characterization of differentiation were CD71FITC (DAKO, Carpinteria, CA), CD34PE (Becton Dickinson, San Jose, CA), CD11bPE (DAKO), and CD15FITC (Becton Dickinson), which were all used according to manufacturer’s instructions.
Cells were also stained in suspension for confocal microscopy analysis. Cells were fixed and permeabilized for flow cytometry. For two-color staining, cells were incubated with p27 polyclonal antibody (Santa Cruz, sc-528, directed at aa 191-198) at 1:50 dilution for 1 hour followed by goat anti-rabbit Alexa-488 (Molecular Probes, Eugene, OR) at 1:500 dilution for 1 hour. After staining for p27, the cells were incubated with monoclonal anti-Ki67 (Oncogene Research Products, Cambridge, MA) for 1 hour followed by goat anti-mouse Cy3-conjugated secondary antibody (Jackson Immunoresearch Labs, Inc, West Grove, PA) at a 1:3,000 dilution for 1 hour. Suspension cells were doubly stained for p27 and CD34 or CD71 by incubating negatively selected cells with phycoerythrin (PE) conjugated CD34 (anti-HPCA-2, Becton Dickinson) or with CD71 FITC (Dako, Inc, Denmark) for 20 minutes followed by fixation and permeabilization. The CD34+ stained cells were then incubated with polyclonal rabbit anti-p27 (Santa Cruz) at 1:50 dilution for 1 hour after goat anti-rabbit Alexa-488 (Molecular Probes) at 1:500 dilution. The CD71-stained cells were then incubated with monoclonal anti-p27 (Neomarkers) at 1:50 dilution, followed by anti-mouse Cy3 (Jackson Immunoresearch Labs) at 1:3,000 dilution for 50 minutes. Alternatively, as indicated, cells were stained with p27 goat polyclonal (Santa Cruz, sc527, directed at N terminal aa 2-21) at 1:50 dilution, followed by donkey anti-goat Cy3 (Jackson Immunoresearch Labs) at 1:500 dilution.
Immunocytochemistry.
Cytospins were stained by fixation in 2% paraformaldehyde and then permeabilized in 0.1% Triton X-100 and 40 μg/mL digitonin, followed by staining for 1 hour with monoclonal antibody (MoAb) to p21 (supernatant CP36, courtesy of Brian Dynlacht, at a ratio of 1:80) or Neomarkers anti-p27 Ab-1 at a ratio of 1:50, followed by four PBS washes, incubation for 30 minutes with biotinylated goat anti-mouse IgG antibody (Vector Laboratories) again, followed by three washes. Immunodetection was enabled by a final 30-minute incubation with Cy3-conjugated-streptavidin at 1:12,000 (Jackson Immunochemicals, West Grove, PA), followed by three PBS washes. Cells were counterstained with Hoechst. In some experiments, the cells were incubated with BrDU for 8 hours before harvest and the slides stained indirectly with anti-BrDU antibody (Amersham, Arlington Heights, IL), biotinylated horse antimouse antibody, and streptavidin AP-conjugate, developed with AEC (Amersham). For BrDU staining and for p27 peroxidase staining, slides were fixed in 50% ethanol for 30 minutes at 4°C, treated with 0.1 N HCl and 0.7% Triton, and heat denatured in H2O.
Confocal microscopy.
To maximize spatial resolution in all three axes, confocal microscopy was used to examine the precise distribution of p27 in cells. After immunohistochemical labeling, cell suspensions were placed onto charged coverslips mounted within a Leica TCS-NT confocal microscope (Leica, Deerfield, IL) and allowed to settle. Subsequently, the cells were scanned with a 100× plan apochromat objective at 1,024 × 1,024 pixel resolution, with a magnification factor of 2× at the scan head. To maximize Z-axis, resolution scans were performed with a small pinhole such that the resolution from a measured point spread function in the Z-axis was less than twice the X-Y resolution (0.35 um). Each scan is the Kalman average of four sequential scans through the middle plane of the cells.
Image analysis.
p21 staining structures in a field were rendered to a grey scale with the Hoechst-stained nucleus as a mask to segregate cells. Profiles of the p21 labeling are extracted and the intensity quantified by using Optimas (Media Cybernetics, Silver Spring, MD).
RESULTS
To characterize p21 and p27 expression during myelopoiesis, we set up an in vitro culture system of CD34+ cells simulating myeloid differentiation. CD34+ cells were harvested by immunoaffinity purification of mononuclear cells obtained from fresh umbilical cord blood. The CD34+ cells were cultured in StemPro (Life Technologies, Gaithersburg, MD) serum-free medium supplemented with 100 ng/mL G-CSF, 50 ng/mL SCF, and 50 ng/mL IL-3. At various time points after the initiation of culture, aliquots of cell suspension were taken for flow cytometric analysis and for cytospins. As shown in Fig 1A, these culture conditions restrict progeny to the myeloid lineage, with mostly myelocytes arising after 4 days in culture and bands and neutrophils predominating after 11 days. Morphological differentiation was accompanied by the expected development of cell-surface markers as shown in Fig 1B. CD71, CD15, and CD11b were used to characterize the emergence of differentiating cells. CD71, a marker of proliferating cells, appeared at 4 days in culture. Single positive CD15 cells were followed by CD15+ CD11b+ and CD15− CD11b+ cells emerging at day 7 as indicated. This progression of markers is similar to previous reports.15
(A) Differentiation of CD34+ cells in StemPro culture medium. Morphology of cells stimulated to differentiate into granulocytes by kit ligand, interleukin-3 (IL-3), and granulocyte-colony-stimulating factor (G-CSF) in StemPro serum-free medium. (B) Surface marker expression by CD34+ cells differentiating under culture conditions. (C) Cell-cycle distribution of differentiating CD34+ cells overtime determined by propidium iodide staining.
(A) Differentiation of CD34+ cells in StemPro culture medium. Morphology of cells stimulated to differentiate into granulocytes by kit ligand, interleukin-3 (IL-3), and granulocyte-colony-stimulating factor (G-CSF) in StemPro serum-free medium. (B) Surface marker expression by CD34+ cells differentiating under culture conditions. (C) Cell-cycle distribution of differentiating CD34+ cells overtime determined by propidium iodide staining.
Differentiation was accompanied by entry of cells into cycle, peaking at 4 to 5 days, followed by a gradual decrease in the percentage of cells in S-phase (Fig 1C). Staining after a 12-hour BrdU pulse confirmed that the percentage of BrdU positive cells declined from 45% at day 6 to 16% at day 10 (data not shown).
p21 and p27 during myelopoiesis.
To first characterize the expression of p21 and p27 in quiescent CD34+ blasts and their differentiating progeny, we immunostained cytospins of CD34+ blasts and of CD34+ cells cultured in cytokines for 6 days. Freshly harvested CD34+ were subjected to immunohistochemical staining for p21 or p27 after overnight culture in low serum to facilitate immunoaffinity bead detachment. Similar results were obtained with immediate staining of cells after chymopapain treatment. As Fig 2 shows, CD34+ blasts were negative for p21 expression when using a sensitive detection system consisting of a biotinylated secondary antibody and streptavidin conjugated Cy3 tertiary staining. Staining with a monoclonal anti-p21 antibody CP36 (courtesy of Brian Dynlacht, MGH, Boston, MA) is shown; similar results were obtained with several commercial antibodies. In contrast to p21, p27 expression is readily evident in nuclei of these cells and can be detected by using either a fluorescent secondary antibody (shown here) or with peroxidase enzymatic detection (see Fig 4). As noted above, the CD34+cells are in G0/G1 phase. The absence of p21 and presence of p27 in the nuclei of these cells suggests that p27, not p21 is contributing to growth arrest of these cells, which is consistent with findings in quiescent lymphocytes.16
Expression of p21 and p27 in CD34+ blasts and in differentiating cells. (A-F) Cytospins of positively selected CD34+ blasts maintained overnight in low serum after harvest were stained for p21 or p27 as described (Neomarkers MoAbs). Some beads used in the immunoisolation are apparent in the isotype control. Nuclear staining of p27 is evident. Cytospins prepared after 6 days of culture in SCF, G-CSF, and IL-3 were simultaneously stained. Nuclear staining of p21 and cytoplasmic staining of p27 is evident. Cells are counterstained with Hoechst. (G-L) Staining of negatively selected CD34+ cells freshly harvested (G) or after 4 days of culture (H-L). (G) Confocal micrograph of cells doubly stained for CD34 (phycoerythrocin, red) and p27 (Alexa-488). A field containing a CD34− cell is shown, enlarged for detail. (H) Photograph of cells doubly stained for p27 (Cy3, red) and CD71 (fluorescein isothiocyanate [FITC]). After days of culture, 86% of the cells were positive for CD71. (I) Confocal micrograph of cells doubly stained for Ki-67 (Cy3) and p27 (Alexa-48). Nuclear Ki-67 and cytoplasmic p27 is evident. Overlapping nuclear signals are yellow. (J) Isotype control for (I). All isotype controls were similarly negative. (K, L) Confocal microscopy of cells for p27, using C-terminal (G) and N-terminal (L) (Santa Cruz) antibodies.
Expression of p21 and p27 in CD34+ blasts and in differentiating cells. (A-F) Cytospins of positively selected CD34+ blasts maintained overnight in low serum after harvest were stained for p21 or p27 as described (Neomarkers MoAbs). Some beads used in the immunoisolation are apparent in the isotype control. Nuclear staining of p27 is evident. Cytospins prepared after 6 days of culture in SCF, G-CSF, and IL-3 were simultaneously stained. Nuclear staining of p21 and cytoplasmic staining of p27 is evident. Cells are counterstained with Hoechst. (G-L) Staining of negatively selected CD34+ cells freshly harvested (G) or after 4 days of culture (H-L). (G) Confocal micrograph of cells doubly stained for CD34 (phycoerythrocin, red) and p27 (Alexa-488). A field containing a CD34− cell is shown, enlarged for detail. (H) Photograph of cells doubly stained for p27 (Cy3, red) and CD71 (fluorescein isothiocyanate [FITC]). After days of culture, 86% of the cells were positive for CD71. (I) Confocal micrograph of cells doubly stained for Ki-67 (Cy3) and p27 (Alexa-48). Nuclear Ki-67 and cytoplasmic p27 is evident. Overlapping nuclear signals are yellow. (J) Isotype control for (I). All isotype controls were similarly negative. (K, L) Confocal microscopy of cells for p27, using C-terminal (G) and N-terminal (L) (Santa Cruz) antibodies.
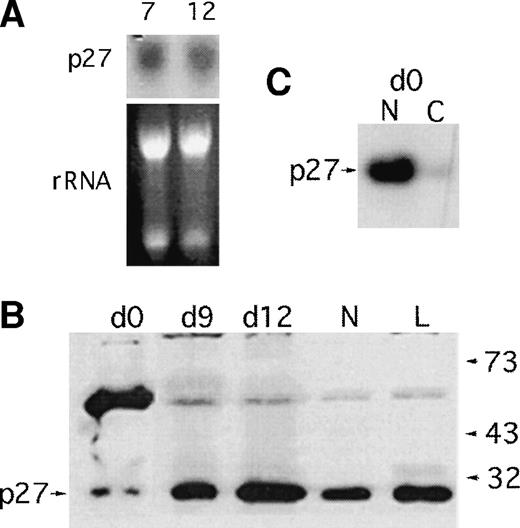
Several interesting findings emerge from staining of cells differentiated for 6 days (predominantly myelocytes under our culture conditions). p21 protein is upregulated in the maturing cells, which is consistent with our previous findings14 and is predominantly localized to the nucleus. Surprisingly, the cells develop strong p27 immunoreactivity in the cytoplasm as they differentiate. A reticular staining pattern is evident. The persistent expression of p27 contrasts with downmodulation of p27 protein levels in lymphocytes and in fibroblasts stimulated to enter the cell cycle.4,16 The cytoplasmic localization of p27 is distinct from the predominantly nuclear localization reported for p27 transfected into mink lung epithelial cells.17 In that study, forced cytoplasmic expression of p27 eliminated cdk4 sequestration by cytoplasmic p15. The persistence of p27 expression in proliferating and differentiating myeloid cells was also confirmed by Western blotting and Northern blotting (Fig 3). A single band of the correct size is recognized in differenting cells by the monoclonal used in immunostaining; the identity of an additional, slowly migrating band most prominent in CD34+ blasts is under investigation. This band is repeatable with other antibodies; we note that ubiquitinated p27 gives a prominent band at this position.18 p27 message is constant during differentiation (Fig 3) in contrast to p21 message, which is upregulated as we have previously reported.1 14
p27 message and protein levels during myelopoiesis. (A) Persistence of p27 message in differentiating CD34+cells. Aliquots of cells grown in the presence of SCF, G-CSF, and IL-3 for 7 or 10 days were harvested for RNA and a Northern blot probed for p27 message as shown. (B) Freshly harvested UC CD34+cells were cultured in the presence of cytokines for 12 days. At days 0, 6, 9, and 12 aliquots of 1 million cells were directly lysed in laemmli buffer and fractionated on a 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel for Western blot analysis. Neutrophils (N) and lymphocytes (L) from the same cord sample were analyzed. p27 immunoblot is shown; p21 levels were undetectable in this assay. (C) p27 immunoblot of CD34+ blasts fractionated for nuclear (N) and cytoplasmic expression of p27.
p27 message and protein levels during myelopoiesis. (A) Persistence of p27 message in differentiating CD34+cells. Aliquots of cells grown in the presence of SCF, G-CSF, and IL-3 for 7 or 10 days were harvested for RNA and a Northern blot probed for p27 message as shown. (B) Freshly harvested UC CD34+cells were cultured in the presence of cytokines for 12 days. At days 0, 6, 9, and 12 aliquots of 1 million cells were directly lysed in laemmli buffer and fractionated on a 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel for Western blot analysis. Neutrophils (N) and lymphocytes (L) from the same cord sample were analyzed. p27 immunoblot is shown; p21 levels were undetectable in this assay. (C) p27 immunoblot of CD34+ blasts fractionated for nuclear (N) and cytoplasmic expression of p27.
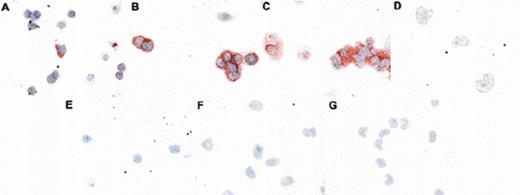
The relocalization of p27 in differentiating cells was confirmed by using confocal microscopy as well as by using antibodies recognizing distinct epitopes and by staining different fixation conditions (Figs 2and 4). Confocal microscopy and Western blotting (Fig 3C) was used to verify that cytospin staining showed p27 in the nuclei of CD34+ blasts rather than in overlying cytoplasm. Figure 2G shows a representative confocal image of a CD34+ and CD34− cell present in a population of cells enriched for CD34+ by negative selection. Nuclear p27 (green) is present in the CD34+ cell; nuclear and cytoplasmic p27 is evident in the larger CD34− cell. p27 persistence and localization was also examined in cells stimulated to proliferate by 4 days of culture in G-CSF, SCF, and IL-3 (Fig 2H-2L). In Fig 2H, cells stained both for p27 and for the CD71 (transferrin receptor), a marker of proliferation, are photographed. A smaller blast and a larger cell positive both for CD71 (green) and p27 (red) are shown. Figure 2I shows confocal microscopy of cells doubly stained for the proliferation marker Ki-67 (red) and for p27 (green). Isotype-control–stained cells are shown in Fig 2J. The presence of p27 in Ki-67+ cells is evident. Moreover, whereas Ki-67 only stains the cell nucleus, p27 is also present in the cytoplasm; cytoplasmic p27 appears to be associated with cellular proliferation. Figures 2K and 2L are representative confocal micrographs of differentiating cells after 4 days of culture stained with C-terminal or N-terminal p27 antibodies, respectively. Both nuclear and cytoplasmic staining is evident when using antibodies specific for either epitope. The relocalization of p27 in differentiating cells was confirmed by using peroxidase as an alternative staining and fixation procedure. A third pattern of immunoreactivity was seen as cells moved into the postmitotic compartment, with nuclear p27 staining and perinuclear accentuation in metamyelocytes and bands (Fig 4).
Altered localization of p27 in differentiating myeloid cells. CD34+ cells were differentiated for 0 (A, E), 6 (B), 9 (C, F), or 15 (D, G) days and aliquots taken at various intervals for p27 immunostaining by using peroxidase detection. Cytospins shown have been indirectly stained either with isotype control (E-F) or anti-p27 antibody (Neomarkers Ab-1; A-D). Altered patterns of p27 immunoreactivity are evident.
Altered localization of p27 in differentiating myeloid cells. CD34+ cells were differentiated for 0 (A, E), 6 (B), 9 (C, F), or 15 (D, G) days and aliquots taken at various intervals for p27 immunostaining by using peroxidase detection. Cytospins shown have been indirectly stained either with isotype control (E-F) or anti-p27 antibody (Neomarkers Ab-1; A-D). Altered patterns of p27 immunoreactivity are evident.
p21 expression in differentiating myeloid cells. Immunostained cytospins of CD34+ cells cultured for 2 days (top), 6 days (middle), or 11 days (bottom). Cells are stained for p21 expression (red, left) by using indirect Cy-3 conjugated antibody staining. Cell nuclei are counterstained with Hoechst (blue) shown as the same field on the left.
p21 expression in differentiating myeloid cells. Immunostained cytospins of CD34+ cells cultured for 2 days (top), 6 days (middle), or 11 days (bottom). Cells are stained for p21 expression (red, left) by using indirect Cy-3 conjugated antibody staining. Cell nuclei are counterstained with Hoechst (blue) shown as the same field on the left.
In contrast to p27, p21 staining remained predominantly nuclear throughout differentiation. Whereas almost all cells were p27+, varying only in staining pattern, the proportion of cells exhibiting p21 positivity varied markedly during differentiation. p21-expressing cells begin to arise 3 days after cytokine stimulation of resting CD34+ cells; the p21 signal peaks at 6 days, just before the emergence of terminally differentiated cells, and then the proportion of cells expressing p21 begins to fall off (Fig5). Image analysis of the stained samples using quantitative laser confocal microscopy gated to Hoechst-positive fields was used to quantitate nuclear p21 (see Materials and Methods). The percentage of nuclei positive for p21 was determined at 0, 3, 6, and 11 days after cytokine stimulation indicated a 31% decline in the percentage of p21-expressing cells between culture days 6 and 11 (Fig6A). The signal intensity of positive cells was also quantitated by averaging the signal within the nucleus and multiplying by the surface area encompassed by the signal (see Materials and Methods). There is a slight decline also in the intensity of the p21 signal in the positive cells (Fig 6B).
Analysis of p21 expression during differentiation. Image analysis of cytospins shown in Fig 6 as described in the text. (A) Proportion of cells positive for p21. Cy3 staining over Hoechst nuclei corrected for background signal are quantified as a percentage of nuclei present. (B) Intensity of p21 staining. p21 fluorescence intensity at different days. Cy3 signal extracted and intensity collected for the mask area for each cell as described in the text. Intensities shown in the figure represent the mean sum of all cell profiles generated.
Analysis of p21 expression during differentiation. Image analysis of cytospins shown in Fig 6 as described in the text. (A) Proportion of cells positive for p21. Cy3 staining over Hoechst nuclei corrected for background signal are quantified as a percentage of nuclei present. (B) Intensity of p21 staining. p21 fluorescence intensity at different days. Cy3 signal extracted and intensity collected for the mask area for each cell as described in the text. Intensities shown in the figure represent the mean sum of all cell profiles generated.
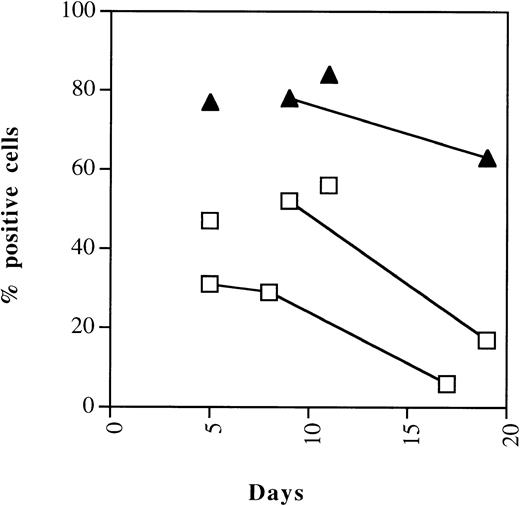
An independent quantitation of changes in the expression of p21 and p27 was undertaken with flow cytometry. Cells were permeabilized and cdki expression determined by indirect immunofluorescence as detailed in Materials and Methods. The cell line K-562, which does not express p21, was used as a negative control. Representative staining of p21 and p27 in CD34+ cells cultured for 5 days in the presence of SCF, IL-3, and G-CSF is shown in Fig 7. Similar results were seen for two different p21 moAbs (Neomarker p21 moAb-2 and moAb-3) and two p27 antibodies (Neomarker moAb-1 and Transduction Laboratories Ab-1 [Transduction Laboratories, Lexington, KY]; not shown). Figure 8 shows the kinetics of p21 and p27 in CD34+ cells over 19 days in culture. The data indicate high levels in the percentage of cells positive for p21 and p27 from days 5 to 11, during which proliferation is occurring. Consistent with image analysis, p21 levels decline sharply starting at day 9, concurrent with increasing number of cells in the postmitotic compartment. p27 levels, in contrast, remain fairly constant.
Detection of p21 and p27 by flow cytometry. Cord blood–derived CD34 cells differentiated for 5 days were permeabilized for staining of nuclear cdki’s p21 and p27. Cells were stained with (Neomarkers) monoclonal p21 Ab-2 (left) or Ab-3 (center), or with p27 Ab-1 (Neomarkers) as indicated by blackened histogram; isotype control is shown as open histogram. Similar staining of p21− K562 cells with anti-p21 antibodies showed no shift from isotype control (not shown).
Detection of p21 and p27 by flow cytometry. Cord blood–derived CD34 cells differentiated for 5 days were permeabilized for staining of nuclear cdki’s p21 and p27. Cells were stained with (Neomarkers) monoclonal p21 Ab-2 (left) or Ab-3 (center), or with p27 Ab-1 (Neomarkers) as indicated by blackened histogram; isotype control is shown as open histogram. Similar staining of p21− K562 cells with anti-p21 antibodies showed no shift from isotype control (not shown).
Kinetics of cdk-inhibitor expression during myelopoiesis. Flow cytometric quantitation of expression of the p21 and p27 cdki’s in differentiating CD34+ cells analyzed overtime. Representative results of two experiments. Samples shown are derived from the same cord.
Kinetics of cdk-inhibitor expression during myelopoiesis. Flow cytometric quantitation of expression of the p21 and p27 cdki’s in differentiating CD34+ cells analyzed overtime. Representative results of two experiments. Samples shown are derived from the same cord.
The fall off in p21 immunoreactive protein contrasts with Northern blot results which indicate a significant upregulation of p21 message as cells proceed through terminal differentiation.14Conceivably, the native protein could be sequestered into immunologically inaccessible complexes at late stages of differentiation; alternatively, posttranscriptional regulation of p21 could alter its production or stability,19 as has been seen in other systems. We have not been able to quantify p21 levels in these cells with Western blotting because of limited sensitivity to the levels in these cells.
The above analyses all indicated that the cdk’s p21 and p27 are not limited in their expression to growth-arrested myeloid precursor cells, because p21 and p27 are expressed at high levels in populations of cells that are growing rapidly. To determine whether p21 and p27 expression was primarily limited to cells in G0/G1 or in cells synthesizing DNA, we doubly stained cells differentiated for various lengths of times for p21 or p27 and for DNA content and subjected the stained cells to FACS analysis. This enabled us to determine cell cycle partitioning of cells that expressed one of these cdki with cells that did not by gating separately on cdki expressing and nonexpressing cells and helped determine DNA profiles of both subpopulations. Dual staining procedures were first optimized by simultaneously staining log-phase K562 cells for the expression of cyclin A and propidium iodide. The vast majority (94%) of cells were positive for cyclin A and were markedly enriched for S-phase cells (33% in G0/G1, 61% in S-phase) compared with cyclin A nonexpressors (70% in G0/G1, 17% in S-phase) (Fig 9).
Partitioning of cyclin A–expressing cells within the cell cycle. K562 cells were double stained for cyclin A expression (indirect FITC-staining) and for DNA content (propidium iodide [PI]). This was a test of our ability to simultaneously determine the expression of an intranuclear antigen by using indirect staining and DNA content. (Top) Flow histogram, PI staining on y-axis; FITC-staining on x-axis. Vertical line denotes 98%-negative isotype control gate. Note most negative cells at left edge of histogram. (Bottom) Cell-cycle distribution of cyclin A-negative cells (as represented by arrow connecting to histogram gate). (Middle) Cell-cycle distribution of cyclin A+ cells. G0/G1 and G2 DNA contents are highlighted in black; S-phase is highlighted with hash marks.
Partitioning of cyclin A–expressing cells within the cell cycle. K562 cells were double stained for cyclin A expression (indirect FITC-staining) and for DNA content (propidium iodide [PI]). This was a test of our ability to simultaneously determine the expression of an intranuclear antigen by using indirect staining and DNA content. (Top) Flow histogram, PI staining on y-axis; FITC-staining on x-axis. Vertical line denotes 98%-negative isotype control gate. Note most negative cells at left edge of histogram. (Bottom) Cell-cycle distribution of cyclin A-negative cells (as represented by arrow connecting to histogram gate). (Middle) Cell-cycle distribution of cyclin A+ cells. G0/G1 and G2 DNA contents are highlighted in black; S-phase is highlighted with hash marks.
Similar analysis was done with CD34+ cells, which were differentiated for 5 days and simultaneously stained for propidium iodide and either p27 or p21. In agreement with cytospin staining results, 94% of cells were positive for p27 (Fig10). Interestingly, the cell-cycle profile of the p27+ cells was nearly identical to that of the 6% of cells that did not express p27 (Table1). p21 was expressed by 61% of the cells; 39% were p21 negative (Fig 10). Again, there was little difference in the cell-cycle expression of p21-expressors and nonexpressors. The intensity of the p21 and p27 signals in positive cells was equally bright in all cell-cycle compartments.
Partitioning of p27-expressing and p21-expressing cells within the cell cycle. Cord blood CD34+ cells differentiated for 5 days were analyzed simultaneously for p27 expression and cell-cycle distribution (left) or for p21 expression and cell-cycle distribution (right). Ninety-three percent of cells were positive and 7% were negative for p27. Sixty-one percent of cells were positive and 39% were negative for p21. Isotype gates and representation of cellular populations are as in Fig 10. p27 (left) and p21 (right)-negative cells at bottom; p27 (left) and p21 (right)-positive cells in middle. As is evident, the cell-cycle profile of cdki-positive and -negative cells is similar.
Partitioning of p27-expressing and p21-expressing cells within the cell cycle. Cord blood CD34+ cells differentiated for 5 days were analyzed simultaneously for p27 expression and cell-cycle distribution (left) or for p21 expression and cell-cycle distribution (right). Ninety-three percent of cells were positive and 7% were negative for p27. Sixty-one percent of cells were positive and 39% were negative for p21. Isotype gates and representation of cellular populations are as in Fig 10. p27 (left) and p21 (right)-negative cells at bottom; p27 (left) and p21 (right)-positive cells in middle. As is evident, the cell-cycle profile of cdki-positive and -negative cells is similar.
Partitioning of p21-Expressing Cells Within the Cell Cycle
| . | G0/G1 . | S . | G2/M . |
|---|---|---|---|
| p21− | 70 | 20 | 10 |
| p21+ | 70 | 26 | 4 |
| p27− | 70 | 26 | 4 |
| p27+ | 72 | 22 | 7 |
| . | G0/G1 . | S . | G2/M . |
|---|---|---|---|
| p21− | 70 | 20 | 10 |
| p21+ | 70 | 26 | 4 |
| p27− | 70 | 26 | 4 |
| p27+ | 72 | 22 | 7 |
Cord blood CD34+ cells differentiated for 5 days were analyzed simultaneously for p21 expression and cell-cycle distribution. As is evident, the cell-cycle profile of p27 or p21 positive and negative cells is identical.
DISCUSSION
This study was designed to investigate the expression patterns of the cdki’s p21 and p27 in normal differentiating myeloid cells by using an in vitro culture system employing SCF, G-CSF, and IL-3. We have found disparate patterns of expression of p21 and p27 both in terms of the prevalence of expression in cells at different stages of differentiation and in the localization of the cdki within the cell.
An advantage of the culture system used was that it recapitulated the progressive appearance of differentiated progeny seen in normal myelopoiesis. Because the cellular population was always somewhat heterogeneous as it moved through stages of maturation, this enabled the comparison at a single time point of cells expressing and those not expressing a given cdki.
Freshly harvested CD34+ cells were in G0/G1 phase and expressed p27 in their nucleus but not p21. This is similar to the cdki expression pattern of lymphocytes and corresponds to in vitro models in which quiescent MO7e cells express little p21 in the absence of cytokine activation.12 In these and other systems,20 cell-cycle entry is associated with downmodulation of p27 protein; it is interesting that this does not appear to occur as the CD34+ cells give rise to precursor cells in our study. A prominent p27 signal is present upon immunoblotting of cellular extracts at different stages of differentiation and is roughly equal to levels seen in neutrophils and in lymphocytes. This suggests that the total level of p27 in cells remains relatively constant during myelopoiesis.
Although p27 levels may not change much, a distinct role for p27 in inhibiting the proliferation of myeloid precursors is suggested by the leukocytosis manifested by p27 knockout mice.21 Whereas these mice exhibit increased numbers of mature hematopoietic cells, the proportions between lineage compartments is normal, suggesting that p27 action is focused on progenitors and early precursors. Because p27 appears not to be eliminated in proliferating myeloid cells, the major determinant of p27 action may relate more to the binding complexes it forms than to its precise level of expression. It has been recognized that in replicating cells, p27 is sequestered into inactive complexes with cyclin D-cdk422; overexpression of p15 results in redistribution of p27 into inhibitory complexes with cdk2.
The finding that p27 changes its subcellular distribution pattern at different stages of myelopoiesis is fascinating, as it suggests a clear mechanism for p27 to switch binding partners and alter its function. Sequestration of p27 out of the nucleus, for example, would be an efficacious way of promoting cell-cycle entry. Conceivably, redistribution of a significant proportion of p27 to the cytoplasm could facilitate formation of cytoplasmic complexes with cdk4 and enable myeloid proliferation mediated by active nuclear cyclinD-cdk2 complexes. In one study, forced expression of a p27 construct lacking a nuclear localization signal resulted in cytoplasmic expression of p27 and led to cytoplasmic p27-cdk4 complex formation.17 Our results raise the possibility that a shift from cdk2 to cdk4-p27 complexes could occur in a stage-specific manner through p27 redistribution. It has recently been reported that p27 is redistributed into the cytoplasm in Barret’s associated adenocarcinoma,23 suggesting that aberrant appropriation of such subcellular control mechanisms could occur in cancer.
Alternatively, novel binding partners might be accessible to cytoplasmic p27, which would engage it in pathways distinct from cell-cycle control. This would also be consistent with the lack of difference in cycling behavior of p27-expressing cells and p27 nonexpressing cells after 5 days in culture (Fig 10). It will be interesting to examine co-localization of p27 binding partners concurrent with these shifts in p27 compartmentalization to test whether p27 acts to shuttle cell cycle or differentiation-regulatory proteins between compartments.
The results of our analysis of p21 expression are interesting in two respects. We show in normal myeloid cells that p21 upregulation is associated with proliferation during differentiation. This extends findings reported in the MO7e in vitro model of myeloid differentiation.12 The expression of p21 is low in growth-arrested CD34+ blast cells and begins to increase around day 2, which is concurrent with cellular expression of CD71 (transferrin receptor), a marker of proliferation. Thereafter, the pattern of p21 expression is inversely proportional to the percentage of cells in G0/G1 (Fig 8), indicating a coupling of p21 to proliferation of myeloid precursor cells. Several laboratories have showed a stoichiometric activity of p21, in which it acts as a cyclin-cdk-assembly factor and promotes proliferation at low concentrations but is inhibitory at high concentrations.9,10 We note that in our system the intensity of p21 signal within positive cells also peaked at day 6, suggesting that the proliferating precursor cells are not simply expressing p21 at a subthreshold concentration for growth arrest (Fig8). Moreover, fractionation of p21-expressing cells by DNA content did not indicate any difference in the intensity of p21 expression between S-phase and G0/G1-phase cells. It is conceivable that p21 function in these cells is largely related to the differentiation process. That would be consistent with the lack of difference in the cell-cycle profiles of p21-expressing cells and nonexpressing cells. Conceivably, a portion of the p21 expressed in these cells could be sequestered in complexes with other binding partners such as casein kinase II,24 SAPK,25 E2F,25 gadd45, or Myd 118,26 which may not as directly relate to cell-cycle effects as to differentiation. Additional studies will be needed to address this concept.
We also find discrepant expression of p21 message, which increases throughout terminal differentiation14 and p21 protein, and appears to decrease in the last stages of differentiation. Because we are studying p21 in its native state, it is conceivable that alterations in protein interactions render p21 inaccessible to our antibodies at this late stage. Alternatively, ubiquitination or translational inhibitory pathways could be activated at this stage. The primary role for p21 could be in choreographing the transition into the differentiated state rather than in maintaining it. Such findings have been reported in muscle differentiation, in which p18 succeeds p21 in playing a dominant role in sustaining growth arrest of terminally differentiated cells.27 Intriguingly, Di Cunto et al28 have recently reported a similar fall off in p21-protein expression during terminal differentiation of keratinocytes and have shown, moreover, that forced p21-protein expression at this stage inhibits terminal differentiation markers. Whether similar stage-specific restrictions on p21 expression occur in terminal myelopoiesis will require further investigation.
ACKNOWLEDGMENT
We thank Brian Dynlacht for the gift of CP36 monoclonal anti-p21 antibody, Julie Goff for CD34+ cells, Eric Loeffert and Donna Shields and for their technical assistance, and Vera Donnenberg for helpful discussions.
Supported in part by grants from Highmark Blue Cross/Blue Shield, the American Cancer Society (JFRA-594), and the National Institute of Health (HL54172-01) to R.A.S.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to R.A. Steinman, MD, PhD, E1052 BST, University of Pittsburgh Cancer Institute, Pittsburgh, PA 15213; e-mail: Steinman+@pitt.edu.


![Fig. 2. Expression of p21 and p27 in CD34+ blasts and in differentiating cells. (A-F) Cytospins of positively selected CD34+ blasts maintained overnight in low serum after harvest were stained for p21 or p27 as described (Neomarkers MoAbs). Some beads used in the immunoisolation are apparent in the isotype control. Nuclear staining of p27 is evident. Cytospins prepared after 6 days of culture in SCF, G-CSF, and IL-3 were simultaneously stained. Nuclear staining of p21 and cytoplasmic staining of p27 is evident. Cells are counterstained with Hoechst. (G-L) Staining of negatively selected CD34+ cells freshly harvested (G) or after 4 days of culture (H-L). (G) Confocal micrograph of cells doubly stained for CD34 (phycoerythrocin, red) and p27 (Alexa-488). A field containing a CD34− cell is shown, enlarged for detail. (H) Photograph of cells doubly stained for p27 (Cy3, red) and CD71 (fluorescein isothiocyanate [FITC]). After days of culture, 86% of the cells were positive for CD71. (I) Confocal micrograph of cells doubly stained for Ki-67 (Cy3) and p27 (Alexa-48). Nuclear Ki-67 and cytoplasmic p27 is evident. Overlapping nuclear signals are yellow. (J) Isotype control for (I). All isotype controls were similarly negative. (K, L) Confocal microscopy of cells for p27, using C-terminal (G) and N-terminal (L) (Santa Cruz) antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2907/4/m_blod409yar02z.jpeg?Expires=1765995380&Signature=L5O3c2MrsQkXTLz76uGcrLQ42fDseuE3iInEPxTip5vvYkR8zpxE1DL3UgOy47Su8TfoodThIpnM7TWLQcYGmy8kZgLU2MRd9D8Xn7rSX4nG9HqCECWlrT4fNUVmYPXC~-a2isRnkZieHqvcMOnMqf0wldUp8Kps3qcaEAtPgsohsMhJaKT4sH0VzN1K~2O~KAhIm6BnexrWkTZQeJNEM7yn2LX8ArSEfbS9lJVlDyasNPgYMPNKCRVEAzr~OH5-1YII7wmLxvBVTF3qyhAPjkeNpMkux56WUoBOeZGYoO4ToyqEN4dlH4WDljGEaKD6FfDnq4jwHrMga1ntcDgpzg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 9. Partitioning of cyclin A–expressing cells within the cell cycle. K562 cells were double stained for cyclin A expression (indirect FITC-staining) and for DNA content (propidium iodide [PI]). This was a test of our ability to simultaneously determine the expression of an intranuclear antigen by using indirect staining and DNA content. (Top) Flow histogram, PI staining on y-axis; FITC-staining on x-axis. Vertical line denotes 98%-negative isotype control gate. Note most negative cells at left edge of histogram. (Bottom) Cell-cycle distribution of cyclin A-negative cells (as represented by arrow connecting to histogram gate). (Middle) Cell-cycle distribution of cyclin A+ cells. G0/G1 and G2 DNA contents are highlighted in black; S-phase is highlighted with hash marks.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.2907/4/m_blod40934009x.jpeg?Expires=1765995380&Signature=rzabN1Oz8d50u6Y8HwtllcdgsSKt4yZ0dApaXez7l2Xf225iDAgLjrJBp0OR63-0YX5eCuldiSn64geB1jmg~wVRJTzto1MFUHymC9ihimAHvmANIsnzuTc1hjgz57IOKdMkKpIjbomq5mvMH4S5o9iiERkTlE4LGbIAcbUdH2xxDqE0GXndecLNSRRbcS2LFgU3G9vXXMzfj16FWEG-yhkfGzOLN7xu-VMIC95lFEvW~efBMdA0jQNmgAlB1XG25uwMZp0ZyIlZtBirFzoqfb~~JiyGycz22VdB3T5d4gRop1LCEz-LZNqujtZWFpqLb~zM8IJ5BMd2wSzguHqdbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal