ANTIPHOSPHOLIPID SYNDROME
ANTIPHOSPHOLIPID antibodies have been associated with a variety of clinical phenomena, including arterial and venous thrombosis, thrombocytopenia, and obstetric complications. The term “antiphospholipid syndrome”1is used to link a variety of thromboembolic events to antibodies against specific proteins involved in blood coagulation. Thrombotic events are reported in approximately 30% of patients with antiphospholipid antibodies,2,3 with an overall incidence of 2.5% patients/yr.4 Deep vein thrombosis of the legs and/or pulmonary embolism account for about two thirds of the thrombotic events, and cerebral arterial thrombosis are the most common arterial complications.2,3 Obstetric complications include recurrent spontaneous miscarriages, fetal deaths, or fetal growth retardations.5 Women with antiphospholipid antibodies are particularly prone to second or early third trimester fetal deaths.6 Hypoxia secondary to spiral arterial vasculopathy is considered to be the cause of the obstetric events.7 A variable degree of thrombocytopenia is reported in as many as 20% to 25% of patients.8 Thrombocytopenia is generally mild and seldom associated with bleeding complications; only 5% to 10% of patients are severely thrombocytopenic (<50 × 109platelets/L).8
Less commonly, hemolytic anemia, livedo reticularis, skin necrosis, dementia or neuropsychiatric events, and the so-called “catastrophic” antiphospholipid syndrome are included in this picture.8 Two types of antiphospholipid syndrome have been described: the “primary” syndrome, which occurs in the absence of an underlying disease,9 and the “secondary” syndrome, which is related to systemic lupus erythematosus, other autoimmune or neoplastic diseases, or other pathological conditions.10
ANTIPHOSPHOLIPID ANTIBODIES
Since the beginning of this decade it has been increasingly appreciated that antiphospholipid antibodies are a large and heterogeneous family of immunoglobulins which, despite their name, do not bind to phospholipids, but are directed at plasma proteins with affinity for anionic (phospholipid) surfaces. Some of the antigenic targets of these antibodies include β2-glycoprotein I,11-13prothrombin,14 high- and low-molecular-weight kininogens,15 annexin V,16 (activated) protein C,17,18 and protein S.17 18
Since most of the antigens are involved in blood coagulation, some antiphospholipid antibodies may hamper the regulation of blood coagulation, thus providing an explanation of the high rate of thrombosis in patients with the antiphospholipid syndrome. Most biological and clinical studies have dealt with anti–β2-glycoprotein I and antiprothrombin antibodies, which are the best known antiphospholipid antibodies. In this report we will focus on antiprothrombin antibodies, particularly their prevalence, immunological and functional properties, clinical significance, and treatment.
HISTORICAL BACKGROUND
In 1959 Loeliger19 described a case whose lupus anticoagulant activity was more pronounced in a mixture of the patient’s plasma with normal plasma than in the patient’s own plasma. The patient’s plasma prothrombin was low. Elegant adsorption experiments of patient’s plasma with BaSO4 led the investigator to suggest prothrombin was the necessary cofactor for the expression of this lupus anticoagulant activity. One year later, Rapaport et al20 reported a case of systemic lupus erythematosus whose lupus anticoagulant was associated with profound acquired hypoprothrombinemia. The patient’s severe bleeding complications were fully described and discussed in relation with reported cases; the investigators concluded that the plasma coagulation disturbances of systemic lupus erythematosus usually resulted from a combination of an inhibitor impeding the activity of the prothrombin activator complex and acquired hypoprothrombinemia. In the subsequent 15 years, many patients were reported with systemic lupus erythematosus, who showed bleeding complications associated with a lupus anticoagulant and acquired hypoprothrombinemia.21-25In none of these cases did the circulating inhibitor neutralize the coagulant activity of prothrombin added to the plasma. In 1972 Feinstein and Rapaport26 reviewed the acquired inhibitors of blood coagulation and concluded that although the lupus anticoagulants impaired clotting in vitro, abnormal bleeding was only seen in cases of severe hypoprothrombinemia and/or thrombocytopenia. Lechner25 and Natelson et al27 provided evidence that the hypoprothrombinemia associated with lupus anticoagulants involved a reduction of both prothrombin activity and prothrombin antigen.
During the 1980s more work was performed to clarify the hypoprothrombinemia of patients with lupus anticoagulants. Bajaj et al28 provided the first evidence that the plasma of patients with lupus anticoagulants and severe hypoprothrombinemia contained nonneutralizing antibodies, which bound prothrombin without inhibiting its conversion to thrombin in the reaction mixtures used to measure plasma prothrombin activity. The investigators postulated that hypoprothrombinemia results from the rapid clearance of prothrombin-antiprothrombin antibody complexes from the circulation.
In 1984 Edson et al29 showed the presence of antiprothrombin antibodies in the plasma of patients with lupus anticoagulants but without severe hypoprothrombinemia in prothrombin crossed immunoelectrophoresis experiments. Using a similar laboratory approach, these findings were confirmed and extended by Fleck et al,30 who found antiprothrombin antibodies in 31 of 42 lupus anticoagulant-positive patients (74%), 15 of whom had prolonged prothrombin time. Adsorption of patients’ plasma with insoluble prothrombin reduced both the immune complexes and the anticoagulant activity. Finally, eluates of the insoluble prothrombin contained IgG that displayed lupus anticoagulant activity. The investigators concluded that these lupus anticoagulant antibodies were polyspecific, because they reacted with negatively charged phospholipids and prothrombin.
DETECTION METHODS AND IMMUNOLOGICAL PROPERTIES
Double immunodiffusion and crossed-immunoelectrophoresis were the first techniques used for screening antiprothrombin antibodies.28-31 Their main advantage lay in the possibility of detecting prothrombin/antiprothrombin immune complexes. This in vitro finding makes it reasonable to assume that such complexes are also present in plasma in vivo, which may be of biologic significance. However, their main disadvantage is that these methods do not provide a quantitative estimate of the antibody. Moreover, in some cases the titer or the affinity of antiprothrombin antibodies is too low to give unequivocal precipitin lines.
Other techniques are based on the impairment of prothrombin activation by antiprothrombin antibodies14 17 (see below). The need for isolated antiprothrombin antibodies and purified cogulation factors, however, makes these methods unsuitable for the routine evaluation of large numbers of patients with antiphospholipid antibodies.
In the last few years, several groups of investigators have developed enzyme-linked immunosorbent assay (ELISA) methods, which are by now the most commonly used techniques. They give a quick determination of the titer and the isotype of antiprothrombin antibodies. Interestingly, the mode of presentation of prothrombin in immunoassays greatly influences its recognition (Table 1). Antiprothrombin antibodies bind to prothrombin coated on γ-irradiated32or high-activated polyvinylchloride (PVC),18,33 but not on plain polystyrene ELISA plates.18,32,33 IgG and/or IgM antibodies to human prothrombin in solid phase have been reported in approximately half of the patients with antiphospholipid antibodies.18,32,33 Antiprothrombin antibodies recognize both human and bovine prothrombin, although the human molecule is a better antigen.32,34 Prothrombin is recognized more efficiently when the protein is bound to phosphatidylserine-coated ELISA plates using calcium ions: the prevalence of positive samples increases up to 90%.33 This may be explained in different ways. Firstly, unlike PVC-bound prothrombin, prothrombin complexed to phosphatidylserine is not likely to be restricted in its lateral movements: this would allow clustering and proper orientation, offering better binding conditions for the antibodies. Alternatively, the ELISA with phosphatidylserine in solid phase may, through the calcium ions, capture the circulating prothrombin-antiprothrombin immune complexes present in some samples. Finally, antiprothrombin antibodies might react with neoepitopes that prothrombin makes available only when bound to phosphatidylserine through calcium ions.
The Mode of Presentation of Prothrombin Influences Its Recognition by Antiprothrombin Antibodies in ELISA Systems
| Human Prothrombin Bound to . | Prevalence (%) . | References . |
|---|---|---|
| Plain polystyrene plates | 0 | 18, 32, 33 |
| γ-Irradiated plates | 55 | 32 |
| High-activated PVC plates | 50-58 | 18, 33 |
| Phosphatidylserine-coated plates | 90 | 33 |
| Human Prothrombin Bound to . | Prevalence (%) . | References . |
|---|---|---|
| Plain polystyrene plates | 0 | 18, 32, 33 |
| γ-Irradiated plates | 55 | 32 |
| High-activated PVC plates | 50-58 | 18, 33 |
| Phosphatidylserine-coated plates | 90 | 33 |
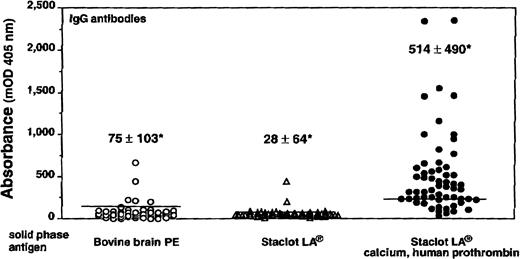
Recent evidence from Rauch35 indicates that antiprothrombin antibodies recognize prothrombin also when bound to hexagonal (II) phase phosphatidylethanolamine, and that the plasma lupus anticoagulant activity is specifically neutralized by the prothrombin/hexagonal (II) phase phosphatidylethanolamine complex. Phosphatidylethanolamine is a neutral phospholipid that can assume nonbilayer configurations under appropriate thermodinamic conditions.36 The hexagonal (II) phase consists of hexagonally packed cylinders of lipid surrounding central aqueous channels toward which the polar head groups are oriented.37 Immunization of mice with hexagonal (II) phase phosphatidylethanolamine induced antiphospholipid antibodies that displayed lupus anticoagulant activity.38 Experiments performed in our laboratory basically confirm these findings. Staclot LA (Stago, Asnieres, France) and bovine brain phosphatidylethanolamine—used as source of hexagonal II phase and lamellar phosphatidylethanolamine, respectively—were coated on ELISA plates, assuming that they maintain their conformational structures in solid phase. Prothrombin bound to Staclot LA, but not to bovine brain phosphatidylethanolamine, in a calcium-dependent fashion (Table2). The presence of IgG antibodies reacting with the calcium-mediated prothrombin/Staclot LA complex was investigated by ELISA in 59 patients with lupus anticoagulants: 41 of them (69%) recognized the complex (Fig 1). The binding was strictly prothrombin and calcium dependent, because in their absence only a minority of samples reacted with Staclot LA coated on the plate (Fig 1).
Human Prothrombin and β2-Glycoprotein I Binding to Phospholipids Depends on Phospholipid Charge and Conformation
| Phospholipids . | Absorbance (mOD, 405 nm) . | ||
|---|---|---|---|
| hPT/Ca2+ . | hPT . | β2-GPI . | |
| PC | 6 | 98 | 1 |
| PS | 1,971 | 6 | 1,994 |
| Bovine brain PE | 103 | 94 | 17 |
| Staclot LA | 810 | 94 | 0 |
| Phospholipids . | Absorbance (mOD, 405 nm) . | ||
|---|---|---|---|
| hPT/Ca2+ . | hPT . | β2-GPI . | |
| PC | 6 | 98 | 1 |
| PS | 1,971 | 6 | 1,994 |
| Bovine brain PE | 103 | 94 | 17 |
| Staclot LA | 810 | 94 | 0 |
Results are expressed as mean value of two replicates. Bovine brain PE was used as a source of lamellar PE; Staclot LA was used as a source of hexagonal II PE.
Abbreviations: PC, phasphatidylcholine; PS, phosphatidylserine; PE, phosphatidylethanolamine. β2-GPI, β2-glycoprotein I; Ca2+, calcium ions; hPT, human prothrombin.
IgG antiphospholipid antibodies binding to phosphatidylethanolamine (PE). When lamellar PE (from bovine brain) was used as the solid-phase antigen in ELISA, only 5 of 59 plasma samples had an absorbance exceeding 2 SD the mean of controls. When hexagonal II PE (Staclot LA) was used as the solid phase antigen, only two samples reacted. When the solid-phase antigen in ELISA plates was represented by the calcium-mediated complex of hexagonal II PE and human prothrombin, 41 samples (69%) had an absorbance exceeding 2 SD the mean of controls. Horizontal lines represent the upper limit of 20 normal controls (ie, mean + 2 SD). *Values represent the mean ± SD of patients’ group.
IgG antiphospholipid antibodies binding to phosphatidylethanolamine (PE). When lamellar PE (from bovine brain) was used as the solid-phase antigen in ELISA, only 5 of 59 plasma samples had an absorbance exceeding 2 SD the mean of controls. When hexagonal II PE (Staclot LA) was used as the solid phase antigen, only two samples reacted. When the solid-phase antigen in ELISA plates was represented by the calcium-mediated complex of hexagonal II PE and human prothrombin, 41 samples (69%) had an absorbance exceeding 2 SD the mean of controls. Horizontal lines represent the upper limit of 20 normal controls (ie, mean + 2 SD). *Values represent the mean ± SD of patients’ group.
The general behavior of antiprothrombin antibodies in ELISA closely resembles that of anticardiolipin antibodies: these antibodies recognize β2-glycoprotein I only when bound to anionic phospholipids or to γ-irradiated polystyrene or high-activated PVC ELISA plates.11-13 The requirements for binding are probably due to the relatively low affinity of anti-cardiolipin antibodies for β2-glycoprotein I; the apparent kd ranges from 10−6 to 10−5.39,40 Kinetic studies have shown that some anticardiolipin antibodies with anticoagulant activity cause a 30- to 40-fold enhancement of β2-glycoprotein I binding to membranes containing 20% phosphatidylserine.41 Furthermore, γ-irradiated ELISA plates increase the surface density of β2-glycoprotein I about 1.5 times39 and induce its conformational change.42The antineoepitope(s) or low-affinity nature of anti–β2-glycoprotein I antibodies remains to be clarified.
Similarly, experimental evidence does not clearly establish whether and which antiprothrombin antibodies are antineoepitope(s) or low-affinity antibodies. With respect to the former possibility, human prothrombin has been shown to undergo a conformational change upon binding to phosphatidylserine-containing surfaces in the presence of calcium ions.43 However, Bajaj et al28 reported rather high values for affinity of antiprothrombin antibodies for human prothrombin—approximately 10−10 to 10−9—in lupus anticoagulant-positive patients with hypoprothrombinemia. In patients with normal antigenic levels of prothrombin one might theoretically expect lower-affinity antibodies. This was indirectly suggested by Fleck et al,30 who reported a patient whose plasma prothrombin was essentially free, not bound to IgG, despite the presence of antiprothrombin antibodies, which could be removed by repeated adsorption with insoluble prothrombin. However, the kd of this type of antibody has not yet been formally determined. Cakir et al44 reported binding of antiprothrombin antibodies to covalently cross-linked prothrombin dimers and multimers coated on an ELISA plate. Cross-linked prothrombin dimers and multimers facilitate bivalent, high-avidity binding of intrinsically low-affinity antibodies. These findings point toward the low affinity, rather than antineoepitope nature of antiprothrombin antibodies.
Antiprothrombin antibodies, like anti–β2-glycoprotein I antibodies, reduce the kd of prothrombin to an artificial anionic phospholipid surface 2.5 to 5.0 times (from 822 ± 150 nmol/L to 184 to 341 nmol/L).45 Table 3 summarizes the main properties of antiprothrombin and anticardiolipin antibodies.
Main Properties of Antiprothrombin and Anticardiolipin Antibodies
| . | Antiprothrombin Antibodies . | Anticardiolipin Antibodies . |
|---|---|---|
| Antigen | Prothrombin | β2-glycoprotein I, cardiolipin (“infectious” antibodies) |
| Epitopes | Fragment 1, prethrombin 1 | Domains 13-150 and 43-150 |
| Species-specificity | Mainly human | Human, bovine, rat, sheep |
| Affinity | Mainly low | Low |
| Lupus anticoagulant activity | Yes (a subgroup) | Yes (a subgroup) |
| Prevalence in the antiphospholipid syndrome | 50-90% (depending on antigen presentation) | 60-90% (depending on antigen presentation) |
| . | Antiprothrombin Antibodies . | Anticardiolipin Antibodies . |
|---|---|---|
| Antigen | Prothrombin | β2-glycoprotein I, cardiolipin (“infectious” antibodies) |
| Epitopes | Fragment 1, prethrombin 1 | Domains 13-150 and 43-150 |
| Species-specificity | Mainly human | Human, bovine, rat, sheep |
| Affinity | Mainly low | Low |
| Lupus anticoagulant activity | Yes (a subgroup) | Yes (a subgroup) |
| Prevalence in the antiphospholipid syndrome | 50-90% (depending on antigen presentation) | 60-90% (depending on antigen presentation) |
EPITOPE MAPPING
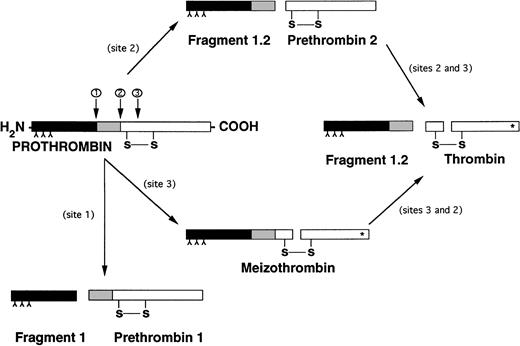
The epitope(s) recognized by antiprothrombin antibodies have not yet been fully defined. Bajaj et al28 reported two cases with lupus anticoagulants and severe hypoprothrombinemia, showing that the plasma of one patient reacted not only with prothrombin, but also with prethrombin 1 (the carboxy-terminal segment of prothrombin) and DIP-α-thrombin (the carboxy-terminal segment of prethrombin 1). No reactivity was seen against fragment 1 (the amino-terminal segment of prothrombin) or fragment 2 (the amino-terminal segment of prethrombin 1) (Fig 2).
Different pathways of prothrombin activation. (*) Indicates active site exposure. Inverted “Y” indicates γ-carboxyglutamic acid.
Different pathways of prothrombin activation. (*) Indicates active site exposure. Inverted “Y” indicates γ-carboxyglutamic acid.
Later, the binding of 14 lupus anticoagulant-positive IgG preparations to prothrombin, prethrombin 1, fragment 1, and thrombin coating ELISA plates was studied45: 11 IgG bound to prothrombin and 8 of them also to prethrombin 1 and fragment 1. None reacted with immobilized thrombin. These data were confirmed by Malia et al,46 who found that antiprothrombin antibodies reacted with prothrombin and its fragment 1-2, but not with the descarboxylated molecule. Finally, Fleck et al30 could not detect binding to purified prothrombin by Western blot, implying that denaturation of prothrombin by sodium dodecyl sulfate (SDS) disrupts essential discontinuous epitopes that are dependent on the tertiary structure of the molecule. However, these investigators did demonstrate that at least some antiprothrombin antibodies bind to epitopes that persist in prothrombin in citrated plasma, regardless of how the three-dimensional structure of prothrombin changes with the markedly reduced availability of calcium ions in citrated plasma. These findings suggest that the majority of antiprothrombin antibodies are of a poly- or oligo-clonal nature.
Because the amino-terminal region of prothrombin shares homology with other vitamin K–dependent proteins, it was suggested that antiprothrombin antibodies recognize a common epitope on this region of prothrombin as well as of protein C and protein S.18 This seems unlikely, in the light of experiments by Rao et al,45who analyzed the binding of 14 IgG fractions from lupus anticoagulant-positive patients to phosphatidylserine in the presence of prothrombin, protein C, or protein S: only prothrombin supported the binding of the IgG preparations to the anionic phospholipids. These investigators obtained similar results when the neutral phospholipid phosphatidylethanolamine was substituted for phosphatidylserine.
Puurunen et al47 reported that antiprothrombin antibodies cross-react with plasminogen in patients with myocardial infarction. Inhibition studies showed that antibody binding to prothrombin was prevented by soluble prothrombin, plasminogen, and synthetic peptides of 20 amino acids from plasminogen kringle 5 and from prothrombin kringle 2.47 This cross-reactivity was confirmed by immunizing mice with either human prothrombin or human plasminogen. All plasma samples from 16 mice immunized with prothrombin had antiprothrombin antibodies and 13 cross-reacted with plasminogen. All plasma samples from 12 mice immunized with plasminogen contained antibodies to plasminogen and 8 cross-reacted with prothrombin.48 It can be hypothesized that antiprothrombin antibodies that cross-react with plasminogen interfere with the fibrinolytic pathway.
ANTICOAGULANT PROPERTIES
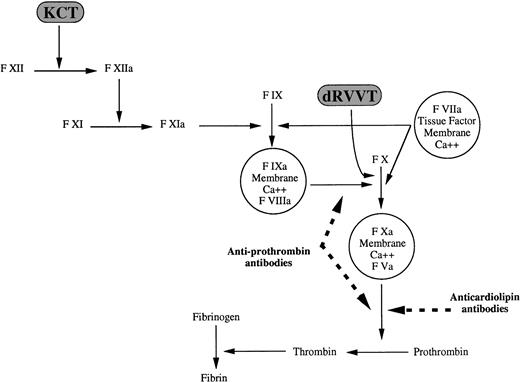
In 1991 our group characterized the lupus anticoagulant activity of two patients with phospholipid-dependent inhibitors of coagulation14: purified IgG antibodies hampered prothrombin activation by coagulation factors Xa and Va on a negatively charged phospholipid surface in the presence of calcium ions. The anticoagulant activity was exerted on human but not on bovine prothrombin and was independent of the source (human or bovine) of factors Xa and Va; anionic phospholipids were an absolute requirement for the expression of this anticoagulant activity. We concluded that antibodies directed at the prothrombin/phospholipid complex were responsible for the lupus anticoagulant activity. Antiprothrombin antibodies also hamper the conversion of factor X by coagulation factors IXa and VIII, provided prothrombin, anionic phospholipids, and calcium are present.49 A schematic representation of the sites of action of antiprothrombin and anticardiolipin, ie, anti–β2-glycoprotein I, antibodies along the coagulation cascade is shown in Fig 3. The anticoagulant activity of antiprothrombin antibodies is expressed mainly, though not exclusively, against prothrombin of human, but not animal, origin.14 49 The reason(s) for this species-specificity are not known.
Sites of action of antiprothrombin and anticardiolipin antibodies along the blood coagulation cascade: antiprothrombin antibodies inhibit the activation of factor X and prothrombin; anticardiolipin antibodies inhibit prothrombin but not factor X activation. Dashed lines indicate inhibitory effect.
Sites of action of antiprothrombin and anticardiolipin antibodies along the blood coagulation cascade: antiprothrombin antibodies inhibit the activation of factor X and prothrombin; anticardiolipin antibodies inhibit prothrombin but not factor X activation. Dashed lines indicate inhibitory effect.
Although most antiprothrombin antibodies behave in vitro as acquired phospholipid-dependent inhibitors of coagulation, some have no anticoagulant activity and can therefore be revealed only by ELISA tests, using prothrombin on high-activated PVC ELISA plates or complexed to phosphatidylserine.33,50 Again, the behavior of antiprothrombin antibodies in coagulation tests resembles that of anticardiolipin antibodies.51 So far, the reason(s) for the generation of antiphospholipid antibodies either with or without anticoagulant properties have not been explained.
Although antiprothrombin and anticardiolipin antibodies display lupus anticoagulant activity, they affect phospholipid-dependent coagulation tests differently (Table 4). The synergistic effect of antiprothrombin antibodies on two consecutive phospholipid-dependent coagulation reactions is shown mainly by overall clotting tests, such as kaolin clotting time (KCT)52 or colloidal silica clotting time (CSCT),53 which proceed through the generation of factor Xa and activation of prothrombin. Phospholipid-dependent tests like the dilute Russell’s viper venom time (dRVVT), that selectively evaluates the conversion of prothrombin to thrombin, are less sensitive to the anticoagulant activity of these antibodies.
Main Laboratory Data of 25 Patients Classified by Their Lupus Anticoagulants
| Test . | Antiprothrombin Antibodies4-150 (n = 14) . | Anticardiolipin Antibodies (n = 11) . | P . |
|---|---|---|---|
| aPTT (ratio) | 1.40 ± 0.25 | 1.52 ± 0.35 | NS |
| KPTT (ratio) | 1.30 ± 0.24 | 1.38 ± 0.30 | NS |
| KCT (ratio) | 2.24 ± 0.41 | 1.50 ± 0.42 | .0002 |
| dRVVT (ratio) | 1.48 ± 0.28 | 2.20 ± 0.42 | .0001 |
| Anticardiolipin antibodies (U) | 61 ± 55 | 147 ± 43 | .0003 |
| Test . | Antiprothrombin Antibodies4-150 (n = 14) . | Anticardiolipin Antibodies (n = 11) . | P . |
|---|---|---|---|
| aPTT (ratio) | 1.40 ± 0.25 | 1.52 ± 0.35 | NS |
| KPTT (ratio) | 1.30 ± 0.24 | 1.38 ± 0.30 | NS |
| KCT (ratio) | 2.24 ± 0.41 | 1.50 ± 0.42 | .0002 |
| dRVVT (ratio) | 1.48 ± 0.28 | 2.20 ± 0.42 | .0001 |
| Anticardiolipin antibodies (U) | 61 ± 55 | 147 ± 43 | .0003 |
Data are expressed as mean ± SD.
Abbreviations: NS, not significant; aPTT, activated partial thromboplastin time; KPTT, activated partial thromboplastin time with kaolin as activator.
The antiprothrombin nature of the 14 lupus anticoagulants was defined according to the behavior of the IgG purified from plasma following adsorption with cardiolipin-containing liposomes in coagulation tests: the Igs retained phospholipid-dependent anticoagulant activity in human but not animal plasma, irrespective of the presence of β2-glycoprotein I (see ref 14).
Data from Galli et al.52
Anticardiolipin antibodies hamper prothrombin activation in a strictly β2-glycoprotein I–dependent fashion,51 but not that of factor X54; consequently, their presence affects the dRVVT more than the KCT or other overall clotting assays.52 Our group reported that the lupus anticoagulant activity caused by antiprothrombin antibodies can be distinguished from that due to anticardiolipin antibodies by use of specific coagulation profiles generated by comparison of the ratios of the KCT and the dRVVT52: if the ratio of the KCT exceeds that of the dRVVT, it is considered a coagulation profile associated with antiprothrombin antibodies; if the relationship is reversed it is considered a “dRVVT” coagulation profile that may be associated with anticardiolipin antibodies. However, both inhibitors may simultaneously contribute to the phospholipid-dependent anticoagulant activity of individual plasmas. Indeed, their high prevalence suggests that both antiprothrombin and anticardiolipin antibodies are often present when a lupus anticoagulant is detected. This is sustained by the recent findings of Horbach et al50 in 28 patients with lupus anticoagulants: the anticoagulant activity was totally dependent on antiprothrombin or on anti–β2-glycoprotein I antibodies in four and seven cases, respectively, whereas in the majority of the plasmas (n = 17) both antibodies contributed to the phospholipid-dependent anticoagulant activity. These findings lead us to suggest that when both inhibitors are present, the stronger one is responsible for the final allocation of the plasma to either the dRVVT or the KCT coagulation profile.
CLINICAL RELEVANCE
A number of retrospective, cross-sectional and prospective clinical studies have established that the presence of lupus anticoagulants constitutes a risk factor for arterial and venous thrombosis.55 The prevalence of patients with thrombosis was found to be retrospectively associated with anticardiolipin antibodies and the related dRVVT coagulation profile rather than with antiprothrombin antibodies and the KCT profile in 25 patients with phospholipid-dependent inhibitors of coagulation52 (Table5).
Main Clinical Data of 25 Patients Classified by Their Lupus Anticoagulants
| . | Antiprothrombin Antibodies (n = 14) . | Anticardiolipin Antibodies (n = 11) . | P . |
|---|---|---|---|
| Sex, M/F | 2/12 | 6/5 | NS |
| Age (yr), median | 42 | 39 | NS |
| Autoimmune diseases | 1 (7%) | 0 | NS |
| Neoplastic diseases | 1 (7%) | 1 (9%) | NS |
| Thrombocytopenia | 5 (36%) | 3 (27%) | NS |
| Recurrent miscarriages (≥2) | 0 | 1 (20%) | NS |
| History of venous and/or arterial thrombosis | 3 (21%) | 8 (73%) | .03 |
| . | Antiprothrombin Antibodies (n = 14) . | Anticardiolipin Antibodies (n = 11) . | P . |
|---|---|---|---|
| Sex, M/F | 2/12 | 6/5 | NS |
| Age (yr), median | 42 | 39 | NS |
| Autoimmune diseases | 1 (7%) | 0 | NS |
| Neoplastic diseases | 1 (7%) | 1 (9%) | NS |
| Thrombocytopenia | 5 (36%) | 3 (27%) | NS |
| Recurrent miscarriages (≥2) | 0 | 1 (20%) | NS |
| History of venous and/or arterial thrombosis | 3 (21%) | 8 (73%) | .03 |
Abbreviation: NS, not significant.
Data from Galli et al.52
These findings have been confirmed and extended by a prospective clinical study performed on 100 patients with lupus anticoagulants classified according to their coagulation profile at diagnosis: 44 of them displayed the KCT coagulation profile, and the other 56 the dRVVT profile.56 Fourteen patients developed 18 thrombotic events during a median follow-up of 37.5 months, with an overall rate of thrombosis of 4.2% patients/yr. Twelve of them had the dRVVT coagulation profile, and the other two the KCT profile (P = .03). Compared with the KCT profile, the dRVVT coagulation profile gave an odds ratio of thrombosis of 5.25 (95% confidence interval, 1.17 to 23.50). Ten of the 14 patients who developed thrombosis during follow-up had already experienced thrombosis: a previous thrombotic event caused an odds ratio of recurrence of 2.72 (95% confidence interval, 0.85 to 8.73) (P = .09).
Therefore, the possibility of distinguishing a patient’s thrombotic risk on the basis of the coagulation profile appears clinically relevant. Care must be exercized when extrapolating these data to current clinical practice, because the reagents and techniques used in the KCT and the dRVVT may greatly influence the predictive value of the coagulation profiles. In this respect, we reported that the CSCT could be used in place of the KCT without loss of the ability to distinguish patients with lupus anticoagulants at different risk of thrombosis.53 Unlike our in-house dRVVT assay system, a commercially available dRVVT kit failed to generate coagulation profiles that identified the thrombotic risk of lupus anticoagulant-positive patients.57 Similar results were reported by Callahan et al.58 Because of the uncertainties about the reproducibility and potential clinical relevance of these coagulation profiles, the ability of several commercially available dRVVT kits to generate coagulation profiles that identify lupus anticoagulant-positive patients at increased risk of thrombosis is presently being investigated by a collaborative study.
The question whether antiprothrombin antibodies increase the risk of thromboembolic events remains unanswered. Horbach et al59studied a large population of patients with systemic lupus erythematosus, showing that IgG and IgM antiprothrombin antibodies (measured by ELISA) were risk factors for venous thrombosis (odds ratio, 2.53 and 2.72; 95% confidence intervals, 1.1 to 5.81 and 1.09 to 6.79 for IgG and IgM antibodies, respectively) but not for arterial thrombosis. However, when multivariate analysis was performed, antiprothrombin antibodies failed to increase the risk of venous thrombosis.59 Association between antiprothrombin antibodies and thrombosis in patients with systemic lupus erythematosus has been confirmed by another retrospective study by univariate analysis.60 Funke et al61 reported that IgG and IgM antibodies directed against the calcium-mediated prothrombin/phosphatidylserine complex conferred an odds ratio of 2.8 (95% confidence intervals, 1.1 to 7.6) for venous thrombosis and an odds ratio of 4.1 (95% confidence intervals, 1.6 to 10.5) for arterial thrombosis in patients suffering from systemic lupus erythematosus. In clinical conditions other than systemic lupus erythematosus a “nested” case-control study estimated that high levels of antiprothrombin antibodies gave an odds ratio of 6.54 (95% confidence intervals, 1.73 to 25.0) of deep vein thrombosis/pulmonary embolism to middle-aged men.62 Other retrospective studies failed to show that antiprothrombin antibodies represent a risk factor for thromboembolic events.18,32 33
The retrospective nature of these studies prevents from drawing definite conclusions. Only one prospective study has been performed that confirmed the association between high titers of antiprothrombin antibodies and an increased risk of developing myocardial infarction,63 which is not one of the typical features of the antiphospholipid syndrome. Therefore, more “cross-sectional” or prospective clinical studies are warranted to establish the clinical relevance of antiprothrombin antibodies.
This uncertainty also holds true at the pathophysiological level. Despite their behavior as lupus anticoagulants in coagulation tests in vitro, antiprothrombin antibodies increase thrombin generation on an endothelial cell surface45 and in a flow system.64 These findings, obtained with a very limited number of Ig samples, are probably due to the stabilizing effect of antiprothrombin antibodies on the binding of prothrombin to a phospholipid surface mentioned above and suggest that antiprothrombin antibodies with lupus anticoagulant activity have a prothrombotic effect. However, in another, rather complex experimental system,65 antiprothrombin antibodies did not show this behavior. Conflicting results have been reported on the effect of antiprothrombin antibodies on the anticoagulant activity of the protein C system. Our group showed that anticardiolipin (ie, anti–β2-glycoprotein I), but not antiprothrombin antibodies hampered the anticoagulant activity of the protein C system.66 On the other hand, Horbach et al67 found a significant impairment of protein C activity by antiprothrombin antibodies in the presence of human prothrombin.
Finally, other additional congenital or acquired factors (ie, factor V “Leiden,” prothrombin gene mutation, hyperhomocysteinemia, increased plasma levels of prothrombin, factor VIII, von Willebrand factor, and decreased protein C and protein S plasma activities) may contribute to the final thrombotic risk regardless of the type of phospholipid-dependent inhibitor of coagulation in some lupus anticoagulant-positive patients.
Regarding the other manifestations of the antiphospholipid syndrome (ie, thrombocytopenia and recurrent miscarriages), the KCT coagulation profile appeared to be retrospectively associated with an unexplained moderate thrombocytopenia (platelet count, 50 to 150 × 109/L) (P = .005).56 We cannot exclude the possibility that antiprothrombin antibodies bind to platelets and cause thrombocytopenia, but we detected antibodies directed to specific platelet glycoproteins in a proportion of patients with antiphospholipid antibodies similar to that reported for patients with idiopathic thrombocytopenic purpura.68 Moreover, antiglycoprotein, but not antiphospholipid, antibodies could be eluted from patients’ platelet. These data suggest that the cause of thrombocytopenia in patients with antiphospholipid antibodies is similar to the cause of idiopathic thrombocytopenic purpura.
Neither coagulation profiles predict the risk of recurrent miscarriages, probably because of the small number of patients with poor obstetric outcome included in the study.56 Rand et al showed that IgG fractions from patients with antiphospholipid antibodies reduced both the expression of annexin V on cultured trophoblasts69 and the binding of annexin V to anionic phospholipid bilayers, frozen thawed washed platelets, activated partial thromboplastin time reagent, and prothrombin time reagent.70 This effect was β2-glycoprotein I–dependent.70 The investigators postulated that, at least in some cases, anticardiolipin antibodies with sufficiently high affinity for β2-glycoprotein I hamper the anticoagulant effect of annexin, thus accelerating coagulation reactions on a phospholipid surface. Although attractive, this model needs further confirmation on a larger number of patients to establish whether anticardiolipin antibodies play a pathophysiological role in the development of recurrent miscarriages or fetal losses. Unfortunately, the effect of antiprothrombin antibodies in this system was not investigated.
The data suggest it is reasonable not to treat patients with antiprothrombin antibodies unless they have severe hypoprothrombinemia with bleeding. Conditions that may prompt treatment are the perioperative state and the bleeding of skin, gums, nose, and urothelium. Corticosteroids are the treatment of choice.71,72 Successful regimens consist of methylprednisolone, 30 mg/kg per day administered intravenously for 3 days, followed by prednisone, 2 mg/kg daily for 14 days72and of 1 g of cyclophosphamide administered intravenously on the first day combined with prednisone, 1 mg/kg daily for 1 month.71Patients who fail to improve have been treated with danazol,73 high-dose intravenous gammaglobulins,74 or cyclophosphamide,73 with variable success.
Treatment of the thrombotic complications of the antiphospholipid syndrome raises two questions in patients with antiprothrombin antibodies. Heparin, oral anticoagulants, or antiplatelet agents may increase the risk of bleeding caused by hypoprothrombinemia. In fact, even though the risk of thrombosis in lupus anticoagulant-positive patients with the KCT coagulation profile seems lower than with the dRVVT profile,52,56 approximately 20% of those with the KCT profile nevertheless have a history of arterial and/or venous thrombosis, with an estimated rate of thrombosis of about 1.2% patients/yr.56 Therefore, care must be exercised when administering antithrombotic drugs. The optimal duration and intensity of oral anticoagulant treatment in these patients is likely to be indicated by the WAPS (Warfarin in the Anti-Phospholipid Syndrome) study, an international randomized trial proposed under the auspices of the SSC Subcommittee for the Standardization of Lupus Anticoagulants/Phospholipid-Binding Antibodies75: patients with arterial and/or venous thrombosis in the last 5 years are randomized either to long-term, high-intensity warfarin treatment (PT International Normalized Ratio, INR, 3.0 to 4.5) or to standard treatment. The study has already recruited more than 100 patients. The estimated date for its completion is December 2000.
The other aspect relates to the monitoring of oral anticoagulation, which is still an unsolved issue in patients with antiphospholipid antibodies. Laboratory control of oral anticoagulant therapy with the PT INR might be inappropriate in lupus anticoagulant-positive patients with hypoprothrombinemia, because the INR may not reflect the true level of anticoagulation. Some groups reported widely varying PT INR values in the plasma of lupus anticoagulant-positive patients under oral anticoagulants, ranging from 2.0 up to 10.0.76-78 This is probably due to the different responsiveness of commercial thromboplastin reagents to the various phospholipid-dependent inhibitors of coagulation.79 Since the studies so far do not provide conclusive information, a multicenter cross-sectional study has been proposed in the setting of the SSC Subcommittee for the Standardization of Lupus Anticoagulants/Phospholipid-Binding Antibodies, to investigate the effect of lupus anticoagulants on the PT INR measured with the most widely used thromboplastins.
CONCLUSIONS AND FUTURE
In conclusion, antiprothrombin antibodies are frequently found in patients with antiphospholipid antibodies. Their immunological and functional properties vary widely, mainly depending on their affinity for human prothrombin. Despite increasing knowledge about their mechanism(s) of action, the clinical relevance of these antibodies has not yet been established, also because their presence has been reported in a number of conditions other than the antiphospholipid syndrome.80 Taking into account the reported association between the KCT coagulation profile (that reflects, at least in part, the in vitro anticoagulant activity of antiprothrombin antibodies) and thrombocytopenia, future work will be aimed at defining whether these antibodies play any role in the pathogenesis of this common complication of the antiphospholipid syndrome. It is even more compelling to distinguish clearly between the contributions of anticardiolipin (ie, anti–β2-glycoprotein I) and antiprothrombin antibodies in the development of arterial and venous thromboembolic events.
ACKNOWLEDGMENT
We thank Drs G. Beretta, G. Brembilla, and G. Bonandrini, and S. Marziali and C. Zanotti for their excellent technical assistance. We are also grateful to J. Baggott for editorial assistance.
REFERENCES
Author notes
Address reprint requests to Monica Galli, MD, PhD, Divisione di Ematologia, Ospedali Riuniti, L.go Barozzi, 1, 24128 Bergamo, Italy; e-mail: ematologia@cyberg.it.