Abstract
2-antiplasmin (2-AP) is the main physiologic plasmin inhibitor in mammalian plasma. Inactivation of the murine 2-AP gene was achieved by replacing, through homologous recombination in embryonic stem cells, a 7-kb genomic sequence encoding the entire murine protein (exon 2 through part of exon 10, including the stop codon) with theneomycin resistance expression cassette. Germline transmission of the mutated allele was confirmed by Southern blot analysis. Mendelian inheritance of the inactivated 2-AP allele was observed, and homozygous deficient (2-AP−/−) mice displayed normal fertility, viability, and development. Reverse transcription-polymerase chain reaction confirmed the absence of 2-AP mRNA in kidney and liver from 2-AP−/− mice, in contrast to wild-type (2-AP+/+) mice. Immunologic and functional 2-AP levels were undetectable in plasma of 2-AP−/− mice, and were about half of wild-type in heterozygous littermates (2-AP+/−). Other hemostasis parameters, including plasminogen activator inhibitor-1, plasminogen, fibrinogen, hemoglobin, hematocrit, and blood cell counts were comparable for 2-AP+/+, 2-AP+/−, and 2-AP−/− mice. After amputation of tail or toe tips, bleeding stopped spontaneously in 2-AP+/+, as well as in 2-AP+/− and 2-AP−/− mice. Spontaneous lysis after 4 hours of intravenously injected 125I-fibrin–labeled plasma clots was significantly higher in 2-AP−/− than in 2-AP+/+ mice when injecting clots prepared from 2-AP+/+ plasma (78% ± 5% v 46% ± 9%; mean ± SEM, n = 6 to 7; P = .02) or from 2-AP−/−plasma (81% ± 5% v 46% ± 5%; mean ± SEM, n = 5; P = .008). Four to 8 hours after endotoxin injection, fibrin deposition in the kidneys was significantly reduced in 2-AP−/− mice, as compared with 2-AP+/+ mice (P ≤ .005). Thus, 2-AP−/− mice develop and reproduce normally; they have an enhanced endogenous fibrinolytic capacity without overt bleeding.
THE MAMMALIAN fibrinolytic system contains a proenzyme, plasminogen, that is converted to the active serine proteinase plasmin by tissue-type (t-PA) or urokinase-type (u-PA) plasminogen activator. Inhibition of the system may occur through neutralization of the plasminogen activators by plasminogen activator inhibitors (mainly PAI-1) or through neutralization of plasmin.1 α2-antiplasmin (α2-AP) is the main physiologic plasmin inhibitor in mammalian plasma, whereas excess plasmin may be inhibited by α2-macroglobulin.2-4 α2-AP is synthesized in the liver and is present in plasma at a concentration of about 1 μmol/L.2-4 Human and murine α2-AP are serpins (serine proteinase inhibitors) with molecular weight (Mr) 65 to 70 kD,2-5 which inhibit plasmin in a very rapid reaction resulting in the formation of a stable inactive complex.6 The cDNA and deduced amino acid sequence,7,8 as well as the gene organization9 10 of both human and murine α2-AP have been elucidated.
The mouse α2-AP gene encodes a 491–amino acid protein, with the NH2-terminus Val of the mature protein corresponding to residue 28,5,10 whereas mature human α2-AP also consists of 464 residues with Met as NH2-terminus.11 The reactive site peptide bond consists of Arg-Met in the inhibitor of both species.7,8 In human and murine plasma, α2-AP occurs as a plasminogen-binding (60% to 70%) and as a non–plasminogen-binding (30% to 40%) form lacking a COOH-terminal fragment, which contains structures with affinity for the lysine-binding sites of plasminogen.5,12-14 The plasminogen-binding form cross-links to fibrin when it is clotted in the presence of Ca2+ and activated factor XIII.15 Binding of α2-AP to plasminogen may prevent subsequent binding of plasminogen to fibrin, and thus result in an antifibrinolytic effect. Low Mr forms of α2-AP have been detected at low concentration (0.05% of the plasma concentration) in the α-granules of blood platelets; their function remains unknown.16
Besides in the removal of fibrin, the fibrinolytic system may also play a role in phenomena such as ovulation, embryogenesis, intima proliferation, atherosclerosis, tumorigenesis, and metastasis.17 α2-AP may thus exert an inhibitory effect at different levels on fibrinolysis, as well as on several other plasmin-mediated biologic processes. Therefore, this inhibitor appears to be an interesting target to study its biologic role directly with the use of mice with specific inactivation of the α2-AP gene. This strategy has successfully been applied to study the biologic function of most other components of the fibrinolytic system.17 We have previously characterized the murine α2-AP gene and constructed a targeting vector for homologous recombination in embryonic stem (ES) cells.10 In this study, we report the generation of homozygous α2-AP–deficient mice and evaluate the biologic effects of α2-APgene disruption.
MATERIALS AND METHODS
Animals, reagents, and assays.
Mice were kept in microisolation cages on a 12-hour day-night cycle and fed a regular chow. They were anesthetized by intraperitoneal injection of 60 mg/kg Nembutal (Abbott Laboratories, North Chicago, IL), and blood was collected by vena cava puncture with a 24-gauge needle. Platelet-poor plasma was prepared by centrifugation of blood collected on citrate at 4,000g for 5 minutes.
Murine α2-AP, plasminogen and plasmin, human plasmin, and two-chain urokinase-type plasminogen activator (tcu-PA) were prepared and characterized as described elsewhere.5 Polyclonal antibodies against purified murine α2-AP and plasminogen were raised in rabbits. Before use, the antiserum against murine α2-AP was adsorbed with 10% to 20% (vol/vol) murine α2-AP–deficient plasma. α2-AP and plasminogen antigen levels were quantitated by enzyme-linked immunosorbent assay (ELISA) using the purified murine proteins for calibration.5 α2-AP activity levels in murine plasma were determined by addition of human plasmin (final concentration 5 nmol/L in 500-fold diluted plasma) and measurement of residual plasmin with the chromogenic substrate S-2403 (final concentration 0.3 mmol/L) (Chromogenix, Antwerp, Belgium) after incubation at 37°C for 10 seconds,5 using a calibration curve constructed with pooled plasma obtained from wild-type C57BL6/J mice. Plasma PAI-1 antigen levels were determined with a specific ELISA using monoclonal antibodies produced in gene-inactivated mice.18 Fibrinogen levels were determined with a clotting rate assay using human thrombin. Plasma levels of murine α2-macroglobulin were determined by rocket electroimmunassay, as described,19 using a polyclonal rabbit antiserum kindly provided by Dr F. Van Leuven (Center for Human Genetics, University of Leuven, Belgium). Calibration curves were constructed using pooled plasma from wild-type male or female mice, for determination of α2-macroglobulin levels in plasma samples from males or females, respectively. White blood cell, red blood cell, and platelet counts, hemoglobin and hematocrit levels, mean corpuscular value, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration were determined on blood collected in trisodium citrate (final concentration, 10 mmol/L) using an automated analyzer.
Endotoxin (Escherichia coli lipopolysaccharide, serotype 0111: B4) was purchased from Sigma Chemical Co (St Louis, MO).
Liver or kidney extracts were prepared by homogenization and extraction with 0.1 mol/L Tris, 0.25% Triton X-100 (Merck, Darmstadt, Germany), pH 8.0, and protein concentration was determined using the BCA protein assay (Pierce, Rockford, IL).
SDS-PAGE without reduction was performed on 10% to 15% gradient gels using the PhastSystem (Pharmacia, Uppsala, Sweden). Immunoblotting, after transfer to nitrocellulose sheets, was performed using antisera against murine α2-AP or plasminogen.
Bleeding times in mice were recorded after amputation of a standardized fragment of the tail (2 cm) or of a toe. Data are reported as mean ± SEM and statistical analysis was performed using the two-tailedt-test (nonparametric, Mann Whitney) for comparison between two groups. Genotype distributions and histopathologic data on fibrin deposition in kidney sections after endotoxin injection were compared by Chi-square analysis.
Generation of chimeric and α2-antiplasmin–deficient mice.
The targeting vector pPNT.α2-AP, in which the neomycin resistance expression cassette replaces a 7-kb genomic fragment comprising exon 2 through part of exon 10 (including the stop codon), which represents the entire sequence encoding mature murine α2-AP, was described previously.10
Electroporation of 129R1 ES cells (obtained from A. Nagy, Samuel Lunenfeld Research Institute, Toronto, Canada) or of 129/SvJ RW4 ES cells (Genome Systems Inc, St Louis, MO) with the linearized targeting vector yielded, respectively, three (out of 127) or eight (out of 93) correctly targeted clones as confirmed by Southern blot analysis of purified genomic DNA with appropriate restriction enzymes and probes.10
Targeted clones were used for aggregation with Swiss morulas (R1 ES clones) or C57BL6/J morulas (RW4 ES clones). Chimeric offspring, identified by the presence of agouti (R1 ES cells) or agouti and/or white (RW4 ES cells) coat pigmentation, were obtained (for both R1 and RW4 targeted clones), whereas germline transmission of ES-cell DNA was only obtained with RW4 ES clones (five germline-competent chimeras originating from three independently targeted clones). Heterozygous α2-AP–deficient germline offspring, identified by Southern blot analysis of tail-tip genomic DNA, were intercrossed to generate α2-AP−/− progeny (yielding a genetic background of 50% 129/SvJ and 50% C57BL6/J).
Southern blot analysis of genomic DNA.
DNA was isolated from mouse tail tips, digested with KpnI and analyzed by Southern blotting using a 3′ probe as described.10
Reverse transcription-polymerase chain reaction (RT-PCR).
Polyadenylated RNA (polyA RNA) was extracted from kidney and liver using the Quick Prep mRNA purification kit (Pharmacia Biotech Benelux, Roosendaal, The Netherlands) and was submitted to first strand cDNA synthesis by oligo(dT) priming using the Ready-to-Go T-primed first strand kit (Pharmacia). The reaction products (RT-cDNA) were then used in PCR amplification with primers annealing in the coding part of exon 10 (sense primer: 5′-AATTGTTCCAGGGCCCAGACCTTCGT-3′, nucleotides (nt) 1109-1134 of murine α2-AP cDNA, GenBank accession numberZ367748; and antisense primer: 5′-GTCCTCCATGATGAAGAAGAGGAAGGG-3′, nt 1302-1276 of murine α2-AP cDNA). The RT-PCR products were analyzed by separation on a 1% agarose gel.
Histopathologic examination.
α2-AP+/+ and α2-AP−/− mice (2 males and 2 females each at 6 and 20 weeks of age) were anesthetized and perfused through cardiac puncture with 0.9% NaCl followed by 4% formalin in 0.07 mol/L sodium phosphate buffer, pH 7.0.
Organs were removed, postfixed in the same fixative for 20 hours, and embedded in paraffin. Representative 7-μm sections of all tissues were examined microscopically after staining with hematoxylin/eosin. The tissue sections included cross sections of brain, heart, thymus, lung, liver, spleen, kidney, small and large intestine, stomach, cecum, leg muscle, reproductive organs (vas deferens, testis and epididymis, or uterus and ovaries), lymph node, adrenal gland, and pancreas.
Immunostaining for fibrin(ogen) was performed by incubating the sections with goat antiserum against murine fibrinogen/fibrin (Nordic, Tilburg, The Netherlands: working dilution 1/500) in 0.01 mol/L Tris, pH 7.6, containing 0.9% NaCl and 0.1% Triton X-100 for 3 hours at room temperature. After rinsing, the sections were incubated consecutively for 60 minutes with biotinylated rabbit antigoat IgG (Dako, Prosan, Ghent, Belgium; dilution 1/400) and with peroxidase-labeled avidin-biotin complex (Dako). Antibody binding was visualized with diaminobenzidine, resulting in a brown staining of the immunoreactive sites. All sections were briefly counterstained with Harris’ hematoxylin (BDH Laboratory Supplies, Poole, England), dehydrated, and mounted with DePex (Prosan, Gentbrugge, Belgium). Specificity of the primary antibodies was tested by adsorption of the antisera with murine fibrinogen.
Endogenous thrombolytic potential.
Lysis of 125I-fibrin–labeled murine plasma clots, injected into age- and weight-matched α2-AP+/+ or α2-AP−/− mice through a jugular vein (and embolized into the pulmonary arteries) was determined essentially as described previously.20 Therefore, 25 μL125I-fibrin–labeled plasma clots, containing ≈70,000 cpm human 125I-labeled fibrinogen, were prepared from a plasma pool of α2-AP+/+ or α2-AP−/− mice, by addition of thrombin (final concentration, 1.5 NIH U/mL) and CaCl2 (final concentration 70 mmol/L). Clot lysis was evaluated by measurement of the residual radioactivity in the heart and lungs ex vivo at 2 hours and 4 hours after injection, and was defined as the amount of radioactivity that had disappeared, expressed as a percent of the total amount of radioactivity injected.
Endotoxin-induced fibrin deposition.
Mice matched for sex, age (12 to 17 weeks), and weight (25 ± 1 g and 25 ± 2 g for α2-AP+/+ and α2-AP−/− mice; mean ± SEM, n = 8) were injected intraperitoneally with endotoxin (2 mg/kg, dissolved in sterile saline). Four or 8 hours after injection, the mice were sacrificed by injection of Nembutal and immediately perfused for 15 to 30 minutes with saline. For protein extraction, one kidney was removed and immediately frozen at −80°C. For immunohistochemical analysis, the other kidney was fixed in 1% paraformaldehyde overnight at 4°C, washed with phosphate-buffered saline, incubated in 70% ethanol overnight at 4°C, and embedded in paraffin.
After immunostaining, the extent of fibrin deposition was given a severity score of 0 to 3.20 Score 0 indicated the absence of fibrin deposits; score 1, the appearance of a few small fibrin deposits, stained very weakly; score 2, the presence of small clearly stained fibrin deposits; score 3, the presence of large and strongly stained fibrin deposits.
RESULTS
Germline transmission of the inactivated α2-APgene.
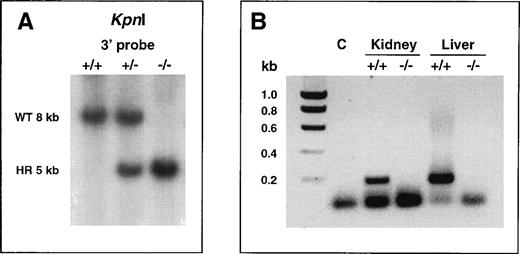
Inactivation of the murine α2-AP gene was achieved by replacing, through homologous recombination in ES cells, a 7-kb genomic fragment comprising the entire coding sequence, with a neomycin resistance cassette.10 Morula aggregation of recombinant RW4 cell clones harboring a disrupted α2-AP gene yielded germline-competent chimeras (male), as indicated by the presence of agouti pups among their offspring after mating with C57BL6/J females. Heterozygous α2-AP–deficient (α2-AP+/−) mice among the agouti offspring were identified by Southern blot analysis of tail-tip DNA (not shown), and were intercrossed, yielding α2-AP+/+, α2-AP+/−, and α2-AP−/− F2 littermates (Fig1A).
Confirmation at the DNA and RNA level of correct targeting of the 2-AP gene. (A) Southern blot analysis of tail-tip genomic DNA of littermates from intercrosses of heterozygous 2-AP–deficient mice. The DNA was digested with KpnI and hybridized with a 3′ probe (probe C in ref 10). The 8-kb and 5-kb bands indicate the presence of the wild-type or mutant allele, respectively. WT, wild-type; HR, homologously recombined. (B) RT-PCR analysis of polyA RNA isolated from liver and kidney of 2-AP+/+ and 2-AP−/− mice. PCR products were generated using PCR primers annealing in the coding part of exon 10 of the murine 2-AP gene (deleted in the disrupted allele), and were separated on a 1% agarose gel. PCR with wild type RT-cDNA yielded the expected 193-bp amplification product (lanes 3 and 5). The absence of signal with 2-AP−/− RT-cDNA (lanes 4 and 6) confirmed the inactivation of the 2-AP gene. Lane 2 (C) represents a negative control PCR reaction performed without DNA template. The lower band present in all lanes represents dimers of the primers.
Confirmation at the DNA and RNA level of correct targeting of the 2-AP gene. (A) Southern blot analysis of tail-tip genomic DNA of littermates from intercrosses of heterozygous 2-AP–deficient mice. The DNA was digested with KpnI and hybridized with a 3′ probe (probe C in ref 10). The 8-kb and 5-kb bands indicate the presence of the wild-type or mutant allele, respectively. WT, wild-type; HR, homologously recombined. (B) RT-PCR analysis of polyA RNA isolated from liver and kidney of 2-AP+/+ and 2-AP−/− mice. PCR products were generated using PCR primers annealing in the coding part of exon 10 of the murine 2-AP gene (deleted in the disrupted allele), and were separated on a 1% agarose gel. PCR with wild type RT-cDNA yielded the expected 193-bp amplification product (lanes 3 and 5). The absence of signal with 2-AP−/− RT-cDNA (lanes 4 and 6) confirmed the inactivation of the 2-AP gene. Lane 2 (C) represents a negative control PCR reaction performed without DNA template. The lower band present in all lanes represents dimers of the primers.
Deficiency in α2-AP in the α2-AP−/− progeny was confirmed at the mRNA level by the absence of signal in RT-PCR analyses of kidney and liver polyA RNA using primers annealing in the coding part of exon 10 (Fig 1B).
Viability, fertility, and growth.
Among 161 F2 littermates from heterozygous crosses that were genotyped at 4 to 5 weeks of age, 23% were α2-AP+/+, 53% were α2-AP+/−, and 24% were α2-AP−/− (Table1). This distribution is similar for males and females, and is not significantly different (by Chi-square analysis) from the expected Mendelian 1:2:1 ratio, thus indicating equal viability.
Genotype Distribution of 4- to 5-Week-Old F2Littermates Obtained by Interbreeding F12-AP+/− Mice
| . | α2-AP+/+ n (%) . | α2-AP+/− n (%) . | α2-AP−/− n (%) . | Total n . |
|---|---|---|---|---|
| Male | 16 (20) | 45 (55) | 21 (25) | 82 |
| Female | 21 (26) | 40 (51) | 18 (23) | 79 |
| Total | 37 (23) | 85 (53) | 39 (24) | 161 |
| . | α2-AP+/+ n (%) . | α2-AP+/− n (%) . | α2-AP−/− n (%) . | Total n . |
|---|---|---|---|---|
| Male | 16 (20) | 45 (55) | 21 (25) | 82 |
| Female | 21 (26) | 40 (51) | 18 (23) | 79 |
| Total | 37 (23) | 85 (53) | 39 (24) | 161 |
Mice were genotyped by Southern blot analysis of tail-tip genomic DNA, as described.
α2-AP deficiency did not affect the growth rate of the mice, as evidenced by weighing the mice at weekly intervals (not shown). Body weights at 5 weeks of age were (mean ± SEM; n = 4), 17 ± 2 g and 15 ± 1 g for α2-AP+/+ and α2-AP−/− mice, respectively, with corresponding values of 22 ± 2 g and 23 ± 1 g at 10 weeks. Mean body weights of α2-AP+/− mice (mean ± SEM; n = 13) were 16 ± 1 g and 22 ± 4 g at 5 and 10 weeks of age, respectively. No macroscopic abnormalities were observed. α2-AP−/− mice (F2 and F3 generations) produced similar sizes of litters+/− as α2-AP+/+ mice with similar time intervals between the litters (Table2).
Size and Frequency of Litters From 2-AP+/+ and 2-AP−/− Breeding Pairs
| . | α2-AP+/+ . | α2-AP−/− . |
|---|---|---|
| Size of litters (mice per litter) | 7 ± 1 (15) | 8 ± 1 (24) |
| Time between litters (d) | 29 ± 3 (11) | 30 ± 3 (18) |
| . | α2-AP+/+ . | α2-AP−/− . |
|---|---|---|
| Size of litters (mice per litter) | 7 ± 1 (15) | 8 ± 1 (24) |
| Time between litters (d) | 29 ± 3 (11) | 30 ± 3 (18) |
The results are mean ± SEM of the number of data points indicated between parentheses.
Hemostasis analysis.
α2-AP antigen levels and other hemostatic parameters are summarized in Table 3. α2-AP antigen levels in plasma were gene-dose–dependent. The functional assay showed an unexpectedly high level of antiplasmin activity in α2-AP−/− plasma as compared with α2-AP+/− and α2-AP+/+ plasma: 22% ± 3% (mean ± SEM; n = 14) versus 47% ± 5% (n = 10) and 94% ± 5% (n = 13), respectively. The levels in male or female α2-AP−/− mice were not significantly different: 18% ± 4.4% versus 26% ± 4.0% (n = 7;P = .16). This rapid reacting plasmin inhibitory activity cannot be due to murine α2-AP activity, because no complexes corresponding to plasmin–α2-AP could be detected on addition of murine or human plasmin to α2-AP−/− plasma (see below).
Hemostasis Analysis of Blood and Plasma Samples
| Parameter . | α2-AP+/+ . | α2-AP+/− . | α2-AP−/− . |
|---|---|---|---|
| α2-Antiplasmin antigen (μg/mL) | 86 ± 9 | 34 ± 1 | ≤1.6 |
| Plasminogen antigen (μg/mL) | 150 ± 10 | 140 ± 9 | 140 ± 10 |
| Fibrinogen (g/L) | 0.37 ± 0.05 | 0.24 ± 0.01 | 0.30 ± 0.04 |
| PAI-1 antigen (ng/mL) | 2.7 ± 0.51 | 1.2 ± 0.15 | 2.9 ± 0.80 |
| Hemoglobin (g/dL) | 12 ± 0.28 | 11 ± 0.13 | 11 ± 0.25 |
| Hematocrit (%) | 34 ± 0.80 | 33 ± 0.45 | 31 ± 0.81 |
| WBC (×109/L) | 4.6 ± 0.56 | 3.7 ± 0.57 | 4.3 ± 0.47 |
| RBC (×1012/L) | 7.5 ± 0.17 | 7.2 ± 0.08 | 6.6 ± 0.13 |
| Platelets (×109/L) | 470 ± 40 | 500 ± 30 | 530 ± 29 |
| MCV (fL) | 45 ± 0.50 | 46 ± 0.70 | 47 ± 0.82 |
| MCH (pg) | 16 ± 0.16 | 16 ± 0.13 | 16 ± 0.28 |
| MCHC (g/dL) | 35 ± 0.42 | 34 ± 0.33 | 35 ± 0.27 |
| Parameter . | α2-AP+/+ . | α2-AP+/− . | α2-AP−/− . |
|---|---|---|---|
| α2-Antiplasmin antigen (μg/mL) | 86 ± 9 | 34 ± 1 | ≤1.6 |
| Plasminogen antigen (μg/mL) | 150 ± 10 | 140 ± 9 | 140 ± 10 |
| Fibrinogen (g/L) | 0.37 ± 0.05 | 0.24 ± 0.01 | 0.30 ± 0.04 |
| PAI-1 antigen (ng/mL) | 2.7 ± 0.51 | 1.2 ± 0.15 | 2.9 ± 0.80 |
| Hemoglobin (g/dL) | 12 ± 0.28 | 11 ± 0.13 | 11 ± 0.25 |
| Hematocrit (%) | 34 ± 0.80 | 33 ± 0.45 | 31 ± 0.81 |
| WBC (×109/L) | 4.6 ± 0.56 | 3.7 ± 0.57 | 4.3 ± 0.47 |
| RBC (×1012/L) | 7.5 ± 0.17 | 7.2 ± 0.08 | 6.6 ± 0.13 |
| Platelets (×109/L) | 470 ± 40 | 500 ± 30 | 530 ± 29 |
| MCV (fL) | 45 ± 0.50 | 46 ± 0.70 | 47 ± 0.82 |
| MCH (pg) | 16 ± 0.16 | 16 ± 0.13 | 16 ± 0.28 |
| MCHC (g/dL) | 35 ± 0.42 | 34 ± 0.33 | 35 ± 0.27 |
Data represent mean ± SEM of determinations in 10 to 13 mice.
Abbreviations: WBC, white blood cells; RBC, red blood cells; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration.
Plasma levels of α2-macroglobulin antigen were higher in α2-AP−/− mice than in α2-AP+/+ mice, for females (128% ± 7%, n = 10, v 101% ± 5%, n = 7, P = .02), and for males (134% ± 13%, n = 10, v 100% ± 9%, n = 6; P = .12). All other measured hemostasis parameters and cell counts were comparable for wild-type mice and for heterozygous and homozygous α2-AP–deficient mice.
Bleeding times after amputation of a toe were variable (range, 0.5 to 7 minutes), but were on average (mean ± SEM) not significantly different between α2-AP+/+ (210 ± 64 seconds; n = 5), α2-AP+/− (180 ± 37 seconds; n = 12) and α2-AP−/−(210 ± 72 seconds; n = 5) mice. Also after tail amputation, bleeding stopped spontaneously in all three genotypes. No significant rebleeding was observed.
Histopathologic examination.
Microscopic analysis of cross-sections through different organs of 6- or 20-week-old α2-AP−/− mice, as described above, did not show any apparent abnormalities or differences with corresponding sections of α2-AP+/+ mice.
Immunocytochemical analysis.
Western blotting of plasma using polyclonal rabbit anti-murine α2-AP antibodies (Fig 2A), showed a positive band with an estimated Mrof 65 kD in plasma of α2-AP+/+ mice but not in plasma of α2-AP−/− mice. In urokinase-activated plasma (incubation with 50 nmol/L tcu-PA for 1 hour at 37°C) of α2-AP+/+ mice, but not of α2-AP−/− mice, an additional band was observed with Mr about 140 kD, which represents plasmin–α2-AP complex, as confirmed by blotting with affinity-purified polyclonal rabbit anti-murine plasminogen antibodies (Fig 2C). After addition of purified plasmin, (1 μmol/L) and incubation for 10 seconds at 37°C, plasmin–α2-AP complex was also detected in α2-AP+/+ but not in α2-AP−/− plasma (Fig 2B). The faint band at Mr about 140 kD observed in α2-AP−/− plasma (Fig 2B, lane 2 and Fig2C, lane 4) does not correspond to plasmin–α2-AP complex, as it is not recognized by the antibodies against murine α2-AP (not shown).
Western blot analysis of murine plasma using an 2-AP–specific (A) or a plasminogen-specific (B and C) antiserum. (A) Lane 1, purified murine 2-AP; lanes 2 and 3, 2-AP+/+ and 2-AP−/− plasma. (B) Lanes 1 and 2, 2-AP+/+ and 2-AP−/− plasma after incubation with human plasmin (final concentration, 1 μmol/L) for 10 seconds at 37°C. (C) Lanes 1 and 2, 2-AP+/+ and 2-AP−/− plasma; lanes 3 and 4, 2-AP+/+ and 2-AP−/− plasma activated with tcu-PA (final concentration, 50 nmol/L) for 1 hour at 37°C; lane 5, purified murine plasminogen.
Western blot analysis of murine plasma using an 2-AP–specific (A) or a plasminogen-specific (B and C) antiserum. (A) Lane 1, purified murine 2-AP; lanes 2 and 3, 2-AP+/+ and 2-AP−/− plasma. (B) Lanes 1 and 2, 2-AP+/+ and 2-AP−/− plasma after incubation with human plasmin (final concentration, 1 μmol/L) for 10 seconds at 37°C. (C) Lanes 1 and 2, 2-AP+/+ and 2-AP−/− plasma; lanes 3 and 4, 2-AP+/+ and 2-AP−/− plasma activated with tcu-PA (final concentration, 50 nmol/L) for 1 hour at 37°C; lane 5, purified murine plasminogen.
α2-AP antigen levels in liver extracts (expressed in ng/mg protein) were 37 ± 4 (n = 5) in α2-AP+/+ mice, 24 ± 1 (n = 11) in α2-AP+/− mice, and below the 1 ng/mg detection level (n = 5) in α2-AP−/−mice.
Endogenous thrombolytic potential.
Spontaneous lysis within 2 to 4 hours of a125I-fibrin–labeled pulmonary plasma clot was always higher in α2-AP−/− than in α2-AP+/+ mice (Fig3). In α2-AP+/+ mice, lysis of a clot produced from α2-AP+/+ or from α2-AP−/− plasma was comparable, indicating that the α2-AP content of the clot does not significantly inhibit lysis in plasma with normal inhibitor concentration. Also in α2-AP−/−mice, lysis of both clot types was not significantly different.
Spontaneous lysis of 125I-fibrin–labeled clots prepared from 2-AP+/+ or 2-AP−/− plasma and injected into 2-AP+/+ or 2-AP−/− mice. The data are mean ± SEM of the number of experiments indicated between parentheses. *P< .05 and **, P < .01 versus 2-AP+/+ mice.
Spontaneous lysis of 125I-fibrin–labeled clots prepared from 2-AP+/+ or 2-AP−/− plasma and injected into 2-AP+/+ or 2-AP−/− mice. The data are mean ± SEM of the number of experiments indicated between parentheses. *P< .05 and **, P < .01 versus 2-AP+/+ mice.
Endotoxin-induced fibrin deposition.
Histopathologic examination and immunostaining of kidney sections after endotoxin injection in α2-AP+/+ mice showed the occasional presence of fibrin deposits in the glomeruli of the outer cortex (Fig 4, IB) and, more frequently, in the capillaries of the medulla (Fig 4, IIB). In α2-AP+/+ mice without endotoxin injection and in α2-AP−/− mice after endotoxin injection, significantly less fibrin deposits were detected in the glomeruli (Fig 4, IA and IC) and in the medulla (Fig 4, IIA and IIC). Semi-quantitative analysis of fibrin deposition in the glomeruli indicated that, 8 hours after endotoxin injection, all 15 sections of α2-AP−/− mice (4 animals) were free of fibrin (score 0), whereas only 9 of 16 sections of α2-AP+/+ were devoid of fibrin (Table 4). In the capillaries of the medulla, fibrin deposits were detected in 4 of 15 sections of α2-AP−/− mice, whereas 14 of 16 sections of α2-AP+/+ mice showed fibrin deposition (Table 4). Chi-square analysis using a two by four contingency table indicated a significant reduction of fibrin deposits in α2-AP−/− mice as compared with α2-AP+/+ mice (P = .11 at 4 hours and P < .05 at 8 hours for the glomeruli; p ≤ .005 at 4 hours and at 8 hours for the medulla).
Immunostaining with a specific antiserum against murine fibrinogen/fibrin of kidney sections taken in the outer cortex (I) or in the medulla (II) (original magnification ×400) from 2-AP+/+ mice before (A) or 4 hours after endotoxin injection (B), and from 2-AP−/− mice 4 hours after endotoxin injection (C). The arrows indicate some of the fibrin deposits in the glomeruli (IB) and in the capillaries (IIB and IIC). The scale bar corresponds to 50 μm.
Immunostaining with a specific antiserum against murine fibrinogen/fibrin of kidney sections taken in the outer cortex (I) or in the medulla (II) (original magnification ×400) from 2-AP+/+ mice before (A) or 4 hours after endotoxin injection (B), and from 2-AP−/− mice 4 hours after endotoxin injection (C). The arrows indicate some of the fibrin deposits in the glomeruli (IB) and in the capillaries (IIB and IIC). The scale bar corresponds to 50 μm.
Extent of Fibrin Deposition as Detected by Immunostaining in Kidney Sections of 2-AP+/+ and 2-AP−/− Mice, 4 or 8 Hours After Injection of 2 mg/kg Endotoxin
| . | Score4-150 . | |||
|---|---|---|---|---|
| 0 . | 1 . | 2 . | 3 . | |
| Cortex/glomeruli | ||||
| 4 h | ||||
| α2-AP+/+ | 11 | 0 | 1 | 4 |
| α2-AP−/− | 15 | 1 | 0 | 0 |
| 8 h | ||||
| α2-AP+/+ | 9 | 1 | 2 | 4 |
| α2-AP−/− | 15 | 0 | 0 | 0 |
| Medulla/capillaries | ||||
| 4 h | ||||
| α2-AP+/+ | 2 | 2 | 3 | 9 |
| α2-AP−/− | 6 | 6 | 4 | 0 |
| 8 h | ||||
| α2-AP+/+ | 2 | 3 | 4 | 7 |
| α2-AP−/− | 11 | 4 | 0 | 0 |
| . | Score4-150 . | |||
|---|---|---|---|---|
| 0 . | 1 . | 2 . | 3 . | |
| Cortex/glomeruli | ||||
| 4 h | ||||
| α2-AP+/+ | 11 | 0 | 1 | 4 |
| α2-AP−/− | 15 | 1 | 0 | 0 |
| 8 h | ||||
| α2-AP+/+ | 9 | 1 | 2 | 4 |
| α2-AP−/− | 15 | 0 | 0 | 0 |
| Medulla/capillaries | ||||
| 4 h | ||||
| α2-AP+/+ | 2 | 2 | 3 | 9 |
| α2-AP−/− | 6 | 6 | 4 | 0 |
| 8 h | ||||
| α2-AP+/+ | 2 | 3 | 4 | 7 |
| α2-AP−/− | 11 | 4 | 0 | 0 |
Four sections were analyzed of kidneys of 4 different animals.
Score 0, no fibrin deposits; score 1, a few small, weakly stained fibrin deposits; score 2, clearly stained, small fibrin deposits; score 3, large, strongly stained fibrin deposits.
PAI-1 levels (mean ± SEM) in extracts of kidney sections taken at the end of the experiments were not significantly different for α2-AP+/+ or α2-AP−/− mice (2.8 ± 0.44 or 2.3 ± 0.32 ng/mg protein at 4 hours, and 0.96 ± 0.34 or 0.90 ± 0.07 ng/mg protein at 8 hours, as compared with <0.1 ng/mg protein in both genotypes without endotoxin injection). Plasma PAI-1 levels after endotoxin injection in α2-AP+/+or α2-AP−/− mice were also comparable (140 ± 26 or 110 ± 14 ng/mL at 4 hours, and 190 ± 93 and 77 ± 14 ng/mL at 8 hours), and were increased more than 50-fold over baseline levels.
α2-AP levels in kidney extracts of α2-AP+/+ mice were not increased after endotoxin injection (7.5 ± 0.12 and 5.6 ± 3.3 ng/mg protein at 4 hours and 8 hours after injection, as compared with 7.0 ± 0.12 ng/mg protein without endotoxin injection). Plasma α2-AP levels in α2-AP+/+ mice were also comparable before (81 ± 5 μg/mL) and 4 hours (73 ± 4 μg/mL) or 8 hours (56 ± 3 μg/mL) after endotoxin injection.
DISCUSSION
Congenital homozygous α2-AP deficiency was reported in patients who presented with hemorrhagic diathesis, whereas several cases of heterozygosity in different families have been described with no or only mild bleeding symptoms.21-29 In all heterozygotes described so far, both α2-AP antigen and activity levels ranged between 40% and 60% of normal, suggesting that the deficiency is due to decreased synthesis of a normal α2-AP molecule. A single case of dysfunctional α2-AP (α2-AP Enschede) associated with severe bleeding tendency has been reported in two siblings in a Dutch family.30 The ability of this α2-AP to bind reversibly to plasminogen was not affected, but it was converted from an inhibitor of plasmin into a substrate, as a result of the insertion of an extra alanine residue in the reactive center loop.31The bleeding tendency in patients with α2-AP deficiency is most likely due to premature lysis of hemostatic plugs, because the half-life of plasmin molecules generated at the fibrin surface may be considerably prolonged in the absence of α2-AP. Acquired α2-AP deficiency associated with enhanced fibrinolysis has been reported in patients with liver disease,32,33disseminated intravascular coagulation,32 and acute promyelocytic leukemia.34 Furthermore, α2-AP levels may be significantly reduced in patients undergoing thrombolytic therapy, especially with nonfibrin-specific agents, as a result of extensive systemic generation of plasmin.35 After exhaustion of plasma α2-AP, excess plasmin may degrade several plasma proteins, including fibrinogen, and eventually lead to bleeding complications. Pathophysiologic observations in humans thus support the relevant role of α2-AP in regulating and controlling plasmin activity. In addition, studies in mice with deficiency of the main components of the fibrinolytic system indicate an important role of plasmin in fibrin surveillance and in maintenance of an intact hemostatic balance.17 To further substantiate these findings, we have generated mice with homozygous deficiency of α2-AP, the main plasmin inhibitor in mammalian plasma.
α2-AP−/− mice develop and reproduce normally. Macroscopic examination and microscopic analysis of cross-sections of different organs did not show significant hemorrhage in 6- to 20-week-old α2-AP−/− mice. Furthermore, after amputation of tail or toe tips, bleeding stopped spontaneously in α2-AP−/−, as well as in α2-AP+/− and α2-AP+/+ mice. The absence of an overt bleeding phenotype in α2-AP−/− mice appears somewhat surprising in view of the observations in humans described above. Several factors may contribute to this apparent difference between humans and mice. First, although the main components of the fibrinolytic system are similar in both humans and mice, important quantitative differences were observed, as a result of which the fibrinolytic system in mice appeared to be very resistant to activation.5 36 Second, the spectrum of proteinase inhibitors in murine plasma, other than α2-AP, may be more efficient toward plasmin than in human plasma, or additional inhibitory mechanisms may contribute. Inhibition of plasmin has indeed been observed by several other plasma-proteinase inhibitors, including α2-macroglobulin and α1-antitrypsin. The residual rapid-reacting plasmin-inhibitory activity in α2-AP–deficient plasma may thus be explained by alternative inhibitory pathways. We have shown that it is not due to interaction with α2-AP. Furthermore, the levels of antiplasmin activity are comparable in male and female α2-AP−/− mice and thus do not correlate with potential sex-related differences in α2-macroglobulin levels. The apparent antiplasmin activity observed in the functional assay, which persisted in the presence of higher S-2403 concentrations or with the use of murine plasmin, may reflect an interaction with plasma proteins rendering the plasmin unavailable for the chromogenic substrate.
The absence of a bleeding phenotype in α2-AP−/− mice, as in many heterozygous patients, probably reflects the fact that the coagulation system adequately prevents bleeding under circumstances where the fibrinolytic system is not dramatically challenged. Extensive activation of the system, in the absence of α2-AP, will, however, result in efficient fibrin degradation, and may thus cause lysis of hemostatic plugs and hemorrhagic complications.
In vivo clot lysis experiments confirm that the endogenous thrombolytic potential is significantly enhanced in α2-AP−/− mice, indicating a physiologic role of α2-AP in fibrin surveillance. Furthermore, the experiments with cross-linked α2-AP+/+ and α2-AP−/− plasma clots in α2-AP+/+ and α2-AP−/− mice suggest that the efficiency of spontaneous thrombolysis is determined primarily by the α2-AP concentration in circulating blood, and not by the amount that is cross-linked to fibrin. This suggests that cross-linking of α2-AP to fibrin, which renders a fibrin clot less susceptible to degradation by plasmin, does not dramatically affect the lysability of the clot by the murine endogenous fibrinolytic system.
Injection of endotoxin in mice was previously shown to result in enhanced PAI-1 levels and in significant fibrin deposition in the kidneys, within 4 to 8 hours after injection.37 Using this model, fibrin deposition was found to be significantly reduced in α2-AP−/− mice as compared with α2-AP+/+ mice. This is most likely not due to a different degree of fibrin formation in both genotypes; furthermore, PAI-1 levels in kidney and plasma were enhanced to a similar degree after endotoxin injection. The observed difference, therefore, would appear to be due to a higher endogenous fibrinolytic capacity in α2-AP−/− mice, again confirming the role of α2-AP in fibrin clearance.
In conclusion, α2-AP−/− mice survive, develop, and reproduce normally, but show an enhanced endogenous fibrinolytic capacity without overt bleeding phenotype.
ACKNOWLEDGMENT
Skillful technical assistance by K. Bijnens, E. Gils, L. Kieckens, T. Vancoetsem, B. Van Hoef, I. Vanlinthout, A. Van Nuffelen, M. Verstreken, and G. Wallays is gratefully acknowledged.
Supported by a grant from the Belgian National Fund for Scientific Research (Project 3.0265.95).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to H.R. Lijnen, PhD, Center for Molecular and Vascular Biology, University of Leuven, Campus Gasthuisberg O&N, Herestraat 49, B-3000 Leuven, Belgium; e-mail:roger.lijnen@med.kuleuven.ac.be.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal