Abstract
Five tyrosine-phosphorylated proteins with molecular masses of 180, 145, 116, 100, and 70 kD are associated with phosphatidylinositol 3-kinase (PI 3-kinase) in erythropoietin (Epo)-stimulated UT-7 cells. The 180- and 70-kD proteins have been previously shown to be IRS2 and the Epo receptor. In this report, we show that the 116-kD protein is the IRS2-related molecular adapter, GAB1. Indeed, Epo induced the transient tyrosine phosphorylation of GAB1 in UT-7 cells. Both kinetics and Epo dose-response experiments showed that GAB1 tyrosine phosphorylation was a direct consequence of Epo receptor activation. After tyrosine phosphorylation, GAB1 associated with the PI 3-kinase, the phosphotyrosine phosphatase SHP2, the phosphatidylinositol 3,4,5 trisphosphate 5-phosphatase SHIP, and the molecular adapter SHC. GAB1 was also associated with the molecular adapter GRB2 in unstimulated cells, and this association dramatically increased after Epo stimulation. Thus, GAB1 could be a scaffold protein able to couple the Epo receptor activation with the stimulation of several intracellular signaling pathways. Epo-induced tyrosine phosphorylation of GAB1 was also observed in normal human erythroid progenitors isolated from cord blood. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and thrombopoietin (TPO) also induced the tyrosine phosphorylation of GAB1 in UT-7 cells, indicating that this molecule participates in the signal transduction of several cytokine receptors.
THE KIDNEY-PRODUCED hormone erythropoietin (Epo) is absolutely required for the production of erythrocytes1 by sustaining the survival and proliferation of the late erythroid progenitors and allowing their terminal differentiation. Epo interacts with a cell surface receptor that belongs to the cytokine receptor family.2 Although stimulation of colony-forming unit-erythroid (CFU-E) progenitors by Epo allows their differentiation into erythrocytes, the Epo receptor does not seem to transduce specific differentiation signals but mainly antiapoptotic and proliferative signals (see Socolovsky et al3 for review). Epo binding to its cognate receptor induces the dimerization of the receptor and the activation of the associated Jak2 tyrosine kinase.4 The Epo receptor is then tyrosine phosphorylated5-8 and recruits various SH2 domain-containing proteins, thereby leading to the activation of several intracellular signaling pathways (see Damen and Krystal9 for a recent review). The activation of the phosphatidylinositol 3-kinase (PI 3-kinase) by Epo has been previously reported.10-13 Several mechanisms have been shown to activate PI 3-kinase in Epo-stimulated cells. PI 3-kinase binds to the last tyrosine residue of the Epo receptor,14 although the peptidic sequence following this tyrosine residue does not match the consensus binding site of the PI 3-kinase SH2-domains.15However, mutation or deletion of this tyrosine residue does not abrogate Epo-induced PI 3-kinase activation,14,16 and two alternative mechanisms for PI 3-kinase activation have been reported. PI 3-kinase could be activated by binding to Vav,17 and we have previously shown that Epo induced the tyrosine phosphorylation of the molecular adapter IRS2 and its association with PI 3-kinase.18
GAB1 is another molecular adapter that has been recently cloned and that exhibits strong homologies with IRS1 and IRS2.19 GAB1 is a 115-kD molecule that seems to play a key role in the intracellular signaling of the hepatocyte growth factor (HGF) receptor.20-23 Moreover, GAB1 is tyrosine-phosphorylated in response to epidermal growth factor (EGF),19insulin,19 nerve growth factor (NGF),23 and interleukin-6 (IL-6).24 After activation by these factors, GAB1 has been shown to associate with the molecular adapter GRB2, the phospholipase Cγ (PLCγ), the phosphotyrosine phosphatase SHP2, and the PI 3-kinase.19,20 23-25
In this report, we show that GAB1 is strongly tyrosine-phosphorylated in Epo-stimulated UT-7 cells and in normal human erythroid progenitors. After tyrosine phosphorylation, GAB1 associates with several signaling molecules including GRB2, PI 3-kinase, SHC, SHP2, and SHIP. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and thrombopoietin (TPO) also induced the tyrosine phosphorylation of GAB1, suggesting that GAB1 could be a signaling molecule involved in the mechanism of action of several cytokine receptors.
MATERIALS AND METHODS
Cell culture and stimulation.
UT-7 cells26 were maintained by diluting twice weekly in α minimum essential medium (MEM) containing 10% fetal calf serum and 2 U/mL Epo. c-mpl–transfected UT-7 cells were obtained from Drs F. Porteu and I. Dusanter (ICGM, Paris, France).27 Highly purified recombinant human Epo (specific activity, 120,000 U/mg) used throughout this study was a generous gift from Dr M. Brandt (Boehringer Mannheim, Mannheim, Germany). Before each experiment, the cells were serum- and growth factor-deprived by overnight incubation in Iscove’s Dulbecco’s modified Eagle’s medium (DMEM) containing 0.1% deionized bovine serum albumin and 25 μg/mL iron-loaded human transferrin. Except when otherwise stated, the cells were stimulated in the same medium for 10 minutes with 10 U/mL recombinant human Epo. The stimulation was stopped by diluting the cells with ice-cold phosphate-buffered saline (PBS) containing 50 μmol/L sodium vanadate.
Preparation of cell extracts, immunoprecipitation, and Western blot analysis.
The cells were solubilized with buffer A containing 1% Nonidet P40 (Boehringer Mannheim) or 1% Brij 98 (Sigma, St Louis, MO; buffer A: 10 mmol/L Tris/HCl, 150 mmol/L NaCl, 5 mmol/L EDTA, 10% glycerol, 1 mmol/L sodium vanadate, 0.02% NaN3, and protease inhibitors from Boehringer Mannheim [catalogue no. 1873580]). For the experiments reported here, the same results were obtained using either Nonidet P40 or Brij 98. After 15 minutes on ice, the extracts were centrifuged for 15 minutes at 27,000g and the supernatants were used for immunoprecipitation. Immunoprecipitating antibodies were incubated with the solubilized cell extracts for 1 hour at 4°C and the mixture was transferred to a protein G-Sepharose pellet. The suspension was rocked for 1 hour at 4°C. The Sepharose beads were washed twice with buffer A containing 1% detergent and twice with buffer A containing 0.1% detergent. They were boiled in Laemmli sample buffer and analyzed by Western blot as previously described,6 except that New England Nuclear (NEN) Renaissance kit (Boston, MA) was used for revelation.
Antibodies.
We used anti–PI 3-kinase antibodies produced by rabbit immunization with a mixture of recombinant proteins corresponding to the N- and C-terminal SH2 domains of p85 fused to GST and commercial anti–PI 3-kinase antibodies from UBI (catalogue no. 06-195, Lake Placid, NY). Anti-GAB1 antibodies (catalogue no. 06-579), anti-Jak2 antibodies (catalogue no. 06-255), anti-SOS antibodies (catalogue no. 06-246), anti–PLC-γ1 antibodies (catalogue no. 05-163), and anti-IRS2 antibodies (catalogue no. 06-506) were from UBI. Anti-GRB2 antibodies (catalogue no. C-23), anti–PLC-γ2 antibodies (catalogue no. Q-20), and anti-SHIP antibodies (catalogue no. V-19) were from Santa Cruz (Santa Cruz, CA). Antiphosphotyrosine antibodies (4G10) and anti-SHP2 antibodies were generous gifts of Dr B. Drucker (Portland, OR) and Dr A. Ullrich (Martinsried, Germany), respectively. Anti-Epo receptor antibodies and anti-SHC antibodies were produced by immunizing rabbits with a recombinant protein composed of the intracellular domain of the human Epo receptor or the SH2 domain of the SHC protein fused to GST. We produced anti-STAT5A and anti-STAT5B antibodies by immunizing rabbits with peptides corresponding to the 12 last amino acids (AGLFTSARSSLS) of STAT5A or the 8 last amino acids of STAT5B (QWIPHAQS) coupled to KLH. These peptidic sequences are specific for STAT5A and STAT5B respectively. A 1/1 mixture of these antibodies was used for immunoprecipitations.
Amplification and purification of normal human erythroid progenitors.
Umbilical cord blood units (mean volume, 85 mL) from normal full-term deliveries were obtained after receiving informed consent of the mothers from the Obstetrics Unit of the Hôpital Saint-Vincent-de-Paul (Paris, France). Cord blood units were diluted with 50 mL PBS and submitted to Ficoll density gradient. Low-density cells were recovered and CD34+ cells were separated by two cycles of positive selection using an immunomagnetic procedure (MACS, CD34 isolation kit; Miltenyi Biotech, Auburn, CA). The cells were then cultured in serum-free Iscove’s DMEM (GIBCO-BRL, Life Technologies, Grand Island, NY) in the presence of 15% of a commercial mixture of bovine serum albumin, insulin, and transferrin (BIT 9500; StemCell Technologies, Vancouver, CA) and 10 ng/mL IL-3, 10 ng/mL IL-6, and 25 ng/mL stem cell factor (SCF). Cells were incubated in 5% CO2 in air at 37°C during 6 days. At day 6, the cells were pelleted by centrifugation and resuspended in PBS containing 0.8% bovine serum albumin. Monoclonal anti-CD36 IgG1 antibody (Immunotech, Marseille, France) was added at a final concentration of 1 μg/106 cells and incubated for 30 minutes at 4°C. Cells were washed twice and then incubated with rat antimouse IgG1 antibody coupled to magnetic microbeads (Miltenyi Biotech), and CD36+ cells were separated on a MACS column. A pure erythroid progenitor cell population composed of CFU-E and late burst forming unit-erythroid (BFU-E) was thus obtained. Indeed, 98% of the cells were CD36+ and CD71high and less than 3% of the cells were CD14+, CD41+, or glycophorin A+. More than 96% of the clonogenic colonies formed by these cells in semisolid culture assays were BFU-E or CFU-E. These cells were cultured again for 72 hours in the same culture medium as described above plus 2 U/mL Epo. A dramatic cell proliferation was observed and led to large numbers of pure erythroid progenitor cells that were 95% to 100% CD36+ and CD71+. The glycophorin A marker progressively appeared from days 2 to 3 of secondary culture. After 72 hours, most cells were immature blasts, and morphologically recognizable erythroblasts appeared after 4 days of secondary culture (S.F., manuscript submitted).
PI 3-kinase assays.
PI 3-kinase assays were performed as previously described.11 Briefly, the cells were stimulated and solubilized using buffer A containing 1% Nonidet P40, and cell extracts were immunoprecipitated with anti-GAB1 antibodies and protein G Sepharose as described above. The Sepharose beads containing immunoprecipitated proteins were washed twice with buffer A containing 1% Nonidet P40, twice with PBS containing 1 mmol/L sodium vanadate, twice with a high salt buffer (0.5 mol/L LiCl, 10 mmol/L Tris/HCl, 1 mmol/L sodium vanadate, pH 7.4), and twice with PI 3-kinase buffer (25 mmol/L HEPES, 5 mmol/L MgCl2, 100 mmol/L NaCl, pH 7.4). The beads were then incubated for 15 minutes at 30°C in 50 μL of PI 3-kinase buffer containing 20 μg of phosphatidylinositol, 20 μg of phosphatidylserine, 10 μmol/L unlabeled ATP, and 20 μCi of32P-γATP. The reaction was stopped with 1 mol/L HCl, and the phospholipids were extracted with methanol/chloroform (1/1 vol/vol). The chloroform extract was washed twice with methanol/1 mol/L HCl (1/1 vol/vol) and evaporated under vacuum. The dried extracts were dissolved in 25 μL methanol/chloroform/1 mol/L HCl (100/200/1, vol/vol/vol), and the phospholipids were separated by thin-layer chromatography on a silica plate with a mobile phase of chloroform/methanol/NH4OH/H2O (45/35/3.3/6.7, vol/vol/vol/vol). Unlabeled phosphatidylinositol monophosphate was run in an adjacent lane to determine the migration position of phosphatidylinositol 3 phosphate. After migration, the plate was dried, exposed to iodine vapor to stain the phosphatidylinositol monophosphate standard, and exposed 1 to 3 days for autoradiography.
RESULTS
Epo induced the association of PI 3-kinase with several tyrosine-phosphorylated proteins.
To determine which tyrosine-phosphorylated proteins were associated with PI 3-kinase in Epo-stimulated UT-7 cells, growth factor-deprived UT-7 cells were stimulated for 10 minutes with Epo and lysed using a mild detergent. PI 3-kinase was immunoprecipitated and the immunoprecipitates were analyzed by Western blot using antiphosphotyrosine antibodies. Five phosphotyrosine-containing proteins with molecular masses of 70, 100, 116, 145, and 180 kD were observed in PI 3-kinase immunoprecipitates (Fig 1). Two of these proteins have been previously identified: the 70-kD protein is the activated Epo receptor14 and the 180-kD protein is the molecular adapter IRS2.18 The identification of the remaining proteins was therefore undertaken.
PI 3-kinase associates with several tyrosine-phosphorylated proteins in Epo-stimulated cells. UT-7 cells were serum- and growth factor-starved for 18 hours and incubated for 10 minutes in the presence (+) or absence (−) of 10 U/mL Epo. The cells were then lysed using 1% Brij 98 and the lysates were cleared by centrifugation (27000g for 15 minutes). Lysates from 107 cells were immunoprecipitated using anti–PI 3-kinase antibodies. Immunoprecipitates were analyzed by Western blot using antiphosphotyrosine antibodies (anti-PY) and anti–PI 3-kinase (anti–PI3-K) antibodies, successively.
PI 3-kinase associates with several tyrosine-phosphorylated proteins in Epo-stimulated cells. UT-7 cells were serum- and growth factor-starved for 18 hours and incubated for 10 minutes in the presence (+) or absence (−) of 10 U/mL Epo. The cells were then lysed using 1% Brij 98 and the lysates were cleared by centrifugation (27000g for 15 minutes). Lysates from 107 cells were immunoprecipitated using anti–PI 3-kinase antibodies. Immunoprecipitates were analyzed by Western blot using antiphosphotyrosine antibodies (anti-PY) and anti–PI 3-kinase (anti–PI3-K) antibodies, successively.
The 116-kD tyrosine-phosphorylated protein associated with PI 3-kinase was GAB1.
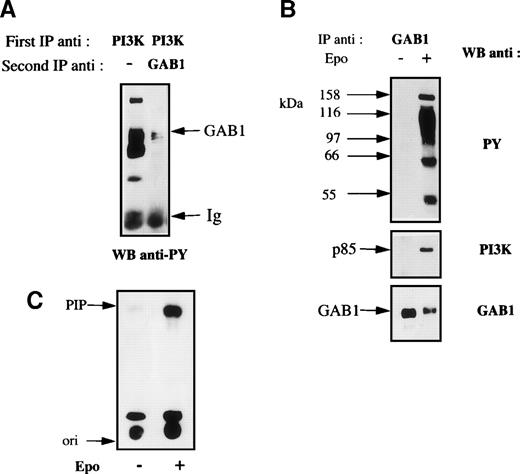
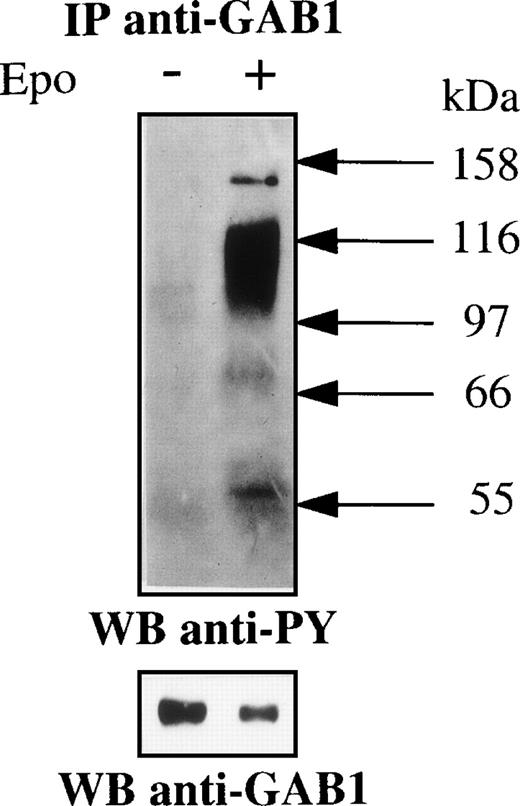
A good candidate for the 116-kD protein is the recently cloned molecular adapter GAB1. Indeed, GAB1 was shown to associate with PI 3-kinase in cells stimulated with various growth factors.19,20,23-25 Moreover, the structure of GAB1 is close to that of IRS2 that we previously showed to associate with PI 3-kinase in Epo-stimulated cells.18 Initial attempts to probe anti–PI 3-kinase immunoprecipitates with anti-GAB1 antibodies were unsuccessful due to the low efficiency of the anti-GAB1 antibodies in Western blot experiments. Consequently, anti–PI 3-kinase immunoprecipitates were denaturated and reprecipitated with anti-GAB1 antibodies. Figure 2A shows that the 116-kD tyrosine-phosphorylated protein was directly recognized by anti-GAB1 antibodies. Then, cellular extracts from Epo-stimulated or unstimulated UT-7 cells were immunoprecipitated with anti-GAB1 antibodies. Western blot analysis of these immunoprecipitates showed that Epo induced the tyrosine phosphorylation of GAB1 and its association with tyrosine-phosphorylated proteins of 145, 66, and 52 kD. In most cases, the subunits of PI 3-kinase are not tyrosine phosphorylated in stimulated cells and PI 3-kinase activation is realized through its binding to tyrosine phosphorylated proteins (see Kapeller and Cantley28 for review concerning PI 3-kinase). To test for the association of PI 3-kinase with GAB1, the blot was probed with anti–PI 3-kinase antibodies and this experiment showed that Epo induced the association of GAB1 with PI 3-kinase (Fig 2B). Reprobing the blot with anti-GAB1 antibodies showed an electrophoretic shift of GAB1 that probably reflects the strong level of GAB1 phosphorylation induced by Epo stimulation (Fig 2B). The lower detection of GAB1 in extracts from stimulated cells was constantly observed. This probably corresponded to a decreased affinity of the anti-GAB1 antibodies for the phosphorylated form of GAB1. Moreover, the association of GAB1 with signaling proteins (see below) after Epo stimulation could also lower the accessibility of GAB1 to anti-GAB1 antibodies. Lastly, GAB1 immunoprecipitates were tested for PI 3-kinase activity. Figure 2C shows that Epo stimulation of UT-7 cells strongly increased the GAB1-associated PI 3-kinase activity.
GAB1 association with PI 3-kinase in Epo-stimulated cells. (A) Anti–PI 3-kinase immunoprecipitates were prepared from Epo-stimulated UT-7 cells as described in Fig 1. Half of the immunoprecipitates were saved for direct analysis by Western blot and the remaining was denatured by boiling in nonreducing Laemmli sample buffer, diluted with solubilization buffer, and reimmunoprecipitated using anti-GAB1 antibodies. Both samples were analyzed by Western blot using antiphosphotyrosine (anti-PY) antibodies. (B) UT-7 cells were serum- and growth factor-starved for 18 hours and incubated for 10 minutes in the presence (+) or absence (−) of 10 U/mL Epo. The cells were then lysed using 1% Brij 98 and cleared lysates from 107 cells were immunoprecipitated using anti-GAB1 antibodies. Immunoprecipitates were analyzed by Western blot using antiphosphotyrosine antibodies (PY). The blot was stripped and reprobed with anti–PI 3-kinase antibodies and anti-GAB1 antibodies, successively. (C) Anti-GAB1 immunoprecipitates from UT-7 cells stimulated for 10 minutes with 10 U/mL Epo or from control cells were prepared and tested for PI 3-kinase activity as described in Materials and Methods. The lipid products were separated by thin-layer chromatography, and the migration position of phosphatidylinositol 3-phosphate (PIP) was determined by comparison with authentic unlabeled PIP run in adjacent lanes and shown by iodine staining. “Ori” indicates the origin of migration.
GAB1 association with PI 3-kinase in Epo-stimulated cells. (A) Anti–PI 3-kinase immunoprecipitates were prepared from Epo-stimulated UT-7 cells as described in Fig 1. Half of the immunoprecipitates were saved for direct analysis by Western blot and the remaining was denatured by boiling in nonreducing Laemmli sample buffer, diluted with solubilization buffer, and reimmunoprecipitated using anti-GAB1 antibodies. Both samples were analyzed by Western blot using antiphosphotyrosine (anti-PY) antibodies. (B) UT-7 cells were serum- and growth factor-starved for 18 hours and incubated for 10 minutes in the presence (+) or absence (−) of 10 U/mL Epo. The cells were then lysed using 1% Brij 98 and cleared lysates from 107 cells were immunoprecipitated using anti-GAB1 antibodies. Immunoprecipitates were analyzed by Western blot using antiphosphotyrosine antibodies (PY). The blot was stripped and reprobed with anti–PI 3-kinase antibodies and anti-GAB1 antibodies, successively. (C) Anti-GAB1 immunoprecipitates from UT-7 cells stimulated for 10 minutes with 10 U/mL Epo or from control cells were prepared and tested for PI 3-kinase activity as described in Materials and Methods. The lipid products were separated by thin-layer chromatography, and the migration position of phosphatidylinositol 3-phosphate (PIP) was determined by comparison with authentic unlabeled PIP run in adjacent lanes and shown by iodine staining. “Ori” indicates the origin of migration.
Epo induced the tyrosine phosphorylation of GAB1.
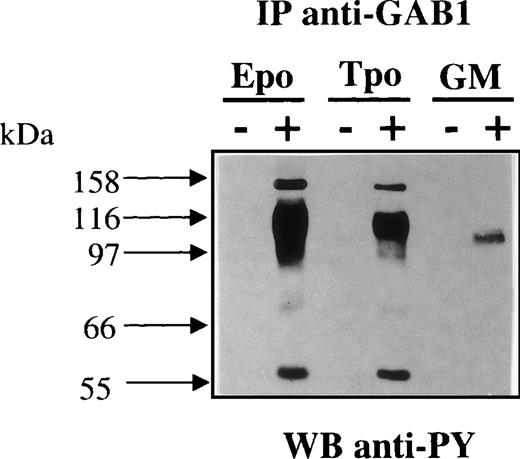
Figure 3 shows the time-course of Epo-induced GAB1 tyrosine phosphorylation. Tyrosine phosphorylation of GAB1 was maximal after 10 minutes of Epo stimulation and then started to decrease, although it remained detectable after 1 hour of stimulation (Fig 3A). Dose-response experiments showed that the Epo-induced tyrosine phosphorylation of GAB1 perfectly correlated with the occupancy of the Epo receptors (Fig 3B). Thus, both the kinetic experiments and the dose-response curves indicated that the Epo-induced tyrosine phosphorylation of GAB1 most likely corresponded to a direct consequence of the Epo receptor activation. In addition to Epo, TPO also induced the tyrosine phosphorylation of GAB1 in c-mpl–transfected UT-7 cells (Fig 4). The same tyrosine-phosphorylated proteins appeared to be associated with tyrosine-phosphorylated GAB1 in Epo- and TPO-stimulated cells. A low level of GAB1 tyrosine phosphorylation was also detected in GM-CSF–stimulated cells (Fig 4). The low efficiency of GM-CSF stimulation was probably due to the reduced number of high-affinity GM-CSF receptors at the cell surface of UT-7 cells. Indeed, we detected only a few hundred high-affinity receptors for GM-CSF, whereas these cells express approximately 7,000 Epo receptors.29
Characteristics of Epo-induced GAB1 tyrosine phosphorylation. Serum- and growth factor-deprived UT-7 cells were stimulated for various times with 10 U/mL Epo (A) or for 10 minutes with various Epo concentrations (B). Cleared lysates were then prepared and immunoprecipitated with anti-GAB1 antibodies. Immunoprecipitates were analyzed by antiphosphotyrosine (anti-PY) antibodies. Assuming an equilibrium constant of dissociation of 200 pmol/L for the Epo receptor in UT-7 cells,29 receptor occupancy is 2% for 10 mU/mL Epo, 18% for 100 mU/mL, 70% for 1 U/mL, 95% for 10 U/mL, and 99.5% for 100 U/mL. Because apparent binding equilibrium was not achieved after 10 minutes of incubation for Epo concentrations less than 1 U/mL (data not shown), receptor occupancy for Epo concentrations of 10 mU/mL and 100 mU/mL was probably slightly lower than the indicated values.
Characteristics of Epo-induced GAB1 tyrosine phosphorylation. Serum- and growth factor-deprived UT-7 cells were stimulated for various times with 10 U/mL Epo (A) or for 10 minutes with various Epo concentrations (B). Cleared lysates were then prepared and immunoprecipitated with anti-GAB1 antibodies. Immunoprecipitates were analyzed by antiphosphotyrosine (anti-PY) antibodies. Assuming an equilibrium constant of dissociation of 200 pmol/L for the Epo receptor in UT-7 cells,29 receptor occupancy is 2% for 10 mU/mL Epo, 18% for 100 mU/mL, 70% for 1 U/mL, 95% for 10 U/mL, and 99.5% for 100 U/mL. Because apparent binding equilibrium was not achieved after 10 minutes of incubation for Epo concentrations less than 1 U/mL (data not shown), receptor occupancy for Epo concentrations of 10 mU/mL and 100 mU/mL was probably slightly lower than the indicated values.
TPO and GM-CSF induced the tyrosine phosphorylation of GAB1. UT-7 cells stably transfected with the TPO receptor (c-mpl) and parental UT-7 cells were stimulated for 10 minutes with 50 ng/mL TPO, 10 U/mL Epo, or 25 ng/mL GM-CSF. Cleared lysates were immunoprecipitated with anti-GAB1 antibodies and immunoprecipitates were analyzed by Western blot using antiphosphotyrosine antibodies (anti-PY).
TPO and GM-CSF induced the tyrosine phosphorylation of GAB1. UT-7 cells stably transfected with the TPO receptor (c-mpl) and parental UT-7 cells were stimulated for 10 minutes with 50 ng/mL TPO, 10 U/mL Epo, or 25 ng/mL GM-CSF. Cleared lysates were immunoprecipitated with anti-GAB1 antibodies and immunoprecipitates were analyzed by Western blot using antiphosphotyrosine antibodies (anti-PY).
GAB1 associates with several signaling proteins in Epo-stimulated UT-7 cells.
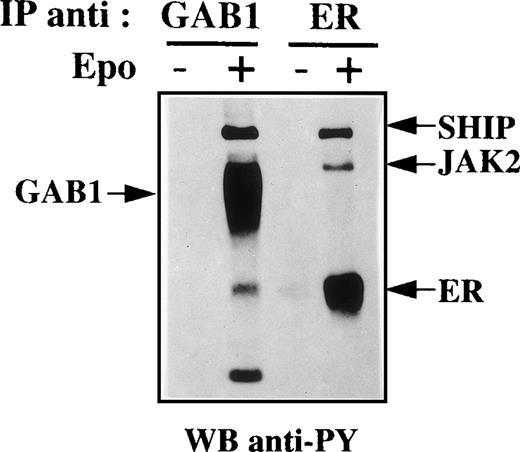
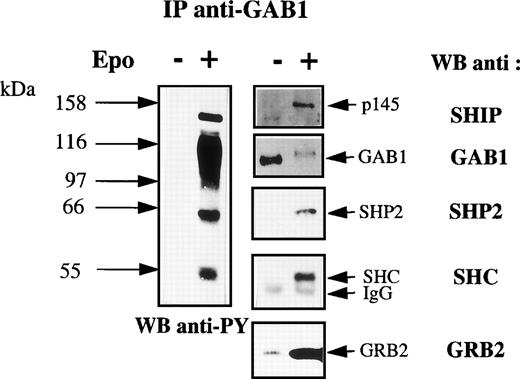
Figure 2B shows that GAB1 was associated with several tyrosine-phosphorylated proteins in Epo-stimulated cells. Three tyrosine-phosphorylated proteins were constantly observed in anti-GAB1 immunoprecipitates. The molecular masses of these proteins suggest that they could be SHC (52 kD), SHP2 and/or the Epo receptor (66 kD), and SHIP (145 kD). GAB1 was not detected in anti-Epo receptor immunoprecipitates (Fig 5). In contrast, anti-SHP2 antibodies recognized a 66-kD protein in GAB1 immunoprecipitates from Epo-stimulated UT-7 cells (Fig 6), and a tyrosine-phosphorylated protein comigrating with GAB1 was also observed in anti-SHP2 immunoprecipitates from Epo-stimulated cells (data not shown). Thus, the 66-kD tyrosine-phosphorylated protein associated with GAB1 was SHP2. The 52-kD protein was recognized by anti-SHC antibodies (Fig 6) and the association between SHC and GAB1 required the Epo-stimulation of the cells. Moreover, a tyrosine-phosphorylated protein comigrating with GAB1 was evidenced in anti-SHC immunoprecipitates from Epo-stimulated cells (data not shown). Thus, the 52-kD tyrosine-phosphorylated protein associated with GAB1 in Epo-stimulated cells was SHC. Probing anti-GAB1 immunoprecipitates with anti-SHIP antibodies showed that the 145-kD protein was SHIP (Fig 6). In addition to these tyrosine-phosphorylated proteins, we tested the association of GAB1 with GRB2. As shown in Fig 6, a low level of association between GAB1 and GRB2 was seen in resting cells and this association was strongly increased in Epo-stimulated cells. This result was reproducibly obtained although the tyrosine phosphorylation of GAB1 was not detected in resting cells, thus suggesting that the association between GRB2 and GAB1 could involve two different mechanisms. We did not detect the presence of Jak2, IRS2, STAT5, Vav, SOS, or PLCγ in anti-GAB1 immunoprecipitates (data not shown).
The Epo receptor is not detected in anti-GAB1 immunoprecipitates. Anti-GAB1 and anti-EpoR immunoprecipitates were prepared from UT-7 cells stimulated for 10 minutes with 10 U/mL Epo or from control cells. These immunoprecipitates were analyzed by Western blot using antiphosphotyrosine (anti-PY) antibodies.
The Epo receptor is not detected in anti-GAB1 immunoprecipitates. Anti-GAB1 and anti-EpoR immunoprecipitates were prepared from UT-7 cells stimulated for 10 minutes with 10 U/mL Epo or from control cells. These immunoprecipitates were analyzed by Western blot using antiphosphotyrosine (anti-PY) antibodies.
Identification of the GAB1-associated proteins. Anti-GAB1 immunoprecipitates were prepared from UT-7 cells stimulated for 10 minutes with 10 U/mL Epo or from control cells and analyzed by Western blot using antiphosphotyrosine (anti-PY) antibodies. The blot was stripped and reprobed with anti-SHIP, anti-SHP2, anti-SHC, anti-GRB2, and anti-GAB1 antibodies, successively.
Identification of the GAB1-associated proteins. Anti-GAB1 immunoprecipitates were prepared from UT-7 cells stimulated for 10 minutes with 10 U/mL Epo or from control cells and analyzed by Western blot using antiphosphotyrosine (anti-PY) antibodies. The blot was stripped and reprobed with anti-SHIP, anti-SHP2, anti-SHC, anti-GRB2, and anti-GAB1 antibodies, successively.
Epo induces the tyrosine phosphorylation of GAB1 in human erythroid progenitors.
We detected the expression of GAB1 in all human leukemic cell lines expressing megakaryocytic or erythroid characteristics such as TF-1, Ku812, K562, or Mo7E (data not shown). However, the level of expression of signaling proteins could be abnormally high in these transformed cell lines, thereby leading to the activation of signaling pathways that are not activated in normal cells. To verify the physiological relevance of Epo-induced GAB1 tyrosine phosphorylation, we used normal erythroid progenitors isolated from human cord blood. More than 95% of the isolated cells exhibited erythroid progenitor characteristics (S.F., manuscript submitted). As shown in Fig 7, Epo also induced the tyrosine phosphorylation of GAB1 in these human primary cells. In addition, the tyrosine-phosphorylated proteins associated with GAB1 previously observed in UT-7 cells were also detectable in anti-GAB1 immunoprecipitates from human erythroid progenitor cells.
Epo induces the tyrosine phosphorylation of GAB1 in normal erythroid progenitors isolated from human cord blood. A purified population of human erythroid progenitor cells was prepared as described in Materials and Methods. The cells were serum- and growth factor-deprived by overnight incubation in Iscove’s DMEM containing 0.4% deionized BSA and 25 μg/mL iron-saturated human transferrin. The cells were then stimulated (+) or not (−) for 10 minutes with 10 U/mL Epo. Lysates from 20 × 106 cells were immunoprecipitated with anti-GAB1 antibodies and analyzed by Western blot using antiphosphotyrosine (anti-PY) and anti-GAB1 antibodies, successively.
Epo induces the tyrosine phosphorylation of GAB1 in normal erythroid progenitors isolated from human cord blood. A purified population of human erythroid progenitor cells was prepared as described in Materials and Methods. The cells were serum- and growth factor-deprived by overnight incubation in Iscove’s DMEM containing 0.4% deionized BSA and 25 μg/mL iron-saturated human transferrin. The cells were then stimulated (+) or not (−) for 10 minutes with 10 U/mL Epo. Lysates from 20 × 106 cells were immunoprecipitated with anti-GAB1 antibodies and analyzed by Western blot using antiphosphotyrosine (anti-PY) and anti-GAB1 antibodies, successively.
DISCUSSION
Although the stimulation of CFU-E cells by Epo is absolutely required for the terminal differentiation of these erythroid progenitors in physiological conditions, recent data strongly suggest that the Epo receptor does not transduce specific differentiation signals, because ectopic expression of other cytokine receptors in these CFU-E cells allows their erythroid differentiation in response to these cytokines (see Socolovsky et al3 for a recent review). Presumably, the Epo receptor essentially transduces antiapoptotic and proliferative signals. PI 3-kinase activation is implicated as a major step in both mitogenic28 and anti-apoptotic30 signaling pathways. These potencies of PI 3-kinase signaling suggest that PI 3-kinase could be a major intracellular signaling pathway in the mechanism of action of Epo. PI 3-kinase activation by Epo has been thoroughly documented previously.10-13 PI 3-kinase activation most generally involves the association of the SH2 domains of the regulatory subunit (p85) with tyrosine-phosphorylated proteins. This association both drives the enzyme close to its substrate by promoting the relocalization of PI 3-kinase to the plasma membrane and probably causes a conformational change in the regulatory subunit that increases the enzyme activity.28,31 We observed the association of PI 3-kinase with five tyrosine-phosphorylated proteins with molecular masses of 70, 100, 116, 145, and 180 kD. According to previously published results, the 70-kD protein is the Epo receptor,10-13 and we recently showed that the 180-kD protein was IRS2.18
In this report, we show that the 116-kD protein is the molecular adapter GAB1. GAB1 is strongly tyrosine phosphorylated in Epo-stimulated cells, and this tyrosine phosphorylation perfectly correlated with the tyrosine phosphorylation of Epo receptors in both kinetics and dose-response studies (Fig 3), strongly suggesting that the tyrosine phosphorylation of GAB1 is a direct consequence of Epo receptor activation. Importantly, Epo-induced GAB1 tyrosine phosphorylation was seen not only in the UT-7 erythroleukemia cell line, but also in normal human erythroid progenitors, demonstrating the physiological relevance of our observation.
HGF-induced GAB1 tyrosine phosphorylation involves the binding of GAB1 to the tyrosine-phosphorylated form of Met both by a novel phosphotyrosine binding domain and through GRB2, which links Met by its SH2 domain and GAB1 by its SH3 domains.20,23 In contrast, the tyrosine residues of gp130 are not required for IL-6–induced tyrosine phosphorylation of GAB1 and gp130 is not precipitated by anti-GAB1 antibodies.24 Our results also indicate that the tyrosine-phosphorylated form of GAB1 was not associated with the Epo receptor (see Fig 5), suggesting that the cytokine receptors induce the tyrosine phosphorylation of GAB1 by a common mechanism, albeit different from that used by the HGF receptor.
Three potential p85 SH2 domain binding sites with a consensus YXXM sequence15 (Y447, Y472, and Y589) are present in GAB1.19 These sites are involved in insulin- and in NGF-mediated activation of PI 3-kinase.25,32 Because PI 3-kinase only associated with the tyrosine-phosphorylated form of GAB1 (Fig 2), this association probably involved these p85 binding sites. In contrast to IRS2, which only associates with PI 3-kinase and SHIP after Epo stimulation,18 we observed the association of GAB1 with several proteins, including GRB2, SHC, SHP2, and SHIP in addition to PI 3-kinase, suggesting that GAB1 could transduce Epo receptor activation to several intracellular signaling pathways. Similarly to PI 3-kinase, SHP2 association with GAB1 was only observed in Epo-stimulated cells. A binding site for the SH2 domains of SHP2 (sequence Y627LDL) is located in the C-terminal domain of GAB1. Whether this sequence is responsible for the association of SHP2 with GAB1 is likely, although this remains to be determined. The association of GRB2 with GAB1 appears to be more puzzling. Indeed, a weak association was detected in unstimulated cells, but it was strongly enhanced by Epo stimulation. The constitutive association could result from the binding of the SH3 domain(s) of GRB2 to proline-rich sequences located in the C-terminal part of the GAB1 molecule. These sequences have been shown to be responsible for most of the association of GAB1 with the HGF receptor.20,23 According to this hypothesis, we have observed the binding of GAB1 to a GST-GRB2 fusion protein containing a deletion in the SH2 domain (data not shown). The Epo-induced association between GAB1 and GRB2 could be realized through several mechanisms that involve the SH2 domain of GRB2. This association could be direct through binding of the GRB2 SH2 domain to a consensus sequence (YKND) present in the N-terminal part of GAB1 or indirect through SHP2 and/or SHC. Indeed, these two proteins possess tyrosine residues able to bind, once phosphorylated, the SH2 domain of GRB2. According to this hypothesis, these GAB1-GRB2 complexes could be able to bind SOS through the SH3 domains of GRB2. This association to SOS could explain the GAB1-mediated activation of ERK that has been previously reported in IL-6–stimulated cells.24 However, we did not detect SOS1 in anti-GAB1 immunoprecipitates, possibly because of the low efficiency of our anti-SOS antibodies. We also observed the association of SHC with GAB1. This is the first demonstration of an association between these two proteins. Whether SHC associated with GAB1 directly or through another GAB1-associated protein such as GRB2 or SHIP remains to be determined.
We have identified the 145-kD protein present in anti-GAB1 immunoprecipitates as SHIP. SHIP was observed in all immunoprecipitates containing PI 3-kinase, namely the anti-IRS2,18 anti-GAB1 (this report), anti–PI 3-kinase, and anti-Epo receptor immunoprecipitates (data not shown). The presence of SHIP in the PI 3-kinase–containing complexes should greatly increase the production of phosphatidylinositol 3,4 bisphosphate and the disappearance of phosphatidylinositol 3,4,5 trisphosphate. SHIP appears to be a negative modulator of intracellular signaling in most cases. However, SHIP−/− mice have a reduced number of bone marrow CFU-E, and it is not established whether SHIP has a positive or negative role in Epo signaling.33 On a molecular basis, the phosphatidylinositol 3,4 bisphosphate production could increase Akt activation,34 which could exert an antiapoptotic function through the phosphorylation of the proapoptotic protein Bad.35 36 However, the disappearance of phosphatidylinositol 3,4,5 could also lead to the inhibition of signaling pathways activated through this second messenger. Overall, our results show that GAB1 could constitute a scaffold protein, allowing the formation of a multimolecular complex containing proteins able to activate several intracellular pathways, including PI 3-kinase in Epo-stimulated cells.
It is now clearly established that PI 3-kinase could be activated in Epo-stimulated cells by several mechanisms that involve or do not involve the tyrosine residues of the Epo receptor. Moreover, the PI 3-kinase inhibitor wortmannin inhibits the Epo-induced proliferation of DA-3 cells expressing wild-type Epo receptors as well as Epo receptors devoid of Tyr479,14 demonstrating the physiological relevance of all these mechanisms. Although in experimental conditions these pathways appear to be redundant, it should be kept in mind that normal erythroid progenitors express lower levels of Epo receptors that the cell lines used as experimental models and that they have to respond in vivo to low Epo concentrations. In these conditions, PI 3-kinase activation by these different pathways could be additive and required to allow a cellular response to a low level of Epo stimulation. Moreover, the importance of the last tyrosine residue of the Epo receptor that directly binds PI 3-kinase is not clearly established. Indeed, it has been reported that this tyrosine residue of the Epo receptor induces a signal transduction pathway sufficient for proliferation and differentiation of fetal liver CFU-E in mice.37 However, truncation of the Epo receptor that also removes this tyrosine residue causes hypersensitivity to Epo and benign erythrocytosis in humans.38 In addition, mice expressing Epo receptors truncated after the first tyrosine residue of intracellular domain exhibit normal hematological parameters despite the removal of the PI 3-kinase binding site (J.N. Ihle, personnal communication of unpublished results). Because it is now demonstrated that all of these receptors induce PI 3-kinase activation, the role of PI 3-kinase in Epo-induced proliferation and/or survival of erythroid progenitors has to be carefully addressed.
C.L.-L. and F.V. contributed equally to this work.
Supported by grants from the Association pour la Recherche sur le Cancer (ARC Contract No. 1373) and from the Ligue Nationale Contre le Cancer. F.V. is supported by the GLAXO WELLCOME Laboratories.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Patrick Mayeux, PhD, ICGM, INSERM U363, Hôpital Cochin, 27 rue du Faubourg Saint Jacques, F75014 Paris, France; e-mail: mayeux@cochin.inserm.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal