Abstract
The high affinity IgE receptor (FcɛRI) expressed on the cell surface of mast cells and basophils is the key molecule in triggering the IgE-mediated allergic reaction. Recently, it was elucidated that the FcɛRI is expressed on a variety of other cells like Langerhans cells, monocytes, and eosinophils, and the functional importance of the FcɛRI expression in Langerhans cells was also shown. Some studies suggest that human platelets may play important roles in allergic inflammation through the cell-surface expression of the FcɛRII and FcγRII. Here, we report that human platelets and megakaryocytes constitutively express the messenger RNA and protein for the FcɛRI. Although the FcɛRI is expressed on the cell surface of human platelets, it is only detected in the cytoplasm of human megakaryocytes. We also confirmed that human platelets express the genes for the , β, and γ chains of the FcɛRI without any defined mutations. Furthermore, stimulation of human platelets via the FcɛRI induced the release of serotonin and RANTES (Regulated on Activation, Normal T Expressed, and presumably Secreted). Taken together, these results suggest a novel and important role for human platelets in perpetuating allergic inflammation through the expression of and activation via the FcɛRI.
THE HIGH AFFINITY RECEPTOR for IgE (FcεRI) is the prototype of antigen recognition receptors, composed of multiple subunits that include T-cell receptor (TCR), B-cell receptor (BCR), Fc gamma (Fcγ), and Fc alpha (Fcα) receptors. Ligand-binding and signal-transducing functions in these Fc receptors are separately performed by distinct subunits.1,2 The FcεRI is a tetrameric structure comprising one α chain, one β chain, and two disulfide-linked γ chains. The extracellular domain of the α chain contains the entire IgE-binding domain,3whereas the β and γ chains are primarily involved in signal transduction. One of the defined functions of the γ chain is to facilitate the cell-surface expression of the FcεRI α chain.4,5 It was recently reported that the γ chain is also found in association with FcγRIII, TCR, FcγRI, and FcαR,6-12 and that the γ chain is a member of a group of functionally related polypeptides, including the TCR ζ and η chains.9,12 Comparisons of the primary sequences of the cytoplasmic domains of signal transducing components of antigen receptors showed a consensus motif, first identified by Reth.13 This region was designated as an immunoreceptor tyrosine-based activation motif (ITAM).14 The ITAM is based on neighboring tyrosine motif (YXXLXXXXXYXXL) that is tyrosine-phosphorylated during signaling13-15 via the receptors.
Crosslinking of allergen-specific IgE bound to the FcεRI expressed on the surface of mast cells, with multivalent allergens, results in the release of both preformed and newly generated mediators, and in the manifestation of allergic symptoms.2,4,5,16 Thus the FcεRI on mast cells and basophils is the key molecule in triggering the IgE-mediated allergic reaction such as in bronchial asthma, atopic dermatitis, and allergic rhinitis. It was recently reported that the FcεRI is expressed not only on mast cells and basophils, but also on dermal Langerhans cells, monocytes, eosinophils, and dendritic cells.17-21
Human platelets are derived from the bone marrow, circulate in the peripheral blood, and play an important role in the coagulation of blood. Recent studies showed that the low affinity receptor for IgG (FcγRIIA/CD32) and the low affinity receptor for IgE (FcεRII/CD23) are expressed on the cell surface of human platelets,22-25and that activation of human platelets by PAF (platelet activating factor) produced by mast cells, results in the perpetuation of the allergic reaction. Activated platelets induce aggregation and form micro-thrombosis, and release chemical mediators such as serotonin, thromboxane A2, PF4 (platelet factor 4), PDGF (platelet-derived growth factor), and cytokines such as RANTES (Regulated on Activation, Normal T expressed, and presumably Secreted).26 In the present study, we showed the expression of the FcεRI in human platelets and megakaryocytes, and that activation of human platelets via the FcεRI induced the release of serotonin and RANTES. These findings show a novel role for human platelets in triggering the allergic reaction through the FcεRI.
MATERIALS AND METHODS
Isolation and purification of human peripheral blood platelets.
Ten mL of venous blood was collected from normal volunteers (healthy subjects) and patients with atopic diseases, in heparinized syringes, and centrifuged at 120g for 15 minutes at room temperature. The PRP (platelet-rich plasma) was resuspended in washing buffer (9 mmol/L Na2 EDTA, 26.4 mmol/L Na2HPO4, 140 mmol/L NaCl) and centrifuged at 800g for 10 minutes at room temperature. Subsequently, the pellet containing the platelets was resuspended again in washing buffer and centrifuged at 200g for 10 minutes at room temperature. The supernatant was centrifuged at 800g for 15 minutes at room temperature and the pelleted platelets were then resuspended in phosphate-buffered saline (PBS) containing 0.02% EDTA. The total yield of platelets was more than 1 × 108 (purity: >99%).
Cell lines.
The human megakaryocyte-like cell line, HML-1, was cultured in α-MEM (minimal essential medium) (GIBCO BRL Life Technologies, Inc, Gaithersburg, MD) supplemented with 10% heat-inactivated fetal calf serum (FCS), 1% bovine serum albumin (BSA). and 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF).
Monoclonal antibodies (MoAbs).
Mouse MoAbs to human FcεRIα chain (CRA2, CRA3: mouse IgG1 and CRA1: mouse IgG2b) were used to detect the expression of the FcεRI α chain in human platelets and megakaryocytes, and for the studies on the release of serotonin and RANTES from human platelets27 28(CRA2 and CRA3 are competitive with IgE and recognize the IgE binding domain of the FcεRI α chain; CRA1 is noncompetitive with IgE). Mouse MoAb to human CD61 (GPIIIa, DAKO A/S, Glostrup, Denmark) was used as the positive control for flow cytometric analyses. PE-conjugated CD61 MoAb (Pharmingen, San Diego, CA) was used for two-color flow cytometric analysis of bone marrow cells. Mouse MoAb to human FcγRII (CD32, IV.3) was used as the positive control for serotonin releasing assay from human platelets.
Analysis of the gene expression for FcεRI α, β, and γ chains by reverse transcription and polymerase chain reaction (RT-PCR).
Total cellular RNA was extracted from purified platelets of healthy individuals and patients with atopic dermatitis, from HML-1 cells, peripheral white blood cells (as positive control) and human erythroleukemic cell line, K562 cells (as negative control) by the phenol-guanidium isothiocyanate method,29 and reverse-transcribed, as previously described.30 FcεRI α, β, and γ chain gene segments from 1 μg of each resultant complementary DNA (cDNA) sample were PCR amplified in the presence of specific sense and antisense primers (1 μmol/L each) (Table 1), 200 μmol/L dNTP, 0.05 U/μL Ampli Taq (Perkin Elmer, Norwalk, CT), 1 U Perfect Match (Stratagene, La Jolla, CA), and PCR buffer (1.5 mmol/L MgCl2, 50 mmol/L KCl, 10 mmol/L Tris-HCl, pH 8.3, 0.001% gelatin) in a final reaction volume of 20 μL. PCR was performed in DNA Thermal Cycler (Perkin Elmer) (α chain: 35 cycles; β chain: 40 cycles; γ chain: 30 cycles), each cycle including denaturation (94°C, 1 minute), annealing (α chain: 50°C, β chain and γ chain: 55°C, 1 minute), extension (72°C, 2 minutes), and final incubation (72°C, 10 minutes) after the last cycle. β-actin cDNA was amplified as internal control and the cDNA was substituted with that of human erythroleukemic cell line, K562, as negative control. The PCR products were electrophoresed on 2% agarose gels and after staining with ethidium bromide, the results were visualized under ultraviolet illumination.
The Sequences of the Oligonucleotides Used for RT-PCR
| FcεRI α chain (796 bp) |
| 118 |
| Forward: 5′-(XhoI) ATGGCTCCTGCCATGGAA-3′ |
| 774754 |
| Reverse: 5′-(NotI) TCAGTTGTTTTTGGGGTTTGG-3′ |
| FcεRI β chain (757 bp) |
| 121 |
| Forward: 5′-(XhoI) ATGGACACAGAAAGTAATAGG-3′ |
| 735705 |
| Reverse: 5′-(NotI)TTATAAATCAATGGGAGGAGACATTTCCCCT-3′ |
| FcεRI γ chain (283 bp) |
| 121 |
| Forward: 5′-(XhoI) ATGATTCCAGCAGTGGTCTTG-3′ |
| 261244 |
| Reverse: 5′-(NotI) CTACTGTGGTGGTTTCTC-3′ |
| FcεRI α chain (796 bp) |
| 118 |
| Forward: 5′-(XhoI) ATGGCTCCTGCCATGGAA-3′ |
| 774754 |
| Reverse: 5′-(NotI) TCAGTTGTTTTTGGGGTTTGG-3′ |
| FcεRI β chain (757 bp) |
| 121 |
| Forward: 5′-(XhoI) ATGGACACAGAAAGTAATAGG-3′ |
| 735705 |
| Reverse: 5′-(NotI)TTATAAATCAATGGGAGGAGACATTTCCCCT-3′ |
| FcεRI γ chain (283 bp) |
| 121 |
| Forward: 5′-(XhoI) ATGATTCCAGCAGTGGTCTTG-3′ |
| 261244 |
| Reverse: 5′-(NotI) CTACTGTGGTGGTTTCTC-3′ |
Sequence analysis of the PCR products for FcεRI α, β, and γ chain genes.
PCR products of the FcεRI α, β, and γ chain genes were sequenced directly on an automated DNA sequencer (Perkin Elmer Applied Biosystems, Model 373A) with Taq dye terminator method using the Taq DyeDeoxy TM Terminator Cycle Sequencing Kit (Perkin Elmer Applied Biosystems). After phenol/chloroform extraction and ethanol precipitation, the products for sequencing were electrophoresed on the 6% polyacrylamide/8.3 mol/L urea sequencing gels.
Analysis for the cell-surface expression of FcεRI on human platelets and HML-1 cells by flow cytometry.
Platelets were isolated as described above. 2 × 107platelets in 100 μL of 0.02% EDTA-PBS were incubated with 10 μg mouse MoAb to human FcεRI α chain on ice for 60 minutes, and 1 × 106 untreated HML-1 cells in 100 μL of 1% BSA-PBS were incubated with 100 ng mouse MoAb to human FcεRI α chain on ice for 60 minutes. After subsequent washing, these cells were incubated on ice for 45 minutes with 100 ng rabbit fluorescein isothiocyanate (FITC)-IgG against mouse IgG+IgM. Fluorescence intensities of stained cells were analyzed by an immunofluorescence cell sorter (Becton Dickinson, San Jose, CA: FACScan). To determine the intracytoplasmic expression of FcεRI in HML-1 cells, the cells were pretreated with cold 70% ethanol on ice for 15 minutes before incubation with mouse MoAb to human FcεRI α chain.
Analysis for the IgE bindability to FcεRI on the cell surface of human platelets by flow cytometry.
2 × 107 purified platelets in 100 μL of 0.02% EDTA-PBS were incubated with 10 μg human myeloma IgE on ice for 60 minutes. After washing, the platelets were incubated with 50 μg/mL FITC-conjugated goat antihuman IgE on ice for 60 minutes. To determine whether IgE binding was competitively inhibited by MoAb to the human FcεRI α chain (CRA2, a MoAb that is competitive with IgE), platelets were pretreated with 100 μg/mL CRA2 or isotype-matched control mouse IgG, for 60 minutes on ice before incubation with the human myeloma IgE. Fluorescence intensities of the stained cells were analyzed using a FACScan.
Immunohistochemical analysis for the expression of the FcεRI α chain in human platelets, and HML-1 cells.
Purified platelets (1 × 106/mL) and HML-1 cells (5 × 104) were fixed with 4% paraformaldehyde, washed in PBS containing 15% sucrose, and cytospinned onto silane-coated slides. The cells were then air dried, rehydrated in Tris-HCl buffer, pretreated with 10% normal rabbit serum, incubated overnight at 4°C in saturating concentration of the mouse antihuman MoAb to the FcεRI α chain (CRA1), and stained by the alkaline phosphatase antialkaline phosphatase method (APAAP) (DAKO APAAP kit) as previously described.30 For the detection of intracellular FcεRI α chain in HML-1 cells, the cells were first permeabilized with 0.1% saponin in PBS (saponin-PBS) and stained as described above.
Analysis for the cell-surface and intracytoplasmic expression of FcεRI α chain in normal human megakaryocytes, by flow cytometry.
For the detection of FcεRI α chain expression in normal human megakaryocytes, 5 mL of bone marrow was aspirated from normal subjects after obtaining their informed consent according to the regulations of the hospital’s ethical board. The bone marrow was aspirated into a heparinized syringe from a single site in the posterior iliac crest, diluted 1:2 in RPMI 1640, mixed with 2.5 mL of 5% Dextran (M.W. 180,000) in saline, and then kept at room temparature for 2 hours. The cell-rich supernatant was collected, centrifuged, and washed twice in Ca++ and Mg++ free PBS. The cells were then treated with 2 mL of lysing solution (Becton Dickinson) for 10 minutes at room temperature, centrifuged and resuspended in PBS at a concentration of 2 × 107/mL. For the detection of cell-surface expression of the FcεRI in megakarocytes, these cells were treated with 5 μg/mL of PE-conjugated antihuman CD61 MoAb (Pharmingen) for 15 minutes at room temparature, washed, and treated with 1 μg/mL of FITC-conjugated anti-FcεRI MoAb (CRA2), or the FITC-conjugated mouse IgG1 (as negative control) for 30 minutes at room temperature. For the detection of intracytoplasmic expression of the FcεRI in megakarocytes, the cells were first treated with 5 μg/mL of PE-conjugated antihuman CD61 MoAb (Becton Dickinson) for 15 minutes at room temperature, followed by treatment with 0.5 mL of the permeabilization solution (Becton Dickinson) for 10 minutes at room temperature (according to the manufacturer’s instructions), and then treated with 1 μg/mL FITC-conjugated anti-FcεRI MoAb (CRA2), or the FITC-conjugated mouse IgG1(as negative control) for 30 minutes at room temperature. Finally, the cells were washed twice, and the FcεRI expression in normal human megakaryocytes was analyzed by two-color flow cytometry using a FACScan (Becton Dickinson) after gating on the polymorphonuclear cells that were CD61 positive (CD61+PMN).
Immunoelectromicroscopic analysis for the expression of the FcεRI α chain in human platelets.
Purified platelets (1 × 106/mL) were fixed with 4% paraformaldehyde, washed in PBS containing 10% sucrose, and then incubated in saponin-PBS for 10 minutes. The cells were then treated with 0.1% H2O2, washed in saponin-PBS, and incubated overnight at 4°C with the mouse antihuman MoAb to the FcεRI α chain (CRA1) at the saturating concentration. Subsequently, the cells were washed and treated with peroxidase-conjugated goat antimouse IgG at the saturating concentration. After four washes, the cells were treated with 0.25% glutaraldehyde for 5 minutes, washed, and treated with diamino benzidine hydrocholride (DAB) in dimethyl sulphoxazide (DMSO) for 30 minutes. The reaction was developed with the DAB substrate. Finally, after washing twice in PBS the cells were treated with 2% osmium tetroxide for 30 minutes, washed and dehydrated in graded series of alcohol (30% to 100%), embedded in epoxy resin, and then polymerized for 72 hours. Ultrathin sections were cut, taken onto fine grids, counterstained briefly with uranyl acetate and lead citrate, and examined with a Hitachi 800 electron microscope.
Assay for FcεRI-mediated serotonin release from human platelets.
Fifty mL of venous blood was collected from atopic patients (patients with atopic dermatitis and chronic urticaria) and healthy individuals with 0.38% sodium citrate. The blood was centrifuged at 120gfor 15 minutes at room temperature and the PRP was collected. The PRP (1 × 108/mL) was incubated with [3H] serotonin (NEN, Boston, MA) at a concentration of 2 μCi/mL and PGI2 (Cayman Chemical, Ann Arbor, MI) at a concentration of 100 ng/mL at room temperature for 60 minutes. The PRP was then centrifuged at 800g for 15 minutes at 4°C, washed, resuspended in HEPES-Tyrode’s buffer (pH 7.4, 142 mmol/L NaCl, 6.2 mmol/L KCl, 6.5 mmol/L HEPES) supplemented with 1% FCS and 100 ng/mL PGI2, and incubated on ice for 45 minutes with mouse antihuman FcεRI α chain MoAb (10 μg/mL) and 0.03% human serum albumin. After washing the platelets were resuspended in HEPES-Tyrode’s buffer supplemented with 1 mmol/L CaCl2 , 0.6 mmol/L MgCl2, and incubated at 37°C for 45 minutes with the rabbit antimouse IgG + IgM at a concentration of 30 μg/mL. We used thrombin at a concentration of 10 U/mL and a mouse MoAb to human FcγRII/CD32 (IV.3) at a concentration of 10μg/mL as the positive control.25 The radioactivity of [3H] serotonin released into the supernatant, and of the total platelet suspension was measured, and the percent release was calculated as: [(a − b)/c] × 100, in which a is the concentration of serotonin released from the stimulated platelets, b is the spontaneous release of serotonin from the unstimulated platelets, and c is the total concentration of serotonin in the cells after lysing the platelets with 1% NP-40.
Assay for FcεRI-mediated release of RANTES from human platelets.
Venous blood (50 mL) was collected from healthy individuals with 0.38% sodium citrate and centrifuged at 120g for 15 minutes at room temperature and the PRP was collected, as described above. The PRP (1 × 108/mL) was washed, resuspended in HEPES-Tyrode’s buffer (pH 7.4, 142 mmol/L NaCl, 6.2 mmol/L KCl, 1 mmol/L CaCl2, 0.6 mmol/L MgCl2, 6.5 mmol/L HEPES) supplemented with 1% FCS and 100 ng/ml PGI2, and incubated at 37°C for 60 minutes with the mouse antihuman FcεRI α chain MoAb at a concentration of 100 μg/mL. We used thrombin at a concentration of 1 U/mL as the positive control. RANTES that was released into the supernatant and of the total platelet suspension was measured by ELISA, using the RANTES specific ELISA kit (Quantikine Human RANTES Immunoassay; R&D Systems, Minneapolis, MN). The percent release was calculated relative to the total concentration of RANTES in the cells as previously described.31
Assay for IgE-mediated release of RANTES from human platelets.
In the meantime, for IgE-mediated stimulation, the PRP (1 × 108/mL) were incubated on ice for 45 minutes with the human myeloma IgE at a concentration of 100 μg/mL, washed, and resuspended in HEPES-Tyrode’s buffer supplemented with 1 mmol/L CaCl2, 0.6 mmol/L MgCl2, and 10% mouse serum. The platelets were then incubated at 37°C for 60 minutes with goat antihuman IgE (CHEMICON International Inc, Temecula, CA) at a concentration of 300 μg/mL. In addition, platelets were incubated at 37°C for 60 minutes with either human myeloma IgE alone (100 μg/mL) or goat antihuman IgE alone (300 μg/mL).31
RESULTS
Gene expression of FcεRI α, β, and γ chains in human platelets and HML-1 cells.
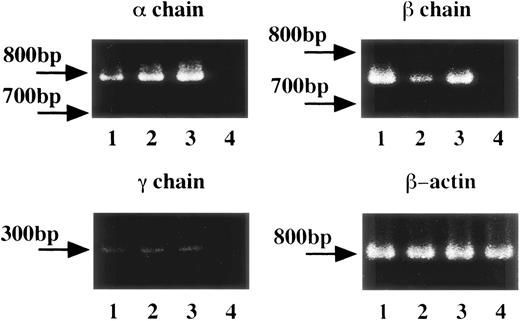
To determine the expression of messenger RNA (mRNA) for FcεRI α, β, and γ chains in human platelets and HML-1 cells, we performed RT-PCR of human platelets obtained from healthy individuals, and of HML-1 cells. Human peripheral white blood cells were used as positive control and the human erythroleukemic cell line, K562 cells, were used as negative control. mRNA expression for FcεRI α chain (796 bp), β chain (757 bp), and γ chain (283 bp) was detected in human peripheral white blood cells, human platelets, and HML-1 cells but not in K562 cells (Fig 1). Moreover, gene expression of FcεRI α, β, and γ chains was detected in human platelets from both healthy individuals and allergic patients (data not shown). To verify the occurrence of any mutations in the FcεRI α, β, and γ chain cDNAs in the RT-PCR products of human platelets of healthy individuals, and HML-1 cells, we performed direct sequencing analyses. Sequencing analyses showed that the PCR products of FcεRI α, β, and γ chains had exactly the same sequences as those described in previous reports.32-34
FcɛRI gene expression in human platelets and HML-1 cells. The FcɛRI gene expression in human platelets (total number: 1 × 108, purity: >99%) and in the human megakaryocyte like cell line, HML-1 cells (total number: 1 × 107) was analyzed by RT-PCR. 1 × 107 human peripheral white blood cells were used as positive control and 1 × 107 of the human erythroleukemic cell line, K562 cells were used as negative control. FcɛRI , β, and γ chain gene segments from 1 μg of each cDNA sample were PCR amplified in the presence of specific sense and antisense primers. Lane 1: human peripheral white blood cells; lane 2: human platelets; lane 3: human megakaryocyte-like cell line, HML-1 cells; lane 4: human erythroleukemic cell line, K562 cells as negative control.
FcɛRI gene expression in human platelets and HML-1 cells. The FcɛRI gene expression in human platelets (total number: 1 × 108, purity: >99%) and in the human megakaryocyte like cell line, HML-1 cells (total number: 1 × 107) was analyzed by RT-PCR. 1 × 107 human peripheral white blood cells were used as positive control and 1 × 107 of the human erythroleukemic cell line, K562 cells were used as negative control. FcɛRI , β, and γ chain gene segments from 1 μg of each cDNA sample were PCR amplified in the presence of specific sense and antisense primers. Lane 1: human peripheral white blood cells; lane 2: human platelets; lane 3: human megakaryocyte-like cell line, HML-1 cells; lane 4: human erythroleukemic cell line, K562 cells as negative control.
Cell-surface expression of the FcεRI α chain in human platelets.
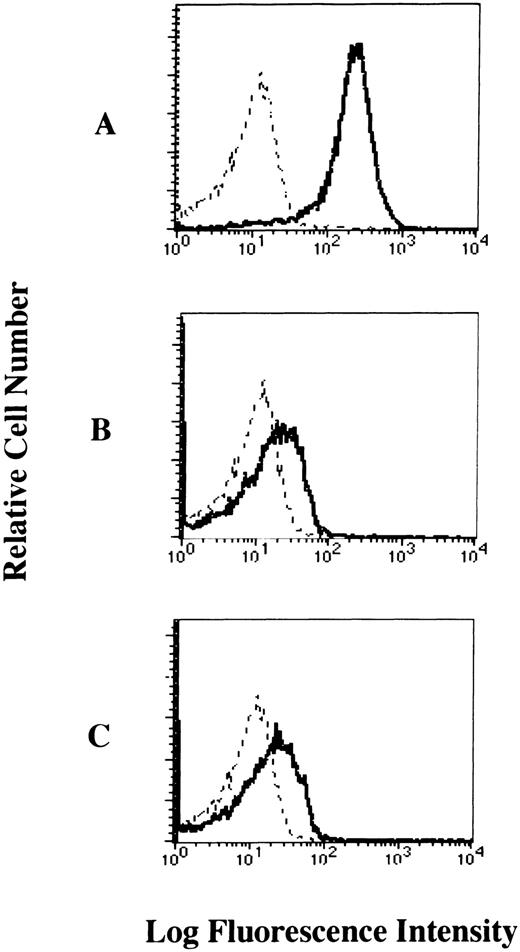
To examine whether the FcεRI α chain is expressed on the cell-surface of human platelets, we performed flow cytometric analyses of human platelets from healthy individuals stained with mouse MoAbs to the human FcεRI α chain (CRA2 and CRA3). Our results showed that the FcεRI α chain was expressed on the cell-surface of platelets from healthy individuals. The levels of FcεRI expression in human platelets when stained with CRA2 and CRA3 MoAbs were 30.3% and 36.1%, respectively (Fig 2). We also confirmed that the FcεRI α chain was expressed on the cell surface of human platelets from patients with atopic dermatitis, but there was no significant difference in the level of FcεRI α chain expression in human platelets between normals and patients (data not shown).
Cell-surface expression of the FcɛRI chain on human platelets analyzed by flow cytometry. The platelets were incubated with mouse MoAbs to (A) human CD61 as positive control, (B) human FcɛRI chain (CRA2), and (C) human FcɛRI chain (CRA3) respectively, and then stained with the FITC-labeled rabbit antimouse IgG + IgM (solid dark line). Mouse IgG1 was used as isotype-matched control antibody (dashed line).
Cell-surface expression of the FcɛRI chain on human platelets analyzed by flow cytometry. The platelets were incubated with mouse MoAbs to (A) human CD61 as positive control, (B) human FcɛRI chain (CRA2), and (C) human FcɛRI chain (CRA3) respectively, and then stained with the FITC-labeled rabbit antimouse IgG + IgM (solid dark line). Mouse IgG1 was used as isotype-matched control antibody (dashed line).
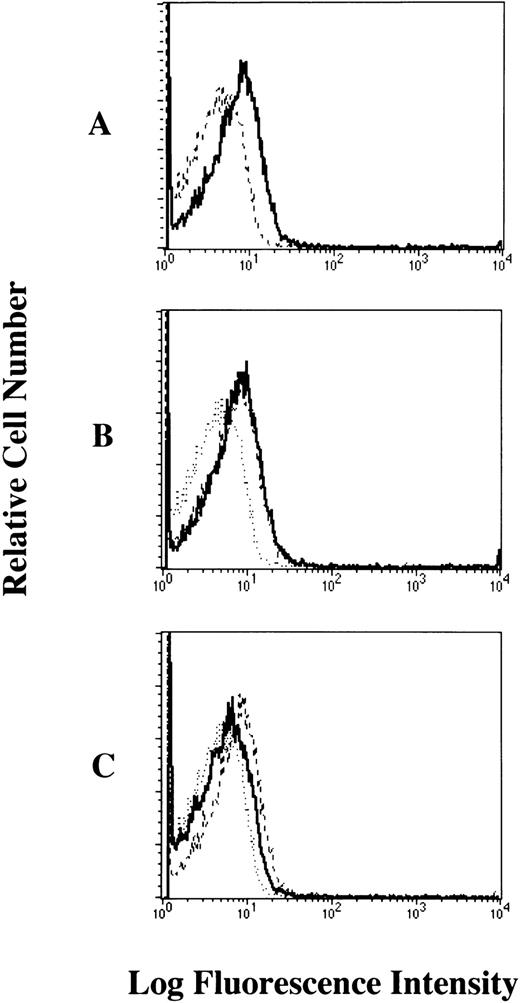
We next examined the levels of cell bound IgE in human platelets of healthy individuals by flow cytometric analyses. Our results showed that human myeloma IgE bound to the cell surface of human platelets (19% positivity) (Fig 3A). We confirmed that this binding was partially inhibited (68.4% inhibition) by treatment with the mouse MoAb to human FcεRI α chain (CRA2, a MoAb that is competitive with IgE) (Fig 3C), but was not inhibited by treatment with the isotype-matched control Ab (Fig 3B). These results confirmed that the human myeloma IgE mainly bound to the cell surface of human platelets via the FcεRI.
Bindability of human IgE to the FcɛRI on human platelets analyzed by flow cytometry. The platelets were (A) untreated (dashed line; only FITC-labeled goat antihuman IgE, solid dark line; human myeloma IgE plus FITC-labeled goat antihuman IgE), (B) pretreated with isotype-matched control Ab (dotted line; only FITC-labeled goat antihuman IgE, dashed line; human myeloma IgE plus FITC-labeled goat antihuman IgE, solid dark line; pretreated with mouse IgG1), (C) pretreated with mouse MoAb to human FcɛRI chain (CRA2) (dotted line; only FITC-labeled goat antihuman IgE, dashed line; human myeloma IgE plus FITC-labeled goat antihuman IgE, solid dark line; pretreated with CRA2). These results shown are representative of 10 independent experiments.
Bindability of human IgE to the FcɛRI on human platelets analyzed by flow cytometry. The platelets were (A) untreated (dashed line; only FITC-labeled goat antihuman IgE, solid dark line; human myeloma IgE plus FITC-labeled goat antihuman IgE), (B) pretreated with isotype-matched control Ab (dotted line; only FITC-labeled goat antihuman IgE, dashed line; human myeloma IgE plus FITC-labeled goat antihuman IgE, solid dark line; pretreated with mouse IgG1), (C) pretreated with mouse MoAb to human FcɛRI chain (CRA2) (dotted line; only FITC-labeled goat antihuman IgE, dashed line; human myeloma IgE plus FITC-labeled goat antihuman IgE, solid dark line; pretreated with CRA2). These results shown are representative of 10 independent experiments.
Expression of the FcεRI α chain in HML-1 cells, normal human megakaryocytes, and human platelets.
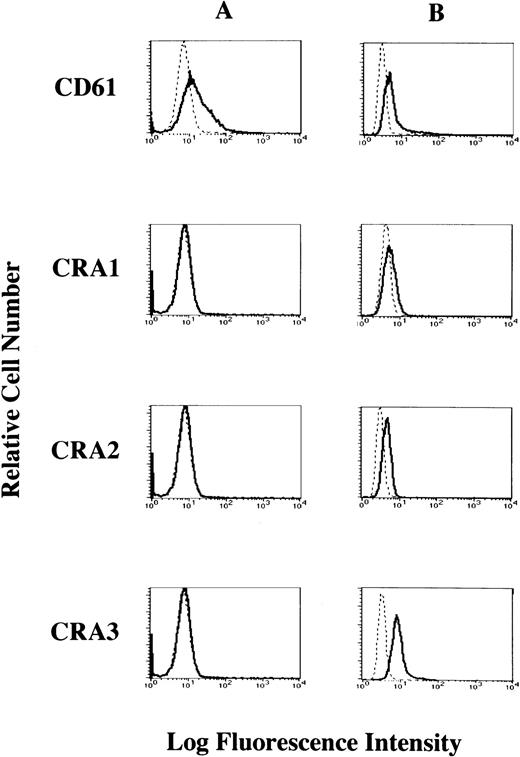
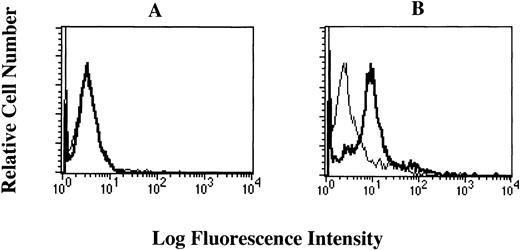
To investigate whether the FcεRI α chain is expressed on the cell surface of HML-1 cells, we performed flow cytometric analyses of HML-1 cells after staining with the mouse MoAbs to the human FcεRI α chain. Mouse MoAb to human CD61 was used as positive control. Although CD61 was expressed on the cell surface of HML-1 cells, these cells did not express the FcεRI α chain on their cell surface (Fig 4A). We next examined whether the FcεRI α chain was expressed in the cytoplasm of HML-1 cells after fixation with ethanol. As shown in Fig 4B, FcεRI α chain was expressed in the cytoplasm of HML-1 cells (CRA1: 25%, CRA2: 51.3%, and CRA3: 93.8%). Furthermore, by immunohistochemistry, immunoreactivity for the FcεRI α chain was not detected on the cell-surface of HML-1 cells (Fig 5A), but was detected in the cytoplasm of these cells (Fig 5B).
Cell-surface expression of the FcɛRI in HML-1 cells. (A) The cell-surface expression of the FcɛRI chain in HML-1 cells was analyzed by flow cytometry. Untreated cells were incubated with mouse MoAb to human CD61 and human FcɛRI chain, respectively, stained with the FITC-labeled rabbit antimouse IgG + IgM. Mouse IgG1 or IgG2b (for CRA1) were used as isotype-matched control antibodies. (B) Intracytoplasmic expression of the FcɛRI in HML-1 cells was analyzed by flow cytometry after pretreatment of the cells with cold ethanol before incubation with primary or secondary antibodies.
Cell-surface expression of the FcɛRI in HML-1 cells. (A) The cell-surface expression of the FcɛRI chain in HML-1 cells was analyzed by flow cytometry. Untreated cells were incubated with mouse MoAb to human CD61 and human FcɛRI chain, respectively, stained with the FITC-labeled rabbit antimouse IgG + IgM. Mouse IgG1 or IgG2b (for CRA1) were used as isotype-matched control antibodies. (B) Intracytoplasmic expression of the FcɛRI in HML-1 cells was analyzed by flow cytometry after pretreatment of the cells with cold ethanol before incubation with primary or secondary antibodies.
Expression of the FcɛRI in HML-1 cells. The cell-surface expression of the FcɛRI in HML-1 cells (A) was analyzed, by immunohistochemistry after staining with the antihuman FcɛRI chain MoAb (CRA1) using the APAAP method. The intracytoplasmic expression of the FcɛRI in HML-1 cells (B) was analyzed, by immunohistochemistry as described above after permeabilization with saponin. Although no immunoreactivity was detected for the FcɛRI chain on the cell surface of HML-1 cells, FcɛRI chain expression was clearly detected in the cytoplasm of HML-1 cells.
Expression of the FcɛRI in HML-1 cells. The cell-surface expression of the FcɛRI in HML-1 cells (A) was analyzed, by immunohistochemistry after staining with the antihuman FcɛRI chain MoAb (CRA1) using the APAAP method. The intracytoplasmic expression of the FcɛRI in HML-1 cells (B) was analyzed, by immunohistochemistry as described above after permeabilization with saponin. Although no immunoreactivity was detected for the FcɛRI chain on the cell surface of HML-1 cells, FcɛRI chain expression was clearly detected in the cytoplasm of HML-1 cells.
By two color flow cytometry, we examined the expression of the FcεRI α chain in normal human magakaryocytes after gating on the CD61+ PMN. Although, we could not detect the expression of the FcεRI α chain on the cell surface of normal human megakaryocytes (Fig 6A), we could detect FcεRI α chain expression in the cytoplasm of these cells (Fig 6B). By immunohistochemistry, we also detected FcεRI α chain on the cell surface of human platelets (data not shown). Furthermore, by immunoelectronmicroscopy after staining with the mouse MoAb to human FcεRI α chain (CRA1), we could precisely localize the FcεRI α chain on the cell surface of human platelets (Fig 7A). However, no staining of the cell surface of human platelets was detected after staining with the isotype-matched Ab (Fig 7B).
Expression of the FcɛRI in normal human megakaryocytes. (A) The cell-surface expression of the FcɛRI chain in normal human megakaryocytes was analyzed by two-color flow cytometry. Cells were isolated from the bone marrow aspirate (as described in the text) and were incubated with PE-conjugated antihuman CD61 MoAb and the FITC-conjugated antihuman FcɛRI chain MoAb (CRA2) (solid line) and analyzed using the FACScan after gating on the polymorphonuclear cells that were CD61 positive (CD61+ PMN). FITC-conjugated mouse IgG1 was used as isotype-matched control antibody. (B) The intracytoplasmic expression of the FcɛRI chain in normal human megakaryocytes. Cells were first incubated with the PE-conjugated antihuman CD61 MoAb, treated with the permeabilization solution (Becton Dickinson) and then incubated with FITC-conjugated antihuman FcɛRI chain MoAb, and analyzed using the FACScan after gating on the CD61+ PMN. FITC-conjugated mouse IgG1 was used as isotype-matched control antibody (thin line).
Expression of the FcɛRI in normal human megakaryocytes. (A) The cell-surface expression of the FcɛRI chain in normal human megakaryocytes was analyzed by two-color flow cytometry. Cells were isolated from the bone marrow aspirate (as described in the text) and were incubated with PE-conjugated antihuman CD61 MoAb and the FITC-conjugated antihuman FcɛRI chain MoAb (CRA2) (solid line) and analyzed using the FACScan after gating on the polymorphonuclear cells that were CD61 positive (CD61+ PMN). FITC-conjugated mouse IgG1 was used as isotype-matched control antibody. (B) The intracytoplasmic expression of the FcɛRI chain in normal human megakaryocytes. Cells were first incubated with the PE-conjugated antihuman CD61 MoAb, treated with the permeabilization solution (Becton Dickinson) and then incubated with FITC-conjugated antihuman FcɛRI chain MoAb, and analyzed using the FACScan after gating on the CD61+ PMN. FITC-conjugated mouse IgG1 was used as isotype-matched control antibody (thin line).
Immunolocalization of the FcɛRI in human platelets. Localization of the FcɛRI chain in human platelets was examined by immunoelectromicroscopy after staining with the antihuman FcɛRI chain MoAb (CRA1), using the indirect immunoperoxidase method. (A) FcɛRI chain was localized to the cell-surface (arrow head) of human platelets. (B) Negative control with isotype-matched control Ab shows no cell-surface staining.
Immunolocalization of the FcɛRI in human platelets. Localization of the FcɛRI chain in human platelets was examined by immunoelectromicroscopy after staining with the antihuman FcɛRI chain MoAb (CRA1), using the indirect immunoperoxidase method. (A) FcɛRI chain was localized to the cell-surface (arrow head) of human platelets. (B) Negative control with isotype-matched control Ab shows no cell-surface staining.
FcεRI-mediated serotonin release from human platelets.
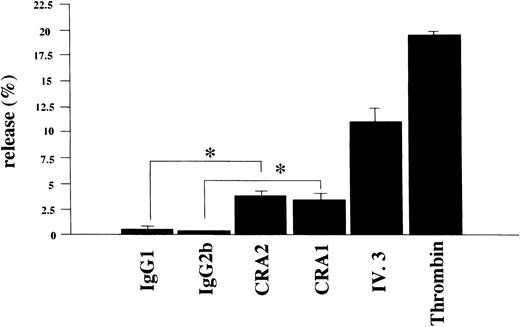
To learn whether the FcεRI α chain expressed in human platelets was functional or not, we examined if activation of human platelets via the FcεRI could induce the release of serotonin. Using the mouse MoAbs to the human FcεRI α chain (CRA1 and CRA2) we stimulated human platelets for the serotonin release. The level of released-serotonin from the platelets stimulated by thrombin was measured as positive control and was always about 20% of the total content. Furthermore, the fraction of the released-serotonin from the platelets by MoAb to FcγRII (also used as positive control) was about 10% of the total content. The fraction of the released-serotonin from platelets by the mouse MoAb to FcεRI α chain was about 4% of the total content (Fig 8). The serotonin release from human platelets by isotype-matched control Abs was always less than 1%, in all experiments performed.
Serotonin release from human platelets. Human platelets (1 × 108/mL) were stimulated with the mouse MoAb to human FcɛRI chain (CRA1 and CRA2, each 10 μg/mL), or with mouse MoAb to human FcγRII/CD32 (IV.3, 10 μg/mL), or with thrombin (10 U/mL). The levels of released serotonin were measured as radioactivity, in the supernatants and the percent release was estimated as described in Materials and Methods. Spontaneous release was always < 1% of the total serotonin content. Results are shown as mean ±SD (n = 3). *P < .01.
Serotonin release from human platelets. Human platelets (1 × 108/mL) were stimulated with the mouse MoAb to human FcɛRI chain (CRA1 and CRA2, each 10 μg/mL), or with mouse MoAb to human FcγRII/CD32 (IV.3, 10 μg/mL), or with thrombin (10 U/mL). The levels of released serotonin were measured as radioactivity, in the supernatants and the percent release was estimated as described in Materials and Methods. Spontaneous release was always < 1% of the total serotonin content. Results are shown as mean ±SD (n = 3). *P < .01.
IgE- and FcεRI-mediated RANTES release from human platelets.
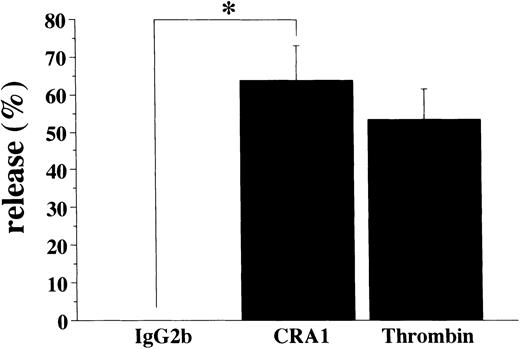
To verify whether the FcεRI expressed in human platelets was functional or not, we also examined whether activation of human platelets via the FcεRI could induce the release of RANTES. Using the mouse MoAb to the human FcεRI α chain (CRA1) we stimulated human platelets and evaluated the RANTES release. The level of released-RANTES from platelets stimulated by thrombin was measured as the positive control. The levels of released RANTES from platelets stimulated by the MoAb to the human FcεRI α chain and thrombin were always more than 50% of the total content (Fig 9). The levels of released-RANTES from human platelets by the isotype-matched control Ab were always less than 1%, in all experiments performed.
FcɛRI-mediated RANTES release from human platelets. Human platelets (1 × 108/mL) were stimulated with mouse MoAb to human FcɛRI chain (CRA1 at 100 μg/mL), or with thrombin (1 U/mL). The levels of released RANTES were measured by ELISA. Spontaneous release was always <1% of the total RANTES content. Results are shown as mean ±SD (n=3). * P < .01.
FcɛRI-mediated RANTES release from human platelets. Human platelets (1 × 108/mL) were stimulated with mouse MoAb to human FcɛRI chain (CRA1 at 100 μg/mL), or with thrombin (1 U/mL). The levels of released RANTES were measured by ELISA. Spontaneous release was always <1% of the total RANTES content. Results are shown as mean ±SD (n=3). * P < .01.
Next, we also examined whether activation of human platelets via IgE and anti-IgE stimulation could induce the release of RANTES. The level of released RANTES from platelets stimulated by human myeloma IgE and goat antihuman IgE Ab was more than 30% (Fig 10). The background RANTES release from human platelets by only human IgE or only goat antihuman IgE Ab was always less than 1%, in all experiments performed.
IgE-anti-IgE–mediated RANTES release from human platelets. Human platelets (1 × 108/mL) were stimulated with human myeloma IgE (100 μg/mL), and/or goat antihuman IgE (300μg/mL). The levels of released RANTES were measured by ELISA. Spontaneous release was always <1% of the total RANTES content. Results are shown as mean ± SD (n = 3). * P < .01.
IgE-anti-IgE–mediated RANTES release from human platelets. Human platelets (1 × 108/mL) were stimulated with human myeloma IgE (100 μg/mL), and/or goat antihuman IgE (300μg/mL). The levels of released RANTES were measured by ELISA. Spontaneous release was always <1% of the total RANTES content. Results are shown as mean ± SD (n = 3). * P < .01.
Taken together, these results confirmed that human platelets could be activated via the FcεRI and IgE, suggesting that the FcεRI expressed on the cell surface of human platelets was functionally active and its probable roles in IgE-mediated diseases.
DISCUSSION
It was not until recently that we have gained an understanding that human platelets play an important role in the allergic reaction and inflammation. Human platelets were reported to express the FcεRII and FcγRIIA, and release chemical mediators such as serotonin, thromboxane A2, PF4, PDGF, and cytokines like RANTES, when activated. However, only most recently M. Capron’s group35reported the expression of FcεRI in human platelets of patients with parasitic infections and showed FcεRI-mediated cytotoxicity forSchistosoma mansoni larvae. In the present study, using samples from normal subjects as well as atopics we showed the functional expression of the FcεRI in human platelets and its intracellular expression in megakaryocytes, and its probable role in allergy based on the IgE-anti-IgE mediated release of RANTES from human platelets.
It was reported that the FcεRI is usually expressed as a tetramer (αβγ2) in mast cells and basophils,1,2,32-34 but that it may be expressed as trimer (αγ2) in human monocytes, epidermal Langerhans cells, and dendritic cells.17-19,21 36 We therefore examined the expression of each subunit of the FcεRI in human platelets and megakaryocytes, and confirmed the expression of mRNA for each subunit of the FcεRI in both kinds of cells. Furthermore, the FcεRI expressed on human platelets was functional as shown by the FcεRI-mediated release of serotonin and RANTES. However, in comparison with thrombin and the MoAb to human FcγRII, the amount of serotonin released from human platelets when stimulated via the FcεRI was relatively less. No significant differences were detected in the levels of FcεRI expression or FcεRI-mediated serotonin release from platelets obtained from healthy individuals and atopic patients (data not shown). Most importantly, we showed that human platelets from healthy individuals released significant levels of RANTES when stimulated via IgE and FcεRI. Presently, we are elucidating the levels of RANTES released from platelets of atopics and nonatopics.
We further confirmed that the mRNA for FcεRI α, β, and γ chains expressed in the human megakaryocyte-like cell line, HML-1 cells, but failed to detect FcεRI α chain on the cell surface of HML-1 cells and normal human megakaryocytes. On the other hand, FcεRI was detected in the cytoplasm of these cells by immunohistochemistry and flow cytometry, using the anti-FcεRI α chain MoAb. Human megakaryocytes have stages of differentiation by endomitosis and FcεRI may be expressed on the cell surface of mature megakaryocytes. But we could not show the expression of FcεRI on the cell surface of HML-1 cells and human megakaryocytes obtained from normal subjects.
In this study, we showed the functional expression of the FcεRI on human platelets and the IgE anti-IgE–induced release of significant amounts of RANTES. These results suggest a novel and important role for human platelets in IgE-mediated allergic inflammation.
ACKNOWLEDGMENT
We thank Hironori Matsuda, Dr Yusuke Suzuki, and Dr Norimichi Tashiro for the experimental help.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Chisei Ra, MD, PhD, Department of Immunology, Juntendo University, School of Medicine, 2-1-1 Hongo, Bunkyo-ku, Tokyo 113-8421, Japan.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal