Abstract
Somatic hypermutation is the most critical mechanism underlying the diversification of Ig genes. Although mutation occurs specifically in B cells during the germinal center reaction, it remains a matter of debate whether the mutation machinery also targets non-Ig genes. We have studied mutations in the 5′ noncoding region of the Bcl6 gene in different subtypes of lymphomas. We found frequent hypermutation in follicular lymphoma (25 of 59 = 42%) (germinal center cell origin) and mucosa-associated lymphoid tissue (MALT) lymphoma (19 of 45 = 42%) (postgerminal center), but only occasionally in mantle cell lymphoma (1 of 21 = 4.8%) (pregerminal center). Most mutations were outside the motifs potentially important for transcription, suggesting they were not important in lymphomagenesis but may, like Ig mutation, represent an inherent feature of the lymphoma precursor cells. Therefore, we investigated their normal cell counterparts microdissected from a reactive tonsil. Bcl6 mutation was found in 13 of 24 (54%) clones from the germinal centre but only in 1 of 24 (4%) clones from the naive B cells of the mantle zone. The frequency, distribution, and nature of these mutations were similar to those resulting from the Ig hypermutation process. The results show unequivocal evidence of non-Ig gene hypermutation in germinal center B cells and provide fresh insights into the process of hypermutation and lymphomagenesis.
THE GENERATION of highly diverse antibodies with high affinity is the key element of acquired immunity. This is primarily achieved by sequential somatic alterations of the Ig genes during B-cell development.1 Functional Ig genes are first generated by recombination of the germline Ig building blocks, ie, the variable (V), diversity (D), and joining (J) segments, during which random nucleotides are inserted into the VD and DJ junctions to increase diversity.1,2 After antigen exposure, somatic hypermutations are introduced into the rearranged Ig genes.1 B cells expressing antibodies with high affinity to exogenous antigens are positively selected, whereas those failing to express high-affinity antibodies or expressing those recognizing autoantigens are eliminated.1 3
Somatic hypermutations occur specifically in B cells during the germinal center reaction and are exclusively found within 2 kb downstream of the Ig promoter, with the great majority in and around the rearranged V genes.3,4 The mutation process appears to target certain specific sequence motifs, such as RGYW (A/G G C/T A/T).5 In addition, the nucleotides vulnerable for mutation may be associated with the features of the neighboring sequences, such as repeat sequences and/or palindromic structures.6,7 Although the mechanisms underlying the mutation process are not fully understood, the research to date indicates that mutation activity is coupled with the transcription process but does not depend specifically on the presence of the Ig promoter or the Ig coding sequence.8 9 Whether the mutation process depends specifically on the Ig enhancer elements remains to be tested. Somatic hypermutation of the Ig gene is believed to be a locus-specific, differentiation-stage specific, and lineage-specific phenomenon. However, in view of the fact that the Ig promoter and coding sequence are not specifically required, the hypermutation process may not target only the Ig gene. We have studied mutations in the 5′ noncoding region of the Bcl6 gene in various B-cell lymphomas and different cell populations microdissected from normal lymphoid follicles and found unequivocal evidence of non-Ig gene mutation in normal germinal center but not in naive mantle B cells.
MATERIALS AND METHODS
Materials.
Frozen and paraffin-embedded tissue blocks from 125 cases of lymphoma were retrieved from the Department of Histopathology, University College London Medical School. They comprised 59 follicular, 45 mucosa-associated lymphoid tissue (MALT), and 21 mantle cell lymphomas. Frozen tonsil tissue from a 25-year-old woman was also retrieved from the departmental tissue bank.
Microdissection and DNA extraction.
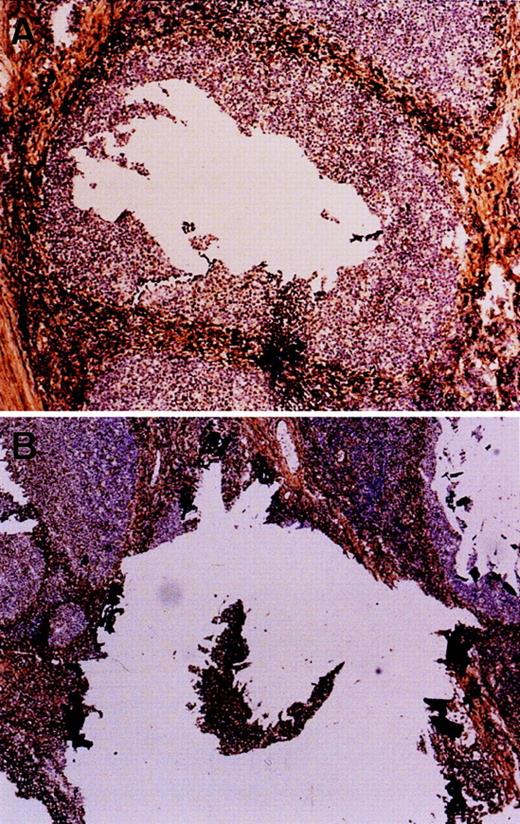
The percentage of malignant cells was estimated by histological examination. The follicular and mantle cell lymphomas contained at least 60% tumor cells, whereas most MALT lymphomas contained only 10% to 60% of tumor cells. For these latter cases, tumor cells were enriched by microdissection.10 Germinal center and mantle B cells were separately microdissected from a frozen section of tonsil immuno-stained for IgD (Fig 1). DNA extraction was performed as described elsewhere.10 11
Microdissection of germinal center (A) and naive B cells (B) of the mantle zone. Frozen sections of a tonsil were immuno-stained for IgD to highlight mantle cells. Germinal center cells were directly microdissected (the hole), whereas mantle cells were first isolated (the island) then harvested.
Microdissection of germinal center (A) and naive B cells (B) of the mantle zone. Frozen sections of a tonsil were immuno-stained for IgD to highlight mantle cells. Germinal center cells were directly microdissected (the hole), whereas mantle cells were first isolated (the island) then harvested.
Polymerase chain reaction-single strand conformation polymorphism (PCR-SSCP).
The 5′ noncoding region (a 1.3-kb fragment 112 bp downstream of the promoter) of the Bcl6 gene was amplified by five different overlapped PCR reactions using the previously published primer sets (E1.7, E1.8, E1.10, E1.11, and E1.12, Fig2A).12 The PCR reaction mix consisted of 1 μL DNA extract, 10 mmol/L Tris (pH 8.3), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.1% Triton X-100, 200 μmol/L of each dNTP, 5 pmol of each primer, 0.001% gelatin, and 0.25 U Taq polymerase (Promega, Southampton, UK) in a total volume of 25 μL. Amplification was performed on a thermal cycler (Hybaid, Teddington, UK) using a hot-start procedure,13 followed by a touch down program, comprising a cycle of 94°C for 30 seconds, 66°C (then reducing 1°C per cycle to 56°C) for 30 seconds and 72°C for 45 seconds, and 35 further cycles with an annealing temperature of 55°C. PCR products (3 μL) were checked for yield and size on 1% agarose gels before further analysis.
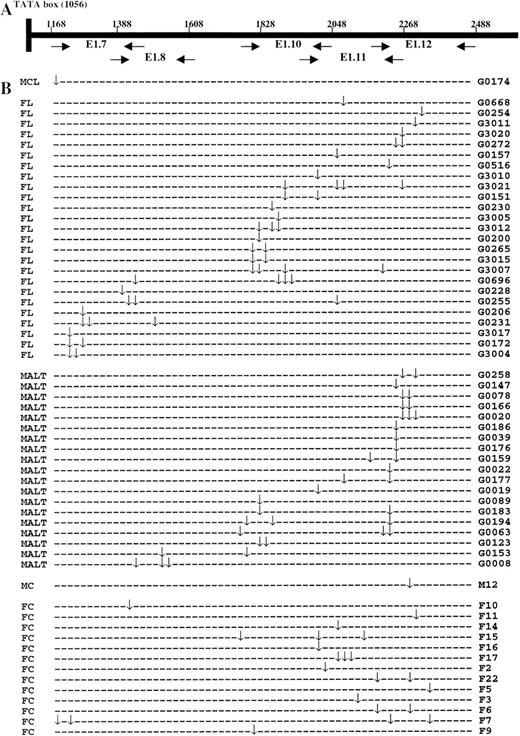
The schematic illustration of the positions of the PCR primers (A) and the distribution of the somatic mutation in the 5′ noncoding region of the Bcl6 gene (B). MCL, mantle cell lymphoma; FL, follicular lymphoma; MALT, MALT lymphoma; MC, mantle cell; FC, follicle centre cell. (↓) Mutation.
The schematic illustration of the positions of the PCR primers (A) and the distribution of the somatic mutation in the 5′ noncoding region of the Bcl6 gene (B). MCL, mantle cell lymphoma; FL, follicular lymphoma; MALT, MALT lymphoma; MC, mantle cell; FC, follicle centre cell. (↓) Mutation.
For cell populations microdissected from tonsil, the 1.3-kb fragment of the 5′ noncoding region of the Bcl6 gene was amplified using primers GGAAAGCAAAGCGCACTC and CACGATACTTCATCTCATC. In addition, a fragment of 151 bp from the coding region of the house-keeping gene iduronate 2-sulfatase (IDS) (humids.gp_pr) was amplified as a control, using primers CCAAAGAAGGGAGGGTCCAC and AGACCAGCTATACGGAGAATCACC. Both Bcl6 and IDS PCR products were cloned into pGEM-T vector and transformed into JM109 competent cells (Promega). The resulting colonies were boiled in 50 μL of distilled water at 95°C for 10 minutes before a brief centrifuge and 1 μL of the subsequent supernatant was used as template for PCR amplification as above.
For SSCP analysis, 2 μL of PCR products was mixed with 4 μL sequencing loading buffer (98% formamide, 10 mmol/L NaOH, 20 mmol/L EDTA, 0.05% bromophenol blue, and 0.05% xylene cyanol FF), denatured at 99°C for 5 minutes on a hot plate, and separated on the Genphor electrophoresis system (Amersham-Pharmacia Biotech, Little Chalfont, UK) under 15 W constant power for 2 to 3 hours at 5°C.
Sequencing and sequence analysis.
The PCR products showing altered SSCP patterns were purified using the Wizard PCR purification system (Promega) and directly sequenced using an ABI 377 sequencer (Perkin Elmer, Warrington, UK) with dye terminators, according to the manufacturer’s protocol. Mutations were identified by comparison with the published Bcl6 gene sequence (Z79581) using web-based BLAST programme (http://www.ncbi.nih.gov/blast). The RGYW hot motif and features of the neighboring sequences, such as repeat sequences and palindromic structures, in the region with mutations were analysed by sequence searching and/or using Wisconsin GCG software (Human Genomic Mapping Project, Cambridge, UK), as described previously.5-7
RESULTS
Bcl6 gene is mutated in lymphomas derived from antigen experienced but not in those from naive B cells.
Following a recent report of somatic mutation in the 5′ noncoding region of the Bcl6 gene in follicular lymphoma,12 we screened this region for mutation in various lymphomas derived from B cells at different maturation stages using PCR-SSCP and sequencing. Mutation was frequently found in follicular (25 of 59 = 42%) and MALT (19 of 45 = 42%) lymphomas, both of which are derived from antigen-experienced B cells. However, mutation was only occasionally observed in mantle cell (1 of 21 = 4.8%) lymphomas, which originate from naive B cells (Table 1). Most mutations were outside the motifs potentially important for transcription. Details of these mutations are presented below.
Frequencies of Bcl6 Mutation in Lymphoma and Normal B Cells
| . | Mutated Cases/Clones (%) . | Mutation Frequency* . | |
|---|---|---|---|
| Whole Region (1.3 kb) . | Hot-Spot Region (603 bp) . | ||
| Lymphoma | |||
| MCL | 1/21 (4.8) | 0.37 × 10−4/bp | 0 × 10−4/bp |
| FL | 25/59 (42) | 6.0 × 10−4/bp | 9.28 × 10−4/bp |
| MALT | 19/45 (42) | 6.0 × 10−4/bp | 11.4 × 10−4/bp |
| Normal | |||
| MC | 1/24 (4) | 0.32 × 10−4/bp | 0.69 × 10−4/bp |
| FC | 13/24 (54) | 7.1 × 10−4/bp | 13.1 × 10−4/bp |
| . | Mutated Cases/Clones (%) . | Mutation Frequency* . | |
|---|---|---|---|
| Whole Region (1.3 kb) . | Hot-Spot Region (603 bp) . | ||
| Lymphoma | |||
| MCL | 1/21 (4.8) | 0.37 × 10−4/bp | 0 × 10−4/bp |
| FL | 25/59 (42) | 6.0 × 10−4/bp | 9.28 × 10−4/bp |
| MALT | 19/45 (42) | 6.0 × 10−4/bp | 11.4 × 10−4/bp |
| Normal | |||
| MC | 1/24 (4) | 0.32 × 10−4/bp | 0.69 × 10−4/bp |
| FC | 13/24 (54) | 7.1 × 10−4/bp | 13.1 × 10−4/bp |
Abbreviations: MCL, mantle cell lymphoma; FL, follicular lymphoma; MALT, MALT lymphoma; MC, mantle cell; FC, follicle center cell.
The mutation frequencies were calculated according to the formula: No. of Mutations/No. of Cases (clones) × Sequence Length Tested.
Bcl6 is mutated in normal germinal center but not in naive B cells.
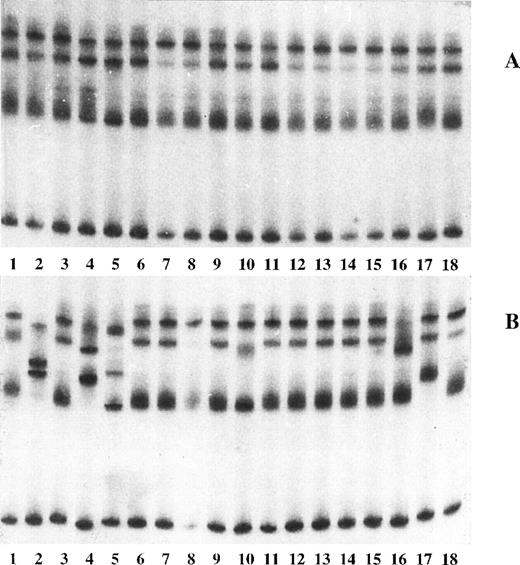
To examine whether Bcl6 mutation also occurs in normal B cells, the 1.3-kb fragment of the 5′ noncoding region of the Bcl6 gene, together with a fragment of 151 bp of the housekeeping gene IDS, was PCR-amplified and cloned from microdissected germinal center and mantle B cells. In initial experiments, DNA from four colonies was mixed and subjected to PCR-SSCP and a total of 96 clones from each cell population were screened for Bcl6 mutation. Single or multiple abnormal SSCP bands were observed in each mixture from germinal center B cells, but in only one from the naive B cells of the mantle zone. Subsequently, we repeated the PCR-SSCP analysis of the Bcl6 gene on 24 single colonies from each cell population, followed by sequencing. Mutation was found in 13 clones (13 of 48 = 54%) from the germinal center but only in 1 (1 of 24 = 4%) from mantle cells (Fig3). However, no mutation was found in 48 clones of the control gene IDS from either cell population.
PCR-SSCP analysis of the 5′ noncoding region E1.11 of the Bcl6 gene in a reactive tonsil. Abnormal SSCP bands were shown in clones 1, 2, 4, 5, 10, 16, and 17 from follicular center cell (B), but in none from mantle cell (A) populations.
PCR-SSCP analysis of the 5′ noncoding region E1.11 of the Bcl6 gene in a reactive tonsil. Abnormal SSCP bands were shown in clones 1, 2, 4, 5, 10, 16, and 17 from follicular center cell (B), but in none from mantle cell (A) populations.
Bcl6 mutations resemble those found in the rearranged Ig gene.
Because the Bcl6 mutations found in follicular and MALT lymphoma most likely inherited from their malignant precursor cells, like the Ig gene mutation, and there was no significant difference in the characteristics of Bcl6 mutation between these lymphomas and germinal center B cells as shown by separate analysis, the data were thus combined. In total, 103 mutations were found among 56 Bcl6 sequences (mutations from mantle cell lymphoma and normal mantle cells were not included). The distribution of these mutations was nonrandom. The great majority (83 of 103 = 80.6%) were localized in a region of 603 bp spanning the E1.10 and E1.12 (Fig 2B) and the overall frequency of mutation in this region was 11.3 × 10−4/bp. The details of the mutation frequency in different lymphomas and normal cell populations of a reactive tonsil are shown in Table 1. Mutations were nearly exclusively single substitutions, with only one being a deletion. The majority of the substitutions (60%) were caused by transition mutations, of which a major portion were T to C. Details of these mutations are presented in Table 2. A substantial proportion of substitutions (48%) were within or flanked by the mutation hot motif RGYW (A/G G C/T A/T) found in the Ig gene on both sides.5 Interestingly, all mutations were also within the stem of an imperfect palindromic (hairpin) structure, and/or close to a repeat sequence, by the previous described standards6 7 (Fig 4). Multiple mutations (2-4), frequently within a short sequence (<100 bp), were observed in 31 Bcl6 sequences, accounting for 54% of the mutants. Among germinal center B cells, two distinct mutations were shared by two pairs of clones, respectively.
Nature of Bcl6 Mutations
| . | A . | C . | G . | T . |
|---|---|---|---|---|
| A → | — | 1 | 6 | 6 |
| C → | 3 | — | 12 | 6 |
| G → | 10 | 5 | — | 1 |
| T → | 8 | 41 | 6 | — |
| Total (%) | 21 (20%) | 47 (45%) | 24 (23%) | 13 (12%) |
| . | A . | C . | G . | T . |
|---|---|---|---|---|
| A → | — | 1 | 6 | 6 |
| C → | 3 | — | 12 | 6 |
| G → | 10 | 5 | — | 1 |
| T → | 8 | 41 | 6 | — |
| Total (%) | 21 (20%) | 47 (45%) | 24 (23%) | 13 (12%) |
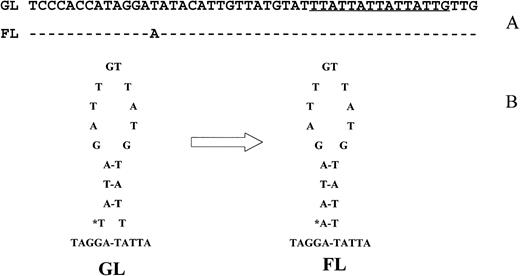
A T > A mutation in a follicular lymphoma (FL). The proximity of the mutation to a (TTA)n repeat sequence (underlined) and its localization to the stem of an imperfect hairpin structure in the germline (GL) sequence were shown in (A) and (B), respectively.
A T > A mutation in a follicular lymphoma (FL). The proximity of the mutation to a (TTA)n repeat sequence (underlined) and its localization to the stem of an imperfect hairpin structure in the germline (GL) sequence were shown in (A) and (B), respectively.
DISCUSSION
Following a recent report of somatic mutation in the 5′ noncoding region of the Bcl6 gene in follicular lymphoma,12 we examined the same region for mutation in various lymphomas derived from B cells at different maturation stages. Mutation was frequently found in lymphomas deriving from antigen-experienced B cells, namely follicular and MALT lymphomas, but only occasionally in mantle cell lymphomas originating from naive B cells. Most of these mutations were outside the motifs potentially important for Bcl6 transcription.14 This, together with the rarity or absence of t(3;14)(q27;q32) and Bcl6 rearrangement in these low-grade lymphomas,15-17 suggests that the observed mutations were not important in lymphomagenesis but may, like Ig mutation, represent an inherent mutation signature of the lymphoma precursor cells.18 To investigate this, we therefore examined their normal cell counterparts.
As expected, similar Bcl6 mutations were found in germinal center but not in naive B cells of the mantle zone. Most or all of these mutations were of somatic origin rather than due to a PCR error, because no mutations were found in 48 IDS clones and the estimated PCR error rate is less than 0.01% in our experimental system.19,20Several features of these Bcl6 mutations resemble those found in the Ig gene as follows. (1) Nonrandom distribution: the majority of Bcl6 mutations localize in a region of 603 bp, 723 bp downstream of the promoter, which is in line with that of the rearranged Ig gene. In addition, 48% of mutations are within or flanked by the Ig mutation hot motif RGYW. (2) High frequency: the incidence of mutation in the hot region of the Bcl6 gene was 11.3 × 10−4/bp, which is far above the estimated PCR error rate in our system,19,20 although about 100 times lower than that of the Ig gene in the germinal center B cells (about 10%).8,21,22 (3) Nature of the mutations: like the Ig mutation, most Bcl6 mutations were single substitutions, mainly due to transitions. (4) Clustering: multiple mutations within a short stretch of sequence were frequently found in the Bcl6 gene. More strikingly, some of the Bcl6 sequences from the germinal center B cells showed both common and unique mutations, resembling the intraclonal variations of the Ig gene found in normal germinal center B cells.22 (5) Effects of neighboring sequences: Mutation of the Bcl6 gene was frequently within the stem of an imperfect hairpin structure or close to a repeat sequence. Although these structures may not template the mutation because mismatch repair is not closely involved in the hypermutation process,23-25 it may induce pausing of RNA polymerase during transcription elongation and then result in the transfer of the putative mutator factor to this region.8Therefore, it appears likely that Bcl6 mutation may result from the Ig hypermutation process.
While preparing this paper, two studies have reported the occurrence of Bcl6 mutations in normal B cells.26 27 Unlike our approach of examining microdissected germinal center and mantle cells, they used isolated normal B cells from peripheral blood or from tonsil and found Bcl6 mutation in 30% to 42% of the memory but none in the naive B cells. The complimentary data from this and the above-mentioned studies clearly indicate that the Bcl6 mutations are introduced in germinal center B cells and are subsequently inherited by their normal and malignant derivatives.
The Ig hypermutation process may also target non-Ig genes of the germinal center cells of non–B-lineage. Zheng et al28examined germinal center T cells of the spleen from immunized mice and found frequent somatic hypermutation in T-cell receptor (TCR) α, but not in β, chain gene. Cheynier et al29 studied T cells of splenic white pulp from human immunodeficiency virus (HIV)-1–positive patients and demonstrated hypermutation in a proportion of TCRβ gene clones. The frequency of these TCRβ mutations (1 to 5 × 10−4/bp) is similar to those seen in the Bcl6 gene of the germinal center B cells. As in the Bcl6 mutations, the TCR gene mutations resemble those of the Ig genes.
Although several features of Bcl6 and TCR mutations resemble those of the Ig genes, there are important differences in both the proportion of germinal center B cells with mutations and the mutation frequency in the affected cells between the Ig and non-Ig genes. In adults, the rearranged Ig gene is mutated in most germinal center cells,18,21 whereas the Bcl6 gene is mutated in only 30% to 50% and the TCR gene is only mutated in 10% to 50% of germinal center T cells.28,29 The frequency of both Bcl6 and TCR mutation are 100 times lower than that of the Ig gene of the germinal center B cells. It thus appears that the Ig hypermutation process may target non-Ig genes in both germinal center B and T cells, but in an inefficient manner. This may explain the heterogeneous incidence of Bcl6 mutations in germinal center cells and their derivatives, and may also underlie the lack of somatic mutations in other genes expressed in germinal centers such as c-myc and S14 shown by Shen et al.26 Study of the cis-acting element shared by the Ig and Bcl6 genes but not by c-myc and S14 may provide fresh insights into the mechanism of Ig hypermutation.26
The finding of non-Ig gene hypermutation in normal germinal center B cells suggests imperfection of the Ig hypermutation process. Theoretically, mutation could occur in the coding region of other cellular genes, which are potentially important in cell growth and survival. It is possible that in the case of lethal mutation, the cell affected will die naturally, whereas in the case of nonlethal mutation causing important cellular biological alterations, such as growth properties, the cell affected will usually be detected and deleted. However, if the mutation escapes from immune surveillance, it may initiate lymphomagenesis. In this context, it is particularly interesting to note that follicular lymphomas, which are derived from germinal center B cells, are the most common B-cell malignancy. Furthermore, a number of B-cell lymphoma subtypes such as follicular and MALT show intimate interaction of tumor cells with lymphoid follicles,30 where tumor cells frequently show high-grade transformation30,31 and occasionally exhibit positive p53 staining.32 It is tempting to speculate that the “infidelity” of the Ig hypermutation machinery to other cellular genes may underlie, at least in part, the genesis and progression of B-cell lymphomas associated with the germinal center microenvironment.
ACKNOWLEDGMENT
We thank Dr T.C. Diss (Department of Histopathology, University College London Medical School, London, UK) for critical reading of the manuscript. We are also grateful to Daniela Papini (Divisione Di Anatomia Patologica, Istituto Nazionale Tumori, Milano, Italy) for her excellent support in sequencing.
Supported by the Cancer Research Campaign (CRC, Grant No. SP1758, United Kingdom) and Leukemia Research Fund (LRF, 9609, United Kingdom), and Associazionr Italiana per la Ricerca sul Cancro (AIRC, Italy).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ming-Qing Du, PhD, Department of Histopathology, University College London Medical School, Rockefeller Bldg, University St, London WC1E 6JJ, UK; e-mail:m.du@ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal