Abstract
The idiotype (Id) determinant on the multiple myeloma (MM) protein can be regarded as a tumor-specific marker. Immunotherapy directed at the MM Id may stem the progression of this disease. We report here on the first 12 MM patients treated at our institution with high-dose therapy and peripheral blood stem cell transplantation (PBSCT) followed by Id immunizations. MM patients received PBSCT to eradicate the majority of the disease. PBSCT produced a complete response in 2 patients, a partial response in 9 patients and stable disease in 1 patient. Three to 7 months after high-dose therapy, patients received a series of monthly immunizations that consisted of two intravenous infusions of Id-pulsed autologous dendritic cells (DC) followed by five subcutaneous boosts of Id/keyhole limpet hemocyanin (KLH) administered with adjuvant. Between 1 and 11 × 106 DC were obtained by leukapheresis in all patients even after PBSCT. The administration of Id-pulsed DC and Id/KLH vaccines were well tolerated with patients experiencing only minor and transient side effects. Two of 12 patients developed an Id-specific, cellular proliferative immune response and one of three patients studied developed a transient but Id-specific cytotoxic T-cell (CTL) response. Eleven of the 12 patients generated strong KLH-specific cellular proliferative immune responses showing the patients’ immunocompetence at the time of vaccination. The two patients who developed a cellular Id-specific immune response remain in complete remission. Of the 12 treated patients, 9 are currently alive after autologous transplantation with a minimum follow-up of 16 months, 2 patients died because of recurrent MM and 1 patient succumbed to acute leukemia. These studies show that patients make strong anti-KLH responses despite recent high-dose therapy and that DC-based Id vaccination is feasible after PBSCT and can induce Id-specific T-cell responses. Further vaccine development is necessary to increase the proportion of patients that make Id-specific immune responses. The clinical benefits of Id vaccination in MM remain to be determined.
DESPITE THE AVAILABILITY of high-dose therapy and peripheral blood stem cell transplantation (PBSCT) for the treatment of multiple myeloma (MM), recurrent disease remains a major problem for patients with MM. Immunotherapy may provide additional control of residual disease. The unique biology of MM allows for the MM idiotypes (Id) and their peptide fragments to serve as tumor-specific antigens and potential targets for adjuvant immunotherapy. One of the most potent means of stimulating immune responses is through the use of dendritic cells (DC). Therefore, we have conducted a feasibility study to evaluate a DC-based Id vaccination strategy for MM patients after PBSCT.
Prior studies by Eisen et al1,2 showed that myeloma-derived Ig can serve as an immunogen in a murine mineral oil-induced plasmacytoma (MOPC) model. When the myeloma protein was used in a vaccine it stimulated a tumor-specific protective immune response.1,2 The specificity of the immune response was restricted to unique determinants (idiotype) located on the antigen-binding sites of the Ig molecule. Other investigators have confirmed Eisen’s findings in various myeloma model systems including a more physiological model based on the spontaneously arising 5T2 murine myeloma.3,4 Studies of the immune mechanisms contributing to Id-specific tumor protection have been discussed in detail and have led to the assertion that Id-specific T cells are important for tumor protection in MM.5 Bogen et al6-8 have shown that Id-specific CD4+ T cells are important for tumor protection in the MOPC 315 model. Others have investigated Id-specific cytotoxic CD8+ T-cell clones, but their role in tumor protection remains to be determined.9,10 The ability to make these anti-Id immune responses, however, was impeded by the presence of circulating myeloma protein.11
The importance of Id-specific T cells in myeloma is not well studied in humans. A small number of Id-specific T cells has been reported in patients with early stages of MM.12 Osterborg et al13 reported on the Id immunization of five MM patients that stimulated T-cell immune responses in all five. Id presented by monocyte-derived DC was able to stimulate a T-cell proliferative response to the MM protein.14 The immunogenicity of a human MM Id protein vaccine in a normal person and the subsequent transfer of Id-specific T cells to a patient has been shown in the setting of allogeneic bone marrow transplantation (BMT).15
Id vaccination for patients with B-cell non-Hodgkin’s lymphoma has succeeded in inducing specific immune responses in these patients.16,17 There was a strong correlation between the presence of an anti-Id immune response and freedom from disease progression as well as overall survival. Furthermore, the patients who were in a complete remission at the time of their vaccination had a greater probability of making an immune response.17
A prospective randomized study has recently shown that high-dose chemotherapy with autologous BMT is superior to standard chemotherapy for the treatment of MM. High-dose therapy resulted in a higher response rate, a prolonged event-free survival, and an increased overall survival.18 Id vaccinations are, thus, likely to be most efficacious in MM patients who have completed a PBSCT and have achieved a status of minimal residual disease (MRD).5
Dendritic cells are capable of antigen uptake, processing, and presentation on major histocompatibility complex (MHC) class I and II molecules. They are efficient in inducing strong and protective immune responses, especially T-cell immune responses.19Immunization with antigen or tumor peptide pulsed autologous DC has been successfully used in various animal models20,21 and has more recently been introduced into clinical trials for lymphoma22 and melanoma.23 Based on these observations we have conducted a study to evaluate the feasibility of DC-based Id vaccination in MM patients after high-dose therapy and PBSCT.
MATERIALS AND METHODS
Patients and PBSCT.
Fourteen eligible MM patients treated at our institution with PBSCT were offered adjuvant immunotherapy on this phase I study. Two patients declined to participate. All patients had laboratory, histopathologic, and radiologic findings consistent with a diagnosis of MM. Institutional review board–approved informed consent was obtained from all study patients. Serum and BM samples were collected early in their disease, preferably before initiation of chemotherapy. Patients received high-dose therapy if they had chemotherapy-sensitive disease (shown by a >50% decrease of monoclonal Ig and a decrease of BM plasma cell involvement to <20% with initial chemotherapy), a Karnofsky performance status of greater than 70%, and no renal, hepatic, cardiac, and pulmonary impairment. PBSC were collected after a single treatment with cyclophosphamide (4 g/m2) followed by granulocyte colony-stimulating factor (G-CSF) and stored as a backup source of stem cells. Twenty-eight days later, a second PBSC collection was obtained after a single treatment with etoposide (2 g/m2) followed by G-CSF. This second cell collection was used for transplantation. The transplant conditioning regimen consisted of fractionated total body irradiation (FTBI) with a total dose of 12 Gy followed by intravenous (IV) administration of melphalan (MEL) at a dose of 140 mg/m2. Patients who received prior ionizing irradiation received carmustine (BCNU) at 550 mg/m2 in place of FTBI and received MEL at a dose of 140 mg/m2 to 180 mg/m2 as part of a dose-escalation study. The patient characteristics and response to high-dose therapy with PBSCT are summarized in Table 1. Partial response (PR) was defined as a reduction of the serum monoclonal spike by greater than 50% of the pretransplant level, a lack of Bence Jones proteinuria and less than 10% plasma cell involvement in BM biopsy and aspirate specimens. Complete response (CR) was defined as the repeated absence of the monoclonal Ig in immunofixation electrophoresis and no evidence of clonal plasma cells in BM specimens.
MM Id Vaccine Patient Characteristics
| Patient (UPN) . | Sex/Age . | Id Isotype . | Prior Therapy (no. of cycles) . | Pre-PBSCT Conditioning . | Post-PBSCT Response . |
|---|---|---|---|---|---|
| 1 (1172) | M/37 | IgAλ | VAD (×5) | FTBI + MEL | CR |
| 2 (1243) | M/44 | IgG1κ | VMCP (×1), VAD (×6), VP16 (×2), PSC-VAD (×5) | BCNU + MEL | PR |
| 3 (1289) | M/42 | IgAκ | VAD (×6) | FTBI + MEL | PR |
| 4 (1353) | M/54 | IgG1κ | VAD (×4) | FTBI + MEL | PR |
| 5 (1397) | M/59 | IgG1κ | MP (×12), VAD (×6) | FTBI + MEL | PR |
| 6 (1396) | F/50 | IgG1κ | VAD (×4), PSC-VAD (×4), MEL 140 (×1) | FTBI + MEL | SD |
| 7 (1445) | M/68 | IgG1κ | MP (×7), VAD (×4) | BCNU + MEL | PR |
| 8 (1441) | F/50 | IgAκ | CVP (×23), BMVP (×13), VAD (×2) | FTBI + MEL | PR |
| 9 (1457) | M/53 | IgG3κ | MP (×12), VAD (×4) | BCNU + MEL | CR |
| 10 (1483) | M/41 | IgG1κ | VAD (×4) | FTBI + MEL | PR |
| 11 (1504) | M/51 | IgG4κ | VAD (×6) | FTBI + MEL | PR |
| 12 (1521) | M/59 | IgG4λ | VAD (×6), PSC-VAD (×2) | FTBI + MEL | PR |
| Patient (UPN) . | Sex/Age . | Id Isotype . | Prior Therapy (no. of cycles) . | Pre-PBSCT Conditioning . | Post-PBSCT Response . |
|---|---|---|---|---|---|
| 1 (1172) | M/37 | IgAλ | VAD (×5) | FTBI + MEL | CR |
| 2 (1243) | M/44 | IgG1κ | VMCP (×1), VAD (×6), VP16 (×2), PSC-VAD (×5) | BCNU + MEL | PR |
| 3 (1289) | M/42 | IgAκ | VAD (×6) | FTBI + MEL | PR |
| 4 (1353) | M/54 | IgG1κ | VAD (×4) | FTBI + MEL | PR |
| 5 (1397) | M/59 | IgG1κ | MP (×12), VAD (×6) | FTBI + MEL | PR |
| 6 (1396) | F/50 | IgG1κ | VAD (×4), PSC-VAD (×4), MEL 140 (×1) | FTBI + MEL | SD |
| 7 (1445) | M/68 | IgG1κ | MP (×7), VAD (×4) | BCNU + MEL | PR |
| 8 (1441) | F/50 | IgAκ | CVP (×23), BMVP (×13), VAD (×2) | FTBI + MEL | PR |
| 9 (1457) | M/53 | IgG3κ | MP (×12), VAD (×4) | BCNU + MEL | CR |
| 10 (1483) | M/41 | IgG1κ | VAD (×4) | FTBI + MEL | PR |
| 11 (1504) | M/51 | IgG4κ | VAD (×6) | FTBI + MEL | PR |
| 12 (1521) | M/59 | IgG4λ | VAD (×6), PSC-VAD (×2) | FTBI + MEL | PR |
Abbreviations: UPN: unique patient number; VAD: vincristine, adriamycin, dexamethasone; VMCP: vincristine, melphalan, cyclophosphamide, prednisone; PSC-VAD: PSC 833, vincristine, adriamycin, dexamethasone; MP: melphalan, prednisone; MEL 140: melphalan at 140 mg/m2; CVP: cyclophosphamide, vincristine, prednisone; BMVP: BCNU, melphalan, vincristine, prednisone; SD: stable disease.
Id purification.
Id proteins were isolated from serum samples obtained before or during initial cytoreductive chemotherapy. Only those patients whose available serum had an M-protein level greater than 0.4 g/dL were eligible for the Id vaccination trial. The myeloma protein isotype and heavy-chain subclass were determined with an enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well MaxiSorb immunosorbent plates (Nalgene Nunc, Rochester, NY) were coated with goat anti-human kappa or lambda antibody (Biosource, Camarillo, CA) in 0.1 mol/L carbonate buffer, pH 9.0, overnight. The plates were then washed several times in normal saline containing 0.1% Triton-X 100 (Sigma, St Louis, MO). Patient samples were serially diluted with phosphate-buffered saline (PBS) containing 2% (wt/vol) bovine serum albumin and incubated on the plates for more than 1 hour at room temperature. The plates were washed again and the bound Ig was detected with peroxidase-conjugated mouse monoclonal antibodies which were specific for the different human heavy-chain isotypes (Southern Biotechnology Assoc, Birmingham, AL).
Id proteins were purified according to their heavy-chain isotype and subclass. IgG1, IgG2, and IgG4 were purified with protein A affinity chromatography followed by ion exchange chromatography. Specifically, serum was diluted with PBS pH 7.5 and passed through a Protein A Sepharose CL-4B column (Sigma). The bound Igs were then eluted with 0.1 mol/L citric acid in sequential pH steps at pH 5.5, 5.0, 4.5, 4.0, and 3.5. IgG2 Igs eluted from Protein A at a higher pH and IgG1 and IgG4 at a lower pH.24 The MM protein containing fractions were pooled and dialyzed against 0.01 mol/L Tris pH 9.0. The Id was then bound to a strong anion exchange (AEX) resin (Macro-Prep High Q Support; Bio-Rad, Hercules, CA) and eluted with a 0 to 0.3 mol/L NaCl salt gradient in 0.01 mol/L Tris pH 9.0. Fractions containing the Id protein were identified by ELISA as above except using a goat anti-human heavy-chain antibody and detected with a peroxidase conjugated goat anti-human kappa or lambda antibody.
Id proteins with an IgG3 isotype were isolated by a combination of Protein G Sepharose, Protein A Sepharose, and AEX chromatography. Serum was diluted with PBS and passed through a Protein G Sepharose (Sigma) column. The bound protein was eluted with 0.1 mol/L citric acid, pH 3.5, and the eluate was then passed over a Protein A column to bind contaminating normal serum IgG1, IgG2, and IgG4 Ig subclasses. The Protein A column flow through which contains IgG3 Igs was further purified using a strong AEX chromatography as above.
IgA Id proteins were purified by precipitating twice with ammonium sulfate (45% vol/vol), dialyzed against 0.01 mol/L Tris, pH 9.0, and separated by strong AEX chromatography as described above. IgA-containing fractions were pooled and then depleted of IgG by Protein A affinity chromatography. Purified Id proteins were extensively dialyzed against sterile normal saline and filtered through a 0.45-μm membrane. The Id proteins were tested for endotoxin using the LAL clotting assay as per the manufacturer’s instructions (Cape Cod Inc, Falmouth, MA) and for sterility.
DC vaccines.
The MM patients received their DC vaccines 3 to 6 months after completing their PBSCT. Each patient was given two Id-pulsed DC immunizations separated by 4 weeks. DC vaccines were prepared as previously described.22 Briefly, mononuclear cells were collected from peripheral blood by leukapheresis. The precursor DC were isolated from the mononuclear cells by a series of density gradient centrifugations. The DC preparations were cultured with purified Id for 36 to 48 hours, collected, washed free of Id protein, and administered IV over 60 minutes in a volume of 100 mL. The composition of DC preparations was monitored by light microscopy and flow cytometry.22 DC were identified by high MHC class II expression and the absence of T- and B-cell markers. An average of 5.1 × 106 ± 2.9 × 106 DC were infused with each immunization.
Id/keyhole limpet hemocyanin (KLH) vaccines.
Five Id/KLH vaccines were administered after the DC vaccines. The Id/KLH vaccines were prepared as previously described.16,17Briefly, purified Id proteins were conjugated with glutaraldehyde to the immunologic carrier KLH at a 1:1 (wt:wt) ratio. The conjugates were dialyzed against normal saline and then mixed with freshly prepared ISAF adjuvant. ISAF consisted of 10% (vol:vol) squalane (Aldrich Chemical, Milwaukee, WI), 5% (vol:vol) pluronic L121 (BASF, Parsippany, NJ), and 0.4% (vol:vol) Tween 80 (Aldrich Chemical, Milwaukee, WI) in PBS.25 26 The Id/KLH vaccinations were started 4 weeks after the second Id/DC vaccine and were administered at 4-week intervals.
Identification of MM Id encoding genes.
The myeloma-derived heavy- and light-chain Ig variable region genes were isolated from BM aspirate specimens obtained before or in the early part of induction chemotherapy. BM mononuclear cells (BM-MNC) were isolated from the aspirate by Ficoll-Paque density centrifugation (Pharmacia, Piscataway, NJ). RNA was isolated from 5 to 10 × 106 BM-MNC with RNAzol B (Tel-test, Friendswood, TX) or with the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturers’ protocols. First-strand cDNA was synthesized from the RNA using random hexamer priming and Superscript RT (Life Technologies, Gaithersburg, MD) as described.27 The myeloma Id heavy-chain variable region genes were amplified from the cDNA using polymerase chain reactions (PCR). Six 5′ primers corresponding to the leader sequences of the six human heavy-chain variable region families (VHL1 to VHL6)28 were used in individual PCR. The 3′ primers used in the PCR corresponded to the appropriate gamma (5′-CTTGACCAGG CAGCCCAGGGC-3′) or alpha heavy-chain constant region (5′-GAGGCTCAGC GGGAAGACCT-3′). PCR conditions were as described,27 except for a few reactions where the annealing temperature was reduced from 55°C to 50°C. In all cases, electrophoretic analysis of the PCR reactions showed a predominant band of the expected length in only one of the six VH leader primer reactions. The PCR product was purified by electrophoresis through agarose and extracting the DNA from the agarose using the QIAquick gel extraction kit (Qiagen). The isolated PCR product was then ligated into the vector, pCRII (Invitrogen, San Diego, CA) and transfected into TOP10F′ bacteria. Plasmid DNA was isolated from cultures derived from individual bacterial colonies and sequenced using dye termination PCR (Perkin-Elmer, Norwalk, CT). A sequence for a heavy-chain variable region was determined to be myeloma derived if it was identified in DNA isolated from three bacterial colonies generated from the cloning of two independent PCR products.
Identification and cloning of the MM-derived light-chain variable (VL) region was performed using the same PCR-based approach as for the VH-chain region. The sequence for the kappa leader primers are: κL1, 5′-ATCACAGATC TCTCACCATG GTGTTGCAGA CCCAGGTC-3′; κL2, 5′-ATCACAGATC TCTCACCATG GRGWCCCCWG CKCAGCT-3′; κL3, 5′-ATCACAGATC TCTCACCATG GACATGAGGG TCCCGCTCA G-3′; and κL4, 5′-ATCACAGATC TCTCACCATG GACACVAGGG CCCCCACTCA G-3′. The sequence for the lambda leader primers are: λL1, 5′-ATCACAGATC TCTCACCATG GCCTGGGCTC TGCTGCTCC-3′; λL2, 5′-ATCACAGATC TCTCACCATG GCCTGGGCTC CACTACTTC-3′; λL3, 5′-ATCACAGATC TCTCACCATG ACCTGCTCCC CTCTCCTCC-3′; λL4, 5′-ATCACAGATC TCTCACCATG GCCTGGACTC CTCTCTTTC-3′; and λL5, 5′-ATCACAGATC TCTCACCATG ACTTGGACCC CACTCCTC-3′. The 3′ primers were derived from the joining regions of either kappa or lambda. The sequence for the kappa joining region (Jκ) is 5′-TGCAGCATCC GTACGTTTGA TCTCGASYTT GGTCC-3′ and the lambda joining region (Jλ) is 5′-CTGACCTAGG ACGGTCASCT BGGTSCC-3′.
Generation of an Id-encoding recombinant adenovirus (IdAd).
A recombinant adenovirus containing a patient’s MM-derived variable region genes was made for selected cases. VH genes used for cloning were obtained by reamplification of Id VH using Pfu DNA polymerase (Stratagene, La Jolla, CA) and leader and constant region primers containing unique restriction sites to aid in the cloning. The primers used for identifying and isolating the VL genes contained restriction sites so that the initial PCR product could be used for cloning into an expression vector without reamplification. The patient’s MM VH and VL genes were cloned into the bicistronic Ig expression vector, pTC.29 This vector allowed for the cloning of the heavy- and light-chain variable region genes in frame with their respective constant regions. Cytomegalovirus promoters were used for each Ig gene. The expression cassette containing the Id genes was then moved from pTC to the adenovirus transfer vector, pXCJL1. The Id-gene–containing pXCJL1 plasmid was cotransfected with the unpackageable adenovirus type V (AdV) plasmid pJM17 into 293 cells as previously described.30 Intracellular recombination between these two plasmids in this AdV permissive cell line resulted in the generation of replication-deficient Ad. The recombinant virus was plaque purified and then expanded on 293 cells. The titers of the adenovirus stocks were determined by limiting dilution. Integrity of the Ig genes was determined by assaying the supernatant of infected 293 cells for Ig expression by ELISA as described above.
T-cell assays.
Id-specific T-cell immune responses were monitored by a T-cell proliferation assay as previously described17 22 except for the use of the serum-free medium, AIM V (GIBCO-BRL, Grand Island, NY) instead of an Iscove’s modified Dulbecco’s medium–based culture medium. Briefly, heparinized blood samples were obtained before each Id vaccination and 4 and 12 weeks after the last vaccination. Peripheral blood mononuclear cells (PBMNC) were isolated from blood using Ficoll-Paque (Pharmacia) density centrifugation. After washing, the PBMNC were plated at 5 × 105 cells per well in a 96-well “U”-bottom plate in AIM V medium supplemented with titrating amounts of either Id protein, an isotype-matched control Ig, or KLH. The PBMNC were cultured for 3 days and then split between two 96-well plates. Fresh medium containing IL-2 was added to the wells such that the final concentration of IL-2 was 30 IU/mL. The plates were incubated for an additional 2 days. One microcurie of 3H-thymidine was added to the wells and the plate was incubated for an additional 12 to 18 hours. The cells were then harvested onto glass filters, which were washed and counted in a scintillation counter. All stimulations were done with triplicate or quadruplicate wells for each protein concentration. T-cell proliferation to the carrier protein KLH was used as an internal control for the T-cellular immune response induced by the Id/KLH vaccination.
Id-specific CTL responses were measured by a 51Cr release assay. Effector cells for the CTL assay were generated by isolating PBMNC as above and stimulating them in bulk cultures at 3 × 106 cells/mL in with either Id or an isotype-matched Ig at 100 μg/mL. The PBMNC were cultured for 12 days, adding 30 IU/mL IL-2 starting on day 4 of the culture and adding fresh medium to the culture every 3 to 4 days. The targets for the CTL assay were generated from autologous fibroblasts (FB) infected with recombinant adenovirus containing the patient’s Id genes. Fibroblasts were derived from 3-mm skin punch biopsy specimens. After several weeks of culture in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, FB cell lines could be expanded to greater than 107cells. Fibroblasts were incubated overnight with titered amounts of recombinant adenovirus to determine the optimal multiplicity of infection (MOI). Ig expression was determined by immunohistochemistry of cytospin preparations using a peroxidase conjugated goat anti-human gamma chain antibody (Biosource, Camarillo, CA). For use as a target (T) in the CTL assay, autologous fibroblasts were seeded at 5 × 103 cells per well in 96-well “U”-bottom plates and then infected with IdAd, isotype-matched irrelevant Ig, or β-galactosidase (AdLacZ) at equivalent MOIs. During the infection, the fibroblasts were labeled by adding 4 μCi 51Cr to the culture medium in each well. Fibroblast targets were carefully washed four times and then coincubated with titered amounts of effector cells (E) for 4 hours. Triplicate wells were used for each E:T ratio. Spontaneous release was determined by the addition of medium and maximal release by the addition of 0.5% Triton-X in medium. Specific lysis was calculated as previously described.22
All T-cell assays were performed with freshly isolated PBMNC obtained from blood drawn when study patients came for their monthly vaccinations; therefore, we measured T-cell responses once at each time point.
RESULTS
Patient description.
Twelve MM patients treated at our institution with a PBSCT were enrolled in this study (Table 1). All of the patients were clinical stage III before treatment.31 Only 2 of the 12 patients were female. The median age of the patients was 50 years. One quarter of the patients had a myeloma protein isotype of IgA. Initial chemotherapy was administered by their referring physician and consisted of a variety of chemotherapy regimens and of varying duration. Most of the patients were treated with multiple chemotherapy regimens before transplant. All patients had chemotherapy-responsive disease before PBSCT. The pre-PBSCT conditioning regimen consisted of high-dose melphalan with either BCNU (3 patients) or FTBI (9 patients). After PBSCT, all but one patient (patient 6) achieved a PR or a CR (patients 1 and 9).
Purification of Id protein.
MM idiotype protein was abundant and relatively easy to prepare from pretreatment or early post initial treatment serum samples. More than 20 mg of Id protein was isolated from 10 mL or less of starting serum, which was sufficient for DC vaccines, Id protein vaccines, and immunological testing. Highly purified Id proteins were isolated using methods optimized for isotype. The Id protein preparations were all greater than 95% pure as determined by ELISA for isotype or light chain restriction and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown).
Id vaccination.
The MM patients were immunized with a combination of Id-pulsed DC vaccines and Id-KLH conjugate vaccines. Ten of the 12 patients completed their series of two Id-pulsed DC vaccinations plus 5 Id/KLH booster injections. Patient 7 received two Id-pulsed DC and two Id/KLH vaccines before developing acute leukemia. Patient 10 developed progressive disease during his Id immunization and elected to withdraw from the study after his second Id/KLH vaccine. Despite numerous cycles of chemotherapy and high-dose chemotherapy with PBSCT, a sufficient number of DC precursors could be routinely isolated from the leukapheresis products (Table 2). The immature DC precursors were enriched approximately 100-fold using a series of density gradient centrifugations resulting in a cell population consisting of 3% to 63% DC. The DC yield from a leukapheresis collection ranged from 1 to 11 × 106 cells. The purity of the DC preparations was somewhat lower than that seen with lymphoma patients,22 which was likely due to increased circulating immature cells which copurified with the precursor DC. After isolation, the immature DC were incubated in medium containing the patient’s Id protein, washed, and administered IV. The patients tolerated the DC infusions well with the vast majority of patients experiencing no side effects or complications. Two of the patients (patients 6 and 8) developed transient low-grade fevers and chills while being infused with their first Id-pulsed DC vaccine and one patient (patient 7) developed a postinfusion thrombophlebitis after his second DC infusion.
Dendritic Cell Vaccine Characteristics
| Patient (UPN) . | Time of 1st Id Vaccine* . | No. of DC Infused (×106) (1st, 2nd vaccine) . | DC Purity (1st, 2nd vaccine)† (%) . |
|---|---|---|---|
| 1 (1172) | 4 | 7.5, 7.5 | 11, 10 |
| 2 (1243) | 3 | 2.1, 1.9 | 30, 50 |
| 3 (1289) | 4 | 3.9, 2.7 | 10, 34 |
| 4 (1353) | 3 | 0.5, 3.5 | 6, 45 |
| 5 (1397) | 6 | 5.5, 8.6 | 63, 12 |
| 6 (1396) | 6 | 8.0, 11.1 | 15, 35 |
| 7 (1445) | 6 | 3.0, 2.7 | 10, 13 |
| 8 (1441) | 6 | 3.0, 3.0 | 21, 8 |
| 9 (1457) | 6 | 3.0, 7.5 | 3, 32 |
| 10 (1483) | 7 | 10.3, 8.4 | 53, 43 |
| 11 (1504) | 6 | 6.6, 6.1 | 27, 53 |
| 12 (1521) | 6 | 3.7, 3.3 | 10, 10 |
| Patient (UPN) . | Time of 1st Id Vaccine* . | No. of DC Infused (×106) (1st, 2nd vaccine) . | DC Purity (1st, 2nd vaccine)† (%) . |
|---|---|---|---|
| 1 (1172) | 4 | 7.5, 7.5 | 11, 10 |
| 2 (1243) | 3 | 2.1, 1.9 | 30, 50 |
| 3 (1289) | 4 | 3.9, 2.7 | 10, 34 |
| 4 (1353) | 3 | 0.5, 3.5 | 6, 45 |
| 5 (1397) | 6 | 5.5, 8.6 | 63, 12 |
| 6 (1396) | 6 | 8.0, 11.1 | 15, 35 |
| 7 (1445) | 6 | 3.0, 2.7 | 10, 13 |
| 8 (1441) | 6 | 3.0, 3.0 | 21, 8 |
| 9 (1457) | 6 | 3.0, 7.5 | 3, 32 |
| 10 (1483) | 7 | 10.3, 8.4 | 53, 43 |
| 11 (1504) | 6 | 6.6, 6.1 | 27, 53 |
| 12 (1521) | 6 | 3.7, 3.3 | 10, 10 |
Time from reinfusion of the PBSC to time of first Id-pulsed DC infusion in months.
Percent of infused cells that were B- and T-cell lineage marker negative and high MHC class II expressors by flow cytometry analysis.
The patients also tolerated the subcutaneous Id/KLH vaccines well. Most patients experienced only minor side effects consisting of local erythema, induration, and soreness at the injection site. These side effects were transient and were controlled with oral acetaminophen.
T-cell proliferation.
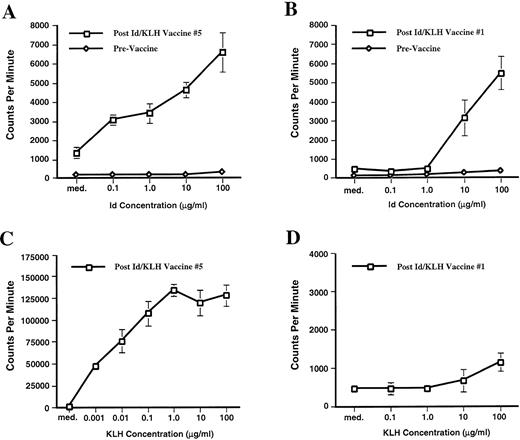
Before immunization, none of the 12 MM patients had a measurable T-cell proliferative response to either their Id protein or KLH. However, the Id immunizations stimulated an Id-specific T-cell proliferative response in two patients. Patient 1 developed Id-specific proliferation that was first detectable after receiving two Id-pulsed DC vaccines and five Id/KLH booster immunizations (Fig 1A). The T-cell proliferation to an irrelevant isotype matched Ig that was isolated in an identical manner was less than one half that seen with the Id protein. The amount of T-cell proliferation measured depended on the dose of stimulating Id. Patient 1 maintained a measurable T-cell proliferative response for more than 3 months after completing the immunizations. Patient 9 developed a T-cellular immune response after two Id-pulsed DC vaccinations and a single Id/KLH boost (Fig 1B). Similar to patient 1, the T-cell response was specific for the patient’s Id protein and dose-dependent. Similar Id-specific T-cell proliferation could be measured after his second and third Id/KLH immunizations.
Id-specific T-cell proliferation. PBMNC were isolated preimmunization and after each immunization and were cultured for 6 days in medium alone (med.) or medium containing Id at 0.1, 1.0, 10, and 100 μg/mL (0.1, 1.0, 10, and 100 respectively). Overnight3H thymidine incorporation into DNA was determined on day 6. (A) T-cell proliferation assay of patient 1 before any immunization (prevaccine) and 3 months post-Id/KLH vaccine no. 5. Proliferation in medium containing an irrelevant isotype-matched Ig at 100 μg/mL was statistically significant less (P = .014) with 3,296 ± 445 cpm. Comparable results were obtained in experiments performed 1 and 2 months post-Id/KLH vaccine no. 5. (B) T-cell proliferation assay of patient 9 before any immunization (prevaccine) and after Id/KLH vaccine no. 1. Proliferation in medium with an irrelevant isotype matched Ig at 100 μg/mL was at 519 ± 93 cpm (P = .05). Id-specific proliferation was also seen after Id/KLH vaccine nos. 2 and 3. (C) T-cell proliferation of patient 1 in response to the immunologic carrier KLH at 3 months post Id/KLH vaccine no. 5. (D) T-cell proliferation of patient 9 in response to the immunologic carrier KLH after Id/KLH vaccine no. 1. The assays were run in triplicate or quadruplicate and mean values are depicted. Error bars denote one standard deviation. Using the Mann-Whitney U test, prevaccine and postvaccine Id-specific responses were significantly different at all Id concentrations studied (P = .014 patient 1,P = .05 patient 9).
Id-specific T-cell proliferation. PBMNC were isolated preimmunization and after each immunization and were cultured for 6 days in medium alone (med.) or medium containing Id at 0.1, 1.0, 10, and 100 μg/mL (0.1, 1.0, 10, and 100 respectively). Overnight3H thymidine incorporation into DNA was determined on day 6. (A) T-cell proliferation assay of patient 1 before any immunization (prevaccine) and 3 months post-Id/KLH vaccine no. 5. Proliferation in medium containing an irrelevant isotype-matched Ig at 100 μg/mL was statistically significant less (P = .014) with 3,296 ± 445 cpm. Comparable results were obtained in experiments performed 1 and 2 months post-Id/KLH vaccine no. 5. (B) T-cell proliferation assay of patient 9 before any immunization (prevaccine) and after Id/KLH vaccine no. 1. Proliferation in medium with an irrelevant isotype matched Ig at 100 μg/mL was at 519 ± 93 cpm (P = .05). Id-specific proliferation was also seen after Id/KLH vaccine nos. 2 and 3. (C) T-cell proliferation of patient 1 in response to the immunologic carrier KLH at 3 months post Id/KLH vaccine no. 5. (D) T-cell proliferation of patient 9 in response to the immunologic carrier KLH after Id/KLH vaccine no. 1. The assays were run in triplicate or quadruplicate and mean values are depicted. Error bars denote one standard deviation. Using the Mann-Whitney U test, prevaccine and postvaccine Id-specific responses were significantly different at all Id concentrations studied (P = .014 patient 1,P = .05 patient 9).
Eleven of the 12 patients developed a strong, dose-dependent and sustained T-cell proliferative response to the carrier protein KLH. The patient who did not mount an immune response to KLH (patient 7) developed acute myelogenous leukemia after his second Id/KLH vaccine. The T-cell proliferative immune responses to KLH were detected after a single Id/KLH immunization in some patients and in all responding patients after three immunizations (Table3). The magnitude of the KLH-stimulated T-cell responses were often significantly higher than that stimulated by Id proteins. Three months post-Id/KLH no. 5, patient 1 had an Id-specific T-cell proliferative response (Fig 1A) that was 6,574 ± 1,017 cpm after Id (100 μg/mL) stimulation compared with 127,430 ± 11,918 cpm after KLH (100 μg/mL) stimulation (Fig 1C). Because immunizations with Id and KLH differ (Id is used in the two DC and subsequent subcutaneous vaccines where the KLH is used only in the subcutaneous immunizations) the KLH response in patient 9 lagged that of the Id-specific T-cell proliferative response. After Id/KLH immunization no. 1, patient 9 Id-specific T-cell proliferation incorporated 5,465 ± 883 cpm when stimulated with 100 μg/mL of Id (Fig 1B) while KLH specific proliferation when stimulated with 100 μg/mL was still low at 1,149 ± 285 cpm (Fig 1D). In all subsequent measurements the magnitude of KLH proliferation was significantly higher than the Id-specific proliferation in patient 9.
Proliferative Responses to the Immunological Carrier KLH
| Patient (UPN) . | Onset of KLH Response . | Peak KLH Response . | cpm at Time of Peak Response3-150 . |
|---|---|---|---|
| 1 (1172) | Post Id/KLH#3 | Post Id/KLH#5 | 177,315 ± 19,518 |
| 2 (1243) | Post Id/KLH#1 | Post Id/KLH#5 | 141,310 ± 4,909 |
| 3 (1289) | Post Id/KLH#1 | Post Id/KLH#1 | 127,250 ± 15,890 |
| 4 (1353) | Post Id/KLH#1 | Post Id/KLH#2 | 58,188 ± 6,561 |
| 5 (1397) | Post Id/KLH#1 | Post Id/KLH#4 | 225,485 ± 2,887 |
| 6 (1396) | Post Id/KLH#1 | Post Id/KLH#5 | 43,465 ± 10,977 |
| 7 (1445) | No KLH response | No KLH response | Background |
| 8 (1441) | Post Id/KLH#3 | Post Id/KLH#4 | 73,815 ± 6,372 |
| 9 (1457) | Post Id/KLH#2 | Post Id/KLH#4 | 14,939 ± 3,198 |
| 10 (1483) | Post Id/KLH#1 | Post Id/KLH#1 | 354,117 ± 4,783 |
| 11 (1504) | Post Id/KLH#2 | Post Id/KLH#3 | 117,556 ± 6,410 |
| 12 (1521) | Post Id/KLH#2 | Post Id/KLH#3 | 83,213 ± 11,185 |
| Patient (UPN) . | Onset of KLH Response . | Peak KLH Response . | cpm at Time of Peak Response3-150 . |
|---|---|---|---|
| 1 (1172) | Post Id/KLH#3 | Post Id/KLH#5 | 177,315 ± 19,518 |
| 2 (1243) | Post Id/KLH#1 | Post Id/KLH#5 | 141,310 ± 4,909 |
| 3 (1289) | Post Id/KLH#1 | Post Id/KLH#1 | 127,250 ± 15,890 |
| 4 (1353) | Post Id/KLH#1 | Post Id/KLH#2 | 58,188 ± 6,561 |
| 5 (1397) | Post Id/KLH#1 | Post Id/KLH#4 | 225,485 ± 2,887 |
| 6 (1396) | Post Id/KLH#1 | Post Id/KLH#5 | 43,465 ± 10,977 |
| 7 (1445) | No KLH response | No KLH response | Background |
| 8 (1441) | Post Id/KLH#3 | Post Id/KLH#4 | 73,815 ± 6,372 |
| 9 (1457) | Post Id/KLH#2 | Post Id/KLH#4 | 14,939 ± 3,198 |
| 10 (1483) | Post Id/KLH#1 | Post Id/KLH#1 | 354,117 ± 4,783 |
| 11 (1504) | Post Id/KLH#2 | Post Id/KLH#3 | 117,556 ± 6,410 |
| 12 (1521) | Post Id/KLH#2 | Post Id/KLH#3 | 83,213 ± 11,185 |
All patients except patients 7 and 10 were vaccinated with two Id-pulsed DC vaccines followed by five Id/KLH vaccines at 4-week intervals. PBMNC samples were stimulated for 6 days in medium containing KLH. Results with the highest KLH concentration investigated (100 μg/mL) are reported as average of triplicate or quadruplicate wells ± 1 SD. 3H thymidine incorporation is reported as counts per minute (cpm). Background proliferation in medium was less than 1,500 cpm.
Cytotoxic T-cell response.
Because MM cells were not available for use as target cells in a CTL assay, we created surrogate target cells which expressed autologous MHC class I and Id proteins. This was accomplished by transducing autologous fibroblasts with IdAd. The Id heavy- and light-chain variable region genes were isolated from BM samples using reverse transcription (RT)-PCR. Two or more independent PCR reactions yielded products with identical sequence, strongly suggesting that the amplified DNA was derived from myeloma cells. The variable region genes were then cloned into a bicistronic expression/adenovirus transfer vector containing the heavy- and light-chain constant regions. This transfer vector was used with an adenovirus plasmid to generate IdAd. Fibroblast lines were established from skin punch biopsy samples obtained from the patients. Sufficient numbers of fibroblasts necessary for the CTL assay could be readily obtained after 3 to 4 weeks of culture. The fibroblasts were infected with the adenovirus without significant cytotoxicity. Approximately 60% to 80% of the fibroblasts expressed the Id protein after adenovirus infection using an MOI of 100 to 1,000. Expression could be detected within 15 hours of infection using immunohistochemistry of cytospin preparations. Infecting with a lower MOI (10 to 100) resulted in fewer fibroblasts (20% to 35%) expressing detectable Id; infecting with a higher MOI caused toxicity. The FB cell lines infected with adenovirus still internalized51Cr well with 5 × 103 cells routinely incorporating 4,000 cpm to 8,000 cpm with a spontaneous release of less than 20%.
As previously reported,32 patient 1 showed an Id-specific CTL response 4 weeks after the second Id-pulsed DC vaccination. The effector cells (E) for the CTL assay were autologous PBMNC that were cultured in medium containing a high concentration of Id protein. After Id stimulation, these effector cells lysed 51Cr labeled Id expressing autologous fibroblast targets (T) in a dose-dependent manner (Fig 2) and the cytotoxic activity required prior stimulation with Id. PBMNC cultured with a control Ig did not stimulate any cytotoxic T-cell activity against Id expressing targets or control targets. The cytotoxic activity was also specific for Id-expressing fibroblast cells. When cultured with the Id-stimulated effector cells, autologous fibroblasts infected with an IdAd released significantly more 51Cr compared to fibroblasts infected with control adenovirus (AdLacZ) (P ≤ .05 at an E:T ratio of 100:1, 33:1, and 11:1 as determined by the Mann-Whitney U test). The requirement for Id stimulation and specific lysis of Id targets strongly suggest that the cytolytic activity is I-specific and not due to nonspecific cytolytic activity, such as that seen with natural killer cells. The fibroblasts targets expressed class I but lacked class II expression as determined by fluorescence-activated cell sorting (data not shown) providing indirect evidence for CD8+ class I– restricted CTLs as the effector cell population. This Id-specific CTL activity was only measurable 1 month after patient 1 received his second Id-pulsed DC vaccine. Because of the complexity of the assay, only three patients were evaluated for Id-specific CTL activity. The other two patients studied (patients 3 and 9) had no measurable Id-specific CTL activity.
Id-specific CTL activity of patient 1. Bulk PBMNC obtained 4 weeks after the second Id-pulsed DC vaccination were stimulated for 12 days with either autologous Id or irrelevant isotype matched control Ig. Stimulated effector cells (E) were coincubated with autologous 51Cr-labeled fibroblasts (FB) targets (T) expressing either Id or a bacterial control gene (LacZ) via Ad transduction. Various effector to target (E:T) ratios were used and specific lysis of FB targets in a 4-hour chromium-release assay was calculated on the basis of triplicate wells. Error bars denote one standard deviation. Id-stimulated effector cells killed significantly more (P = .05) IdAd expressing targets than control targets at an E to T ratio of 100:1, 33:1, and 11:1 (Mann-Whitney U test).
Id-specific CTL activity of patient 1. Bulk PBMNC obtained 4 weeks after the second Id-pulsed DC vaccination were stimulated for 12 days with either autologous Id or irrelevant isotype matched control Ig. Stimulated effector cells (E) were coincubated with autologous 51Cr-labeled fibroblasts (FB) targets (T) expressing either Id or a bacterial control gene (LacZ) via Ad transduction. Various effector to target (E:T) ratios were used and specific lysis of FB targets in a 4-hour chromium-release assay was calculated on the basis of triplicate wells. Error bars denote one standard deviation. Id-stimulated effector cells killed significantly more (P = .05) IdAd expressing targets than control targets at an E to T ratio of 100:1, 33:1, and 11:1 (Mann-Whitney U test).
Clinical observations.
The date of the last follow-up is May 31, 1998, with a minimum follow-up of 16 months from PBSCT and 3 months from last Id-KLH injection. Of the 12 treated patients, 9 are alive 16 to 30 months after transplantation. Two patients have maintained a CR at 30 months (patient 1) and 17 months (patient 9) after PBSCT. One patient (patient 6) had stable disease after PBSCT and is now 20 months after PBSCT. Without any further chemotherapy treatment, this patient’s M-spike has gone from 2.3 g/dL before her Id vaccination to 1.8 g/dL after completing her vaccinations. The myeloma progressed in 5 patients at 8 to 17 months after PBSCT. Two of these patients (patients 3 and 4) died at 11 and 22 months after PBSCT. One patient (patient 7) died 10 months after PBSCT of a secondary acute myeloid leukemia.
DISCUSSION
Compared to conventional chemotherapy, high-dose therapy with autologous stem cell support offers improvements in CR rates, freedom from disease progression, and overall survival,18,33 and is emerging as the therapy of choice to achieve MRD. Unfortunately, the majority of MM patients relapse after PBSCT, even those patients who obtain CRs and good PRs. Additional chemotherapy dose intensification is unlikely to significantly improve the survival of MM patients. New treatment approaches are needed for this disease. The rationale for immunizing MM patients after PBSCT has been discussed5 and is based on several premises. The Id protein is a unique immunologic marker for the malignant MM cells and an immune response directed against the Id protein may stem the regrowth of MM. However, the immune system’s ability to mount a response to the MM Id is likely greatest when at a stage of minimal disease. In mouse MM models, the tumor burden directly correlated to the development of Id-specific T-cell unresponsiveness or anergy.11 Therefore, Id immunizations may be the most efficacious in the post-PBSCT period.
The work presented in the report shows that DC-based Id vaccination is feasible. We isolated sufficient quantities of individual Id proteins and DC to immunize 12 patients. The vaccinations were well tolerated with no serious toxicity and 10 of 12 patients finished the planned course of immunizations. Id immunizations stimulated Id-specific T-cell proliferative responses in two patients and an Id-specific CTL response was detected in one of these patients. While this patient (patient 1) developed a measurable cytolytic immune response after only two Id/DC IV vaccinations, the Id-specific proliferative response was not detectable until completion of the subsequent 5 Id/KLH booster immunizations. The Id-specific proliferative response of patient 9 developed already after two DC/Id immunizations. The ability to stimulate or at least detect an anti-Id immune response correlated with the clinical status of the patient post-PBSCT. The two patients in CR after PBSCT were the ones who made a detectable immune response. One patient had a decrease in M-protein while receiving Id immunization.
We used a DC-based vaccine approach because of the potent immunostimulatory capacity of DC. We were able to detect Id-specific immune responses in only two of the vaccinated patients. It is possible that our T-cell proliferation assay is not sufficiently sensitive and more patients may have made immune responses. Using a single-cell assay (ELISPOT), Bergenbrant et al34 found Id-reactive T cells in three of five MM patients immunized with Id mixed in alum. When they used granulocyte-macrophage (GM)-CSF instead of alum, they were able to detect Id-reactive T cells by ELISPOT in all five of the immunized MM patients.13 Only 1 of the 5 Id/GM-CSF vaccinated patients made an Id-specific T-cell response that could be detected using a T-cell proliferation assay. Yi et al12immunized patients with early stage MM (stage I and II) because of their earlier finding of Id-reactive T cells in similar MM patients. It is possible that patients with early stage MM are better able to make an immune response to their Id than patients with more advanced disease, especially after high-dose chemotherapy. However, we were able to detect strong cellular immune responses to KLH in nearly all of our immunized patients, showing that these patients have an ability to make an immune response to new antigens. These T-cellular immune responses were clearly detectable in 11 of 12 patients after one to three Id/KLH vaccines and peaked usually within less than 1 year after high-dose therapy with PBSCT (Table 3). This is in line with a recent review on immune reconstitution after autologous hematopoietic stem cell transplantation.35 Other investigators have immunized MM patients with Id after high-dose therapy and have also been able to detect T-cell immune responses. Using a cytokine release assay, Kwak et al36 found that four of five Id vaccinated MM patients made an immune response. Similarly, Wen et al14 describe immunizing an MM patient with Id-pulsed DC pre- and post-PBSCT, which stimulated a T-cell response.14
When similarly prepared DC-based Id immunizations were administered to low-grade lymphoma patients, a much higher proportion of patients made an anti-Id immune response.22 One major difference between the two groups of patients is that MM patients often have easily detectable circulating Id protein. This circulating Id may have prevented the development or possibly the detection of a T-cell immune response. This observation is consistent with the observation of Bogen11 where Id-specific T cells become anergic or even undergo apoptotic death when exposed to soluble Id protein. It is intriguing that the two Id-specific T-cell responses we observed were found in the two patients who entered a CR after PBSCT. However, others have observed T-cell immune responses in immunized patients with substantial levels of serum Id.13,14,34 37 Differences in MM patient characteristics and the method of immunization may account for some of the differing observations. More immunized MM patients need to be evaluated before we can determine whether residual M-protein inhibits the development of an immune response.
The DC used in our protocol come from DC precursors isolated from leukapheresis products by sequential density centrifugation steps22,38,39 (Table 2). In prior experiments, these cells proved to have an immunophenotype of immature DC as determined by high class II, CD80, and CD1a expression and a lack of monocyte, B- and T-cell surface markers,38,39 and to have unique DC functions.39 Immature DC in peripheral blood are relatively rare constituting approximately 0.1% of the blood mononuclear cells. Our DC preparations yielded an average of 5.1 × 106 DC (Table 2), which is limited by practical constraints. If the ability to stimulate an immune response or the magnitude of the immune response is related to the number of DC used in the vaccine, it may be possible to stimulate Id immune responses in a greater proportion of patients if more DC are used in the vaccine. Others have explored the use of functional DCs or “dendrophages” derived from monocytes isolated from peripheral blood.14 Monocytes differentiate into DC-like cells when they are cultured with the appropriate cytokines.40,41 Because monocytes are far more abundant than DC precursors in peripheral blood, an apheresis procedure is not necessary to obtain large numbers of monocyte-derived DC. The morphologic and functional characteristics of these cells seem to be comparable with the cells used in our study. The feasibility of generating DC starting from apheresis bulk PBMNC and even “left-over” cells from CD34+ enrichment procedures of MM patients has been recently shown by Tarte et al.42
Further studies are necessary to show the clinical effectiveness of an Id-pulsed DC vaccination approach for MM patients. Future experimental protocols will focus on other sources of DCs, alternative methods of antigen pulsing of DC, and strategies to induce clinically significant Id-specific cellular immune responses in a larger cohort of MM patients.
ACKNOWLEDGMENT
We thank Shoshana Levy, PhD, for scientific advice. We thank Anette Grabski, RN, Ranjani Rajapaksa, BS, Debbie Czerwinski, BS, Adrienne van Beckhoven, RN, Maria Fazio, RN, the nurses and physicians of the BMT day hospital, the Stanford Medical School Blood Center, and the General Clinical Research Center at the Stanford University Medical Center for continuous support.
Supported in part by the National Institutes of Health (NIH) Program Project Grant No. PO1 CA49605 “Bone Marrow Grafting for Leukemia and Lymphoma” and NIH Grant No. CA33399. V.L.R. was supported by a grant of Dr. Mildred Scheel Stiftung für Krebsforschung, C.Y.O. was supported by a Howard Hughes Physician Fellowship, A.L. is a postdoctoral fellow of the Department of Hematology, University of Perugia, Italy, and R.L. is an American Cancer Society Clinical Research Professor.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ronald Levy, MD, Division of Oncology, Department of Medicine, Stanford University School of Medicine, 300 Pasteur Dr, Stanford, CA 94305.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal