Alglucerase, a macrophage-targeted enzyme replacement therapy for Gaucher disease, has been successfully used for several years to improve clinical symptoms and reverse disease progression. As part of an immunosurveillance program, 1,122 Gaucher patients were monitored for antibody response to glucocerebrosidase, the active component of alglucerase. Seroconversion was detected in 142 patients (12.8%) by enzyme-linked immunosorbent assay (ELISA) and confirmed by radioimmunoprecipitation. The majority (75%) of the seroconverted population had no detectable levels of circulating inhibitory antibody as assessed by in vitro inhibition of enzymatic activity of the therapeutic molecule. Of the remaining patients with putative inhibitory antibodies, the majority had only low levels of serum inhibitory activity, which was transient. A very small number of patients were identified as developing true neutralizing antibodies, as defined by the development of antibodies that impacted clinical efficacy. Many of the patient antibody responses were also diminished with time. Eighty-two of the 142 seroconverted patients have stopped producing antibody to the molecule and appear tolerized. The mean time for humoral tolerization was 28 months from initiation of therapy. Of 64 seroconverted patients followed for at least 30 months of therapy, the tolerization rate was 93%. These results show that although 12.8% of the patients on therapy developed antibodies to the molecule, 90% of these patients became tolerized over time.
IN 1882, PHILIP GAUCHER was the first to describe the finding of unusual large foamy cells in a patient with an epithelioma of the spleen, now recognized as a hallmark of Gaucher disease. Subsequently, lipid abnormalities, related enzymology, and autosomal recessive inheritance were described.1-4 Gaucher disease is the most prevalent lysosomal storage disease affecting 20,000 to 30,000 individuals worldwide.5 The pathology of the disease is associated with a marked accumulation of complex lipids, glucocerebrosides, in tissues in conjunction with a defective activity of the enzyme glucocerebrosidase (GCR). This results in an accumulation of glucocerebrosides in cells of the spleen, liver, bone, and infrequently, the brain, which ultimately impairs organ function.3 In 1991, the Food and Drug Administration (FDA) approved alglucerase enzyme replacement therapy for Gaucher disease. The active component consists of purified placental-derived GCR modified to expose α-mannose residues on the oligosaccharide. These modifications improved the efficiency of targeting to the mannose receptor on macrophages in the reticuloendothelial system.6 7 Imiglucerase, a replacement therapy using a recombinant form of this enzyme, was subsequently approved.
A safety issue associated with the use of any therapeutic protein is the immunogenicity of the molecule and potential development of immune-mediated complications.8 Parameters that impact a molecule’s ability to elicit an immune response include the size of the molecule, the structural or sequence difference from the native protein, as well as the dose, frequency, and route of administration. An additional consideration for patients with genetic diseases is that the endogenous molecule may be absent, mutated, or produced in a conformation that renders it nonfunctional. Consequently, the native protein could be recognized as foreign and elicit an immune response. Immunologic responses to both natural and recombinant therapeutic proteins have been reported. Serum proteins such as insulin,9 growth hormone,10,11 and Factor VIII,12 as well as cytokines such as interferon α,13 interferon-β,14 interleukin (IL)-2,15 IL-3,16 and granulocyte-macrophage colony-stimulating factor (GM-CSF)17 have been reported to induce antibody formation. Some of these proteins also elicited the development of neutralizing antibodies,11-19 which impaired the clinical efficacy of the molecule. The incidence of neutralizing antibodies can be significant. For example, the development of neutralizing antibodies varied from 30% to 50% of the patient population treated with interferon.13 20 Immunologic reactivity is a key determinant in product efficacy.
During alglucerase therapy, Gaucher patients generally received frequent infusions (often every 2 weeks) of milligram quantities of GCR. It is recommended that patients be monitored for a period of at least 18 months to assess their immunological response to therapy. The development of assays and immunosurveillance of 262 patients treated with alglucerase has been previously reported.21 These findings indicated that within the first year of therapy 13% of the patients developed antibodies to the molecule and that a trend toward humoral unresponsiveness was observed with several patients. This report describes the updated findings for over 1,100 patients treated over the course of 5 years.
MATERIALS AND METHODS
Patients
Patients with documented Gaucher disease were treated with periodic infusions of alglucerase (approximately 15 to 60 U/kg body weight, generally every 2 weeks). Blood was always drawn before the start of infusion. Samples were obtained from patients before the start of therapy (baseline) and at different times after initiation of therapy, generally at 3, 6, 9, 12, and 18 months. Serum samples were collected by centrifugation and stored at −80°C until analyzed. The majority of the samples were obtained as part of an immunosurveillance program (provided by Genzyme Corp).
Eighty-four percent (1,003 of 1,183) of patients initiating therapy with alglucerase in the Gaucher registry have been tested for antibody status. Eleven percent (113 of 1,003) of those tested were found to be antibody positive.
Though participation in the Gaucher Registry is voluntary and submission of data may be incomplete, it is reasonable to assume that data included in this analysis are representative of the population of patients treated for Gaucher disease.
Evaluation Criteria
To be included in this evaluation, patients were required to have at least one serum sample drawn at least 30 days past the first infusion of alglucerase. Patients with only a baseline sample, as well as those treated exclusively with imiglucerase, were excluded from this analysis. Samples from patients receiving alglucerase who subsequently were treated with imiglucerase were included in the study up to the time they changed therapy.
GCR
Clinical grade, unformulated, placental-derived GCR was used in all the analyses. The enzyme’s carbohydrate structure was enzymatically modified to yield a molecule with a terminal mannose residue for enhanced macrophage targeting. The GCR was further purified over a Protein A (Pierce, Rockford, IL) column, as this preparation contained small amounts of contaminating human IgG that could give false positive results in the enzyme-linked immunosorbent assay (ELISA).
Patient Immune Response to GCR
ELISA IgG response.
An ELISA developed as a screening assay for the detection of antibodies in patients’ sera has been previously described and validated.21 Briefly, 96-well microtiter plates were coated overnight with GCR followed by 2 hours blocking with human serum albumin (HSA, Baxter Healthcare, Glendale, CA) in phosphate-buffered saline (PBS). Wells were also coated with HSA because it is used as an excipient in the formulation. Plates were incubated with patient sera at a 1/100 dilution in PBS-Tween, HSA for 2 hours at 37°C. Plates were washed followed by a subsequent 1-hour incubation at 37°C with a horseradish peroxidase-labeled goat antihuman IgG Fc specific antibody (Jackson Immunoresearch, West Grove, PA). O-phenylenediamine (OPD; Sigma, St Louis, MO) was used as a substrate and the color reaction measured at 490 nm. Assay controls included three different pooled sera prepared from seronegative Gaucher patients, seroconverted Gaucher patients’ sera, and normal human sera. The upper limit of reactivity to GCR was defined as 2 standard deviations above the mean value of commercially available pooled human sera (Scantibodies Laboratory, Santee, CA). All serum samples with values above the established normal range were subsequently tested for confirmation of specific antibodies to GCR in the radioimmunoprecipitation assay.
Radioimmunoprecipitation.
A radioimmunoprecipitation (RIP) assay developed as a confirmatory assay for antibodies to GCR has been previously described.21 Briefly, GCR was iodinated using Iodobeads (Pierce) following the protocol recommended by the manufacturer. Patient sera was incubated with [I125] GCR overnight at 4°C with constant mixing. The following day Sepharose bound Protein A beads (Sigma) were added to the mixture and incubated for 1 hour. After extensive washing, the immune complex was precipitated by centrifugation, the pellet resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (sepraSol, ISS, Natick, MA), boiled, and the samples separated through a 10% separating gel (Biorad, Hercules, CA). After drying the gel, the radioimmunoprecipitate was visualized via autoradiography. Pooled sera from seroconverted patients and nonseroconverted patients, as well as pooled normal human sera, were used for controls. Once a patient had been shown to be positive for antibody to GCR by RIP, all subsequent samples from that patient were evaluated by RIP regardless of the ELISA results.
In vitro inhibition assay.
An assay to measure inhibition of in vitro enzymatic activity of the GCR enzyme was developed and qualified. Serum samples were diluted 1/10 in substrate buffer (0.01 mol/L phosphate buffer, 2.3 mmol/L sodium taurocholate, 0.15% Triton X-100, 0.02% sodium azide, pH 5.9). A twofold dilution series of the sera was incubated overnight at 4°C with GCR (17.5 mU diluted from the stock solution in substrate buffer; one unit is defined as the amount of enzyme required to hydrolyze 1 μmol of substrate in 1 minute at 37°C). The GCR activity was determined by subsequently incubating a 1/10 dilution of the mixture (in substrate buffer) with a 6.6-mmol/L solution of pNP-β-glucopyranoside (pNP, Sigma) in substrate buffer for 2 hours at 37°C with shaking. Enzymatic activity was interpolated from a standard curve (3.5 to 0.3 mU) of GCR. Serum inhibition was expressed as percentage of activity remaining relative to the control GCR incubated without serum. Pooled normal human sera, pooled sera from Gaucher patients with inhibitory activity, and pooled sera from Gaucher patients without inhibitory activity were included as controls. During qualification of this assay, it was established that 10% of normal human sera and pretreatment Gaucher patients’ sera inhibited 10% to 20% of enzyme activity. Therefore, levels of up to 20% of inhibition were considered nonspecific.
Adverse event testing.
In addition to the methods described above, patients who experienced hypersensitivity symptoms during treatment had their sera further analyzed for the presence of serum tryptase, C3 products, and development of IgE to GCR. These assays were performed as described previously.21
Clinical Response Parameters
Four clinical response parameters: platelet counts, hemoglobin, liver volume, and spleen volume (expressed as multiple of normal organ volumes) were examined to identify statistically significant differences in response between patients with different antibody status.
Statistical Analysis
Test of differences in frequency of category of disease type, genotype, and dose frequency were performed by Pearson χ2 test.
Comparison of response trends were conducted for patients who were antibody negative versus those who were antibody positive and those who had inhibitory antibodies. Using a weighted analysis of variance, groups were compared to determine whether there were differences in responses achieved over time on enzyme therapy in any of the four clinical parameters. The group comparisons included antibody positive versus antibody negative patients, patients with inhibitory antibodies versus those without inhibitory antibodies, and patients with inhibitory antibodies versus those without antibodies. Patients included in these group analyses had intact spleen at the start of therapy. In addition, evaluation time points were excluded following periods of interruption in enzyme therapy of greater than 3 months.
RESULTS
Seroconversion
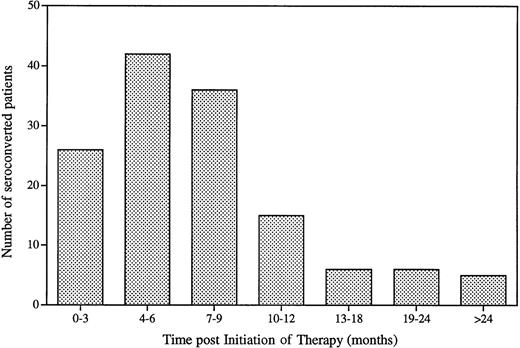
A patient was considered to have developed a circulating immune response to GCR (ie, seroconverted) if both the ELISA and the RIP assay were positive. GCR-specific circulating antibodies were not detected in any of the patients before the start of therapy. Of the 1,122 evaluable patients (Table 1), a total of 142 patients (12.8%) seroconverted while on therapy. These patients’ sera all had above normal range ELISA values, and the presence of GCR-specific antibody was subsequently confirmed by RIP. The time course of seroconversion is shown in Fig 1. Most patients (109 of 142) seroconverted within 3 to 9 months after initiation of therapy. The median time to seroconversion was 6 months with a mean time of 8.2 months. The majority (87.5%) of the patients seroconverted within the first year of therapy. Seroconversion occurring more than 12 months after initiation of therapy may actually have occurred earlier, as serum samples drawn within the first year of therapy were not always available for analysis. None of the patients in this study developed antibodies to HSA, an excipient used in the alglucerase formulation.
Seroconversion and Adverse Events for Gaucher Patients Treated With Alglucerase
| Total no. of patients with serum sample tested | 1,430 |
| Patients with at least one serum sample beyond baseline | 1,122 |
| No. of patients seroconverted while on therapy | 142 |
| % of seroconverted patients | 12.65 |
| No. of patients with reported adverse events | 74 |
| No. of seroconverted patients with reported adverse events | 34 |
| Total no. of patients with serum sample tested | 1,430 |
| Patients with at least one serum sample beyond baseline | 1,122 |
| No. of patients seroconverted while on therapy | 142 |
| % of seroconverted patients | 12.65 |
| No. of patients with reported adverse events | 74 |
| No. of seroconverted patients with reported adverse events | 34 |
Immunosurveillance of Gaucher patients treated with alglucerase. Summary of occurrence of seroconversion and immune-mediated adverse events of patients evaluated.
Time to seroconversion for Gaucher patients treated with alglucerase. Sera from patients with at least one sample beyond initiation of therapy were analyzed by ELISA and RIP. A patient was considered seroconverted if a positive ELISA result was confirmed by RIP.
Time to seroconversion for Gaucher patients treated with alglucerase. Sera from patients with at least one sample beyond initiation of therapy were analyzed by ELISA and RIP. A patient was considered seroconverted if a positive ELISA result was confirmed by RIP.
A statistical analysis was performed to determine whether the disease type, genotype, or dose frequency had any impact on seroconversion. The majority of both antibody positive and antibody negative groups reported type I disease (935 patients). The distribution of disease types did not differ between those with antibodies and those without (χ2, P = .37).
Patients who developed antibodies were as likely to have the N370S allele as those who did not develop antibodies (χ2,P = .71). The same was true for the L444P allele (χ2, P = .23). Additionally, N370S homozygoity was equally prevalent in both the antibody positive and antibody negative groups (χ2, P = .28). Regarding dose frequency, every 2 weeks was the most common initial dose frequency (81%) among registry patients who were tested for antibodies. Those who developed antibodies were as likely to be treated at this frequency as any other (χ2, P = .60).
Inhibitory Antibodies
The presence of inhibitory antibodies was evaluated by the ability of patient’s serum to interfere with the in vitro enzymatic activity of GCR using pNP as a substrate. The patient’s sera were incubated overnight with GCR having a known enzymatic activity. The percent inhibition represents the loss of activity due to antibody binding. This assay does not discriminate between inhibitory and neutralizing antibodies, ie, an antibody binding the catalytic site of GCR and/or an antibody causing steric hindrance versus an antibody that interferes with the clinical effect of the therapeutic molecule. Validation of this assay demonstrated that the sera of normal individuals, as well as pretreatment sera of the Gaucher population, inhibited GCR enzymatic activity at levels ranging from 0% to 20%. Only patients who were found to have seroconverted were tested for potential presence of inhibitory antibodies.
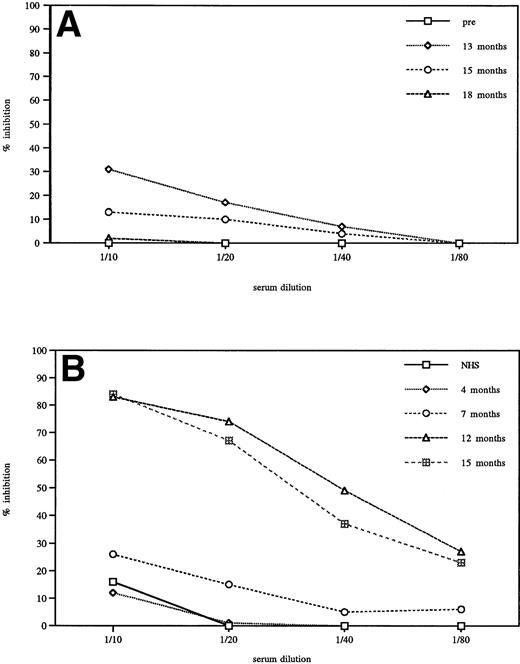
No inhibitory activity was detected in sera of most seroconverted patients (75%). Sera from an additional 15% of the seroconverted patients inhibited GCR enzymatic activity by <50%. This low level of inhibition was generally transient in these patients. This finding is illustrated in Fig 2A. Sera from one patient representative of the group with low levels of inhibition were serially diluted (1/10 to 1/80) and analyzed for inhibition of GCR activity. A small level of inhibition was detected at 13 months after initiation of therapy. By 15 months, it was below 20% and not detectable at 18 months. Only 10% of the patients (1.2% of the total tested population) who seroconverted were found to have antibodies that could inhibit more than 50% of the in vitro activity of the enzyme. Figure 2B illustrates inhibition results for a patient representative of that group. It is significant to note that the presence of inhibitory antibodies, as determined by the in vitro assay, did not always correlate with a decrease in clinical efficacy. A review of these individual cases indicated that antibodies may have had an impact on less than 1% of the treated population. Very few patients required a specific change in therapy due to the presence of inhibitory antibodies and therefore developed true neutralizing antibodies. One of these patients had type III Gaucher disease. Three patients with neutralizing antibodies have been recently described.22 23Interestingly, analysis of a recent serum sample of one of the described patients showed a decrease in reactivity to the molecule and disappearance of neutralizing antibodies.
Titration of in vitro inhibition of GCR enzymatic activity by sera of seroconverted Gaucher patients. (A) Representative patient sera showing <50% in vitro inhibitory activity. Transient inhibition was detected when pretreatment, 13, 15, and 18 months posttreatment sera were compared. (B) Patient sera showing >50% inhibition, comparison to normal human serum (NHS) and different time points in therapy.
Titration of in vitro inhibition of GCR enzymatic activity by sera of seroconverted Gaucher patients. (A) Representative patient sera showing <50% in vitro inhibitory activity. Transient inhibition was detected when pretreatment, 13, 15, and 18 months posttreatment sera were compared. (B) Patient sera showing >50% inhibition, comparison to normal human serum (NHS) and different time points in therapy.
Clinical Analysis
Clinical data regarding hematologic and organ volume response were available for review through the Gaucher Registry for a substantial number of patients who had demonstrated in vitro inhibition of enzyme. Fifteen patients who exhibited >50% in vitro inhibition were examined. Three of these patients were receiving immunosuppressive therapy to manage the inhibitory antibodies and were clearly thought by their physicians to have had either inadequate clinical responses or deterioration of clinical responses. Eight of the remaining patients had sufficient data for evaluation (Table2). For these patients, the mean time to detection of inhibitory antibodies was 11 months with a range of 3 to 19 months. The mean time to detection of initial IgG antibodies was 6 months with a range of 3 to 12 months. Three of these eight patients tolerized after 59, 17, and 17 months, respectively. Two of the eight patients showed a deterioration of clinical response in the parameters available. One of these patients experienced a decreased response in platelet count. However, the resulting value was only slightly below normal. Of important note, this patient’s dose was reduced 50% 2 to 3 months after development of inhibitory antibodies; this dose reduction may have also played a role in the decreased response. The second patient who showed a deterioration in response had only platelet counts available for assessment. This patient’s platelet count decreased 31% to 97,000/mm3. The patient’s dose of enzyme was reported to have been increased after the development of inhibitory antibodies and the platelet count subsequently improved.
Individual Clinical Responses Pre- and Postconversion in High Titer Patients (n = 8)
| Patient . | Spleen . | Time to a/b (mo)* . | Treatment History† . | Hemoglobin . | Platelet . | Acid Phos . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B . | C . | P . | B . | C . | P . | B . | C . | P . | ||||
| A | Intact | 7 | 120, ↓60 @ 17 mo | 11.5 | 12.8 | 13.6 | 61 | 155 | 142 | 17.9 | 6.2 | 5.3 |
| B | Total Splenectomy | 14 | 120, ↓60 @ 17 mo ↑ 117 @ 40 mo | 14.6 | 15.2 | 14.4 | 163 | 243 | 144 | 17.1 | 8.9 | 9.4 |
| C | Intact | 9 | 120 × 29 mo | 9.9 | 12.0 | 13.4 | 21 | 22 | 37 | 13.3 | 6.8 | 5.4 |
| D | Intact | 10 | 120,‡ 100 @ 9 mo | 11.8 | 12.4 | 12.3 | 107 | 139 | 150 | 7.6 | 4.3 | 4.4 |
| E | Intact | 12 | 120, ↓60 @ 11 mo | 11.6 | 14.2 | 13.4 | 97 | 142 | 141 | 1.5 | 0.7 | 0.7 |
| F | Intact | 3 | 320 × 14 mo2-153 | 5.3 | 11.0 | 12.9 | 53 | 97 | 143 | NA | ||
| G | Intact | 19 | 120, ↓60 @ 4 mo2-155 | 10.2 | 9.2 | 10.7 | 61 | 90 | 87 | 88.7 | 70.3 | 74.1 |
| H | Intact | 10 | 120, ↑180 @ 14 mo2-154 | NA | 61 | 140 | 97 | NA | ||||
| Patient . | Spleen . | Time to a/b (mo)* . | Treatment History† . | Hemoglobin . | Platelet . | Acid Phos . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B . | C . | P . | B . | C . | P . | B . | C . | P . | ||||
| A | Intact | 7 | 120, ↓60 @ 17 mo | 11.5 | 12.8 | 13.6 | 61 | 155 | 142 | 17.9 | 6.2 | 5.3 |
| B | Total Splenectomy | 14 | 120, ↓60 @ 17 mo ↑ 117 @ 40 mo | 14.6 | 15.2 | 14.4 | 163 | 243 | 144 | 17.1 | 8.9 | 9.4 |
| C | Intact | 9 | 120 × 29 mo | 9.9 | 12.0 | 13.4 | 21 | 22 | 37 | 13.3 | 6.8 | 5.4 |
| D | Intact | 10 | 120,‡ 100 @ 9 mo | 11.8 | 12.4 | 12.3 | 107 | 139 | 150 | 7.6 | 4.3 | 4.4 |
| E | Intact | 12 | 120, ↓60 @ 11 mo | 11.6 | 14.2 | 13.4 | 97 | 142 | 141 | 1.5 | 0.7 | 0.7 |
| F | Intact | 3 | 320 × 14 mo2-153 | 5.3 | 11.0 | 12.9 | 53 | 97 | 143 | NA | ||
| G | Intact | 19 | 120, ↓60 @ 4 mo2-155 | 10.2 | 9.2 | 10.7 | 61 | 90 | 87 | 88.7 | 70.3 | 74.1 |
| H | Intact | 10 | 120, ↑180 @ 14 mo2-154 | NA | 61 | 140 | 97 | NA | ||||
| Patient . | Liver Volume . | Spleen Volume . | Bone . | ||||
|---|---|---|---|---|---|---|---|
| B . | C . | P . | B . | C . | P . | ||
| A | NA | NA | NA | ||||
| B | NA | NA | ↑Uptake at the 16-month evaluation | ||||
| C | 1.8x | 1.11 | 1.17 | 23.6x | 18.6x | 12.5x | NA |
| D | NA | NA | Baseline: R hip infarct and pain. | ||||
| MRI improved and bone pain resolved at 18-month evaluation. | |||||||
| E | 1.17x | 1.04x | 0.9x | 6.8x | 4.49x | 3.9x | NA |
| F | NA | NA | NA | ||||
| G | NA | Improved by PE | NA | ||||
| H | NA | NA | NA | ||||
| Patient . | Liver Volume . | Spleen Volume . | Bone . | ||||
|---|---|---|---|---|---|---|---|
| B . | C . | P . | B . | C . | P . | ||
| A | NA | NA | NA | ||||
| B | NA | NA | ↑Uptake at the 16-month evaluation | ||||
| C | 1.8x | 1.11 | 1.17 | 23.6x | 18.6x | 12.5x | NA |
| D | NA | NA | Baseline: R hip infarct and pain. | ||||
| MRI improved and bone pain resolved at 18-month evaluation. | |||||||
| E | 1.17x | 1.04x | 0.9x | 6.8x | 4.49x | 3.9x | NA |
| F | NA | NA | NA | ||||
| G | NA | Improved by PE | NA | ||||
| H | NA | NA | NA | ||||
BCP denotes Baseline (B), Conversion (C), and Postchange (P) values. Postvalues are 4 to 8 months postconversion, unless otherwise indicated.
Abbreviations: NA, not applicable; PE, physical exam.
Time to detection of inhibiting antibodies.
Treatment history indicates initial dose in U/kg/4 weeks followed by dose adjustment and timing of dose adjustment.
Patient (D) had a dose interruption for 1 month after 4 months of ERT. On resuming therapy, the patient received 34 to 80 U/kg/4 weeks during months 6 to 8. The dose was then increased to 100 U/kg/4 weeks. Spleen volume was 3 cm below costal margin at pretreatment. After 19 months on ERT, spleen was nonpalpable. Bone involvement improved on MRI and bone pain resolved.
Patient F has Type III disease.
Patient G interrupted therapy after 5 months on ERT. The interruption lasted for 8 months. The dose at the time of therapy resumption was 60 × 2.
Patient H has platelet count data available only. Patient refuses other evaluations. Dose increase at 14 months was reported to be due to inadequate response.
Of the 22 patients who had low levels (<50%) of inhibitory antibodies detected, there were 12 for whom sufficient data was available to evaluate the impact of these antibodies on clinical response. The mean time to detection of inhibitory antibodies for the 12 evaluable patients was 9 months with a range of 3 to 17 months (median, 8 months). Nine of these 12 patients tolerized after a mean time of 24 months on therapy with a range of 7 to 53 months (median, 20 months). Only one of the 12 patients evaluated appeared to have clearly not maintained responses in the clinical parameters after the development of inhibitory antibodies. Two additional patients had variable clinical responses, which are somewhat difficult to interpret.
Four clinical response parameters, two laboratory parameters (percent change in platelet count [mm3]) and absolute change in hemoglobin [g/dL]), and two visceral organ parameters (percent change in liver volume [multiples of normal (MN)] and percent change in spleen volume [MN]) were examined to identify statistically significant differences in clinical response between patients with different antibody status. Registry patients on alglucerase therapy with an intact spleen at baseline and known antibody status were included in the analysis. Data was restricted to that collected while on alglucerase therapy and prior to any interruption in therapy of more than 3 months. Organ volume multiples of normal refer to normal volume predicted for body weight,24 approximately 2.5% and 0.2% of body weight for liver and spleen, respectively.
Two comparisons were performed: antibody negative patients (n = 406) versus those who were antibody positive (n = 60) and antibody negative patients (n = 406) versus those antibody positive patients with inhibitory antibodies (n = 20). Two sample t-test was used to compare baseline values of clinical response parameters. To compare response over time in each of the clinical response parameters, the slope of change from baseline for each patient was determined by simple linear regression. Both percent and absolute changes were examined. The differences between the slopes of groups differing in antibody status were assessed by weighted analysis of variance (ANOVA), weighted by the number of observations within patient contributing to the slope.
There were no statistically significant differences in any of the four clinical response parameters at baseline within either the two groupings (two-sample t-test, all P > .05).
Comparisons of clinical responses of patients with antibodies (n = 60) to patients without antibodies (n = 406) showed no significant differences in platelet count response (% change mm3;P = .13), hemoglobin response (absolute change g/dL;P = .58), liver volume (% change MN; P = .48), spleen volume (% change MN; P = .32). Clinical responses over time for patients with inhibitory antibodies (n = 20) were not different compared with those patients without antibodies (n = 406) in any of the four parameters: platelet count response (% change MN; P = .22), hemoglobin response (absolute change g/dL; P = .78), liver volume (% change MN; P= .75) spleen volume (% change MN; P = .59). No meaningful differences were observed in mean clinical responses between antibody positive and antibody negative patients when examined as groups. Patients with inhibitory antibodies achieved only slightly lesser responses at 12 and/or 18 months that were not clinically different from patients with noninhibitory antibodies or antibody-negative patients. The slight differences were not observed at later time intervals when the number of patients with observations was sufficient for conclusions, ie, greater than three. Using a weighted ANOVA, no significant differences were observed between responses in any of the group comparisons (P > .05).
In summary, the presence of inhibitory antibodies in Gaucher patients treated with alglucerase does not necessarily denote reduced effectiveness of therapy. Rather inhibition of clinical effectiveness was restricted to a small fraction of patients, particularly those with inhibition of >50% in the assay described.
Immunologically Related Adverse Events
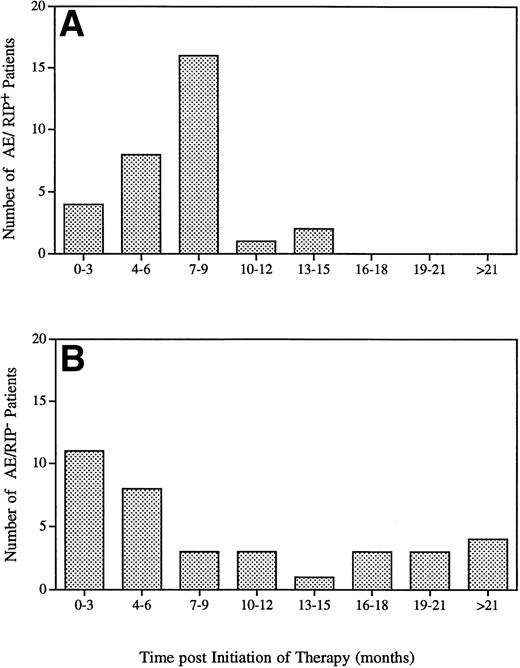
Seventy-four patients (about 5% of the total treated population) reported symptoms suggestive of immediate hypersensitivity during the time of infusion or shortly thereafter (Table 1). The majority of these patients had mild cutaneous symptoms, which included urticeria, flushing, and pruritis. All patients recovered quickly and without sequalea. Patients were able to continue treatment and their symptoms were managed by slowing the rate of infusion as well as administration of antihistamines. Patients were tested for the presence of IgG or IgE specific to GCR. While 34 of the 74 patients who experienced symptoms of hypersensitivity developed IgG-specific antibodies to GCR, only one patient was found to have IgE antibodies (0.06% of the total treated population). Most of the adverse events occurred during the first year of therapy (Fig 3). A difference was observed between the time of onset of an adverse event and patient antibody status. Patients who seroconverted generally experienced adverse events within the first 14 months of therapy, with a median time of 7 months and a mean of 6.6 months (Fig 3A). Nonseroconverted patients experienced adverse events at different times ranging from the day of first infusion up to 48 months poststart of therapy with a median time of 6 months and a mean of 10.9 months (Fig 3B).
Time to adverse event for Gaucher patients treated with alglucerase in seroconverted patients (A) and in nonseroconverted patients (B).
Time to adverse event for Gaucher patients treated with alglucerase in seroconverted patients (A) and in nonseroconverted patients (B).
Humoral Tolerization
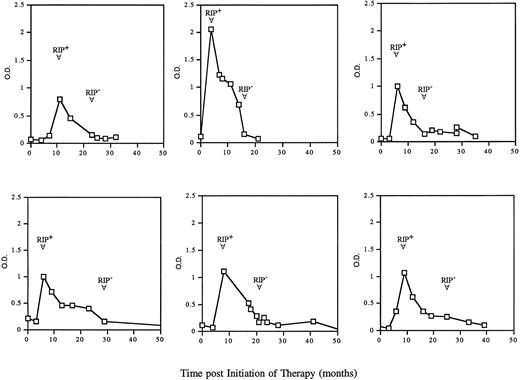
After seroconversion, many patients exhibited an increase in the levels of antibodies to GCR, as demonstrated by an increase in ELISA absorbency values and a sustained or increased RIP signal. However, with ongoing treatment, a gradual decrease in the assay signals was observed. Figure 4 is a composite of six representative patients on therapy. This decrease in reactivity was initially manifested by a reduction in intensity in the ELISA, followed by a subsequent decrease in intensity of the RIP signal. Over time, the patients’ serologic reactivity to GCR was no longer detectable and the patients were therefore considered tolerized. Generally, patients were considered tolerized if after seroconversion, both the ELISA and the RIP assay were negative for two consecutive samples.
ELISA reactivity and tolerization to GCR in six representative seroconverted patients. Sera from individual patients were analyzed during their course of therapy by ELISA and RIP. The arrows indicate the time of seroconversion (RIP+) and the time of tolerization (RIP-). A patient was considered seroconverted if a positive ELISA result was confirmed by RIP and tolerized if after seroconversion, both ELISA and RIP of subsequent sera samples were negative.
ELISA reactivity and tolerization to GCR in six representative seroconverted patients. Sera from individual patients were analyzed during their course of therapy by ELISA and RIP. The arrows indicate the time of seroconversion (RIP+) and the time of tolerization (RIP-). A patient was considered seroconverted if a positive ELISA result was confirmed by RIP and tolerized if after seroconversion, both ELISA and RIP of subsequent sera samples were negative.
Humoral tolerization was evaluated in patients treated for at least 1 year (Table 3). Of the 122 seroconverted patients followed for at least 1 year from the start of therapy, 82 were found to be subsequently negative in both the ELISA and the RIP and were therefore considered tolerized. The median time to tolerization was 24 months, while the mean time was 28 months from start of therapy. Because not all seroconverted patients had samples tested for extended periods beyond the initiation of therapy, seroconverted patients with samples beyond 30 months of therapy (past the mean time of tolerization) were analyzed. Of the 142 seroconverted patients, 69 had samples beyond 30 months of therapy, and 64 of those patients (93%) were tolerized to the therapeutic molecule. Five patients were still showing positive ELISA and RIP reactivity. Two patients receiving immunochemotherapy were excluded from this analysis because this treatment inherently affected their ability to produce antibodies.
Tolerization for Gaucher Patients Treated With Alglucerase
| Time Postinitiation of Therapy . | Tolerized . | Nontolerized . | % Tolerized . |
|---|---|---|---|
| >12 months therapy (n = 122) | 82 | 40 | 62.7 |
| >30 months therapy (n = 69) | 64 | 5 | 93 |
| Time Postinitiation of Therapy . | Tolerized . | Nontolerized . | % Tolerized . |
|---|---|---|---|
| >12 months therapy (n = 122) | 82 | 40 | 62.7 |
| >30 months therapy (n = 69) | 64 | 5 | 93 |
A decrease and ultimately absence of antibody to GCR (tolerization) was noted in seroconverted patients on prolonged treatment with alglucerase. A patient was considered tolerized if after seroconversion, both ELISA and RIP of subsequent sera samples were negative.
DISCUSSION
The use of alglucerase enzyme therapy for Gaucher disease has proven to be efficacious in treating this genetic disorder. Visceromegaly or hematologic abnormalities were arrested in most patients with concomitant improvement in quality of life. Bone involvement, although less understood, also can be improved with enzyme therapy.25 An ongoing concern with any protein therapeutic is the immunologic response to the treatment molecule. We previously reported in a study of 262 patients21 that approximately 13% of the Gaucher patient population developed an antibody response to GCR, which became diminished with time in some patients. This report describes our immunologic findings from 1,122 treated patients, confirming our previous results that only a relatively small percentage of patients (12.8%) produce antibodies to the therapeutic molecule. This percentage appears to be lower than what has been reported in the literature for other therapeutic molecules. Interferon alpha given subcutaneously in chronic hepatitis patients resulted in 50% of the patients developing antibodies,20 while given intramuscularly to metastatic renal carcinoma patients, it resulted in 63% of the population developing antibodies.13 The general incidence of antibody production for insulin given subcutaneously was reported as 20%.9 However, a study administering insulin intraperitoneally indicated that 40% of the patients had antibodies in serum within 1 year of treatment,26 emphasizing the importance of route of administration. Experience with growth hormone has been varied primarily due to differences in the source and purity of the molecule. However, an incidence of up to 60% of seroconversion has been documented.27 Recently, it has been reported that administration of a genetically engineered humanized anti-tumor necrosis factor (TNF) antibody gave rise to specific antibodies in 30% of the patients.28
The lower incidence of antibody production to GCR may reflect several parameters related to immunogenicity. Of significance is the fact that the enzyme is generally not absent in Gaucher patients, merely present in a nonactive mutated form. Second, many therapies are given subcutaneously and thereby come in contact with antigen-presenting cells. Alglucerase is given intravenously over a period of hours, and the enzyme gets readily distributed to target tissues. Last, because the molecule is targeted to the mannose receptor on macrophages, these cells do not “see” the molecule in the context of major histocompatability complex (MHC) presentation and may more readily recognize GCR as a self-antigen.
As more protein therapeutics are becoming available, reports in the literature citing the development of antibodies that can neutralize the efficacy of the protein are more common. Some of the first reports of inhibitory antibodies were for Factor VIII replacement therapy. The prevalence has been reported at 14%, with the most severely affected patients developing predominantly IgG4 subclass.12 To overcome those inhibitory antibodies, immunosuppressive therapy, combined with a high dose of factor VIII, has been the treatment of choice. More recently, it has been reported that recombinant staphylokinase given to patients with myocardial infarctions can rapidly induce neutralizing antibodies, even within 2 weeks of treatment.29 The development of neutralizing antibodies have also been reported in cytokine therapy. Injections of IL-3, given subcutaneously or intravenously, resulted in 50% of the animals developing neutralizing antibodies.16 Similar results have been reported for IL-2 in humans, although it has been suggested that the use of the natural molecule was not inhibited by the antibodies raised to the recombinant molecule.15 Extensive studies performed with subcutaneous injections of interferon alpha or beta, reported that 30% to 50% of the patients develop neutralizing antibodies.13,18 20
The development of neutralizing antibodies to GCR has been rare, in contrast, most of the antibodies elicited to GCR were not inhibitory. Only a small percentage of the seroconverted patients had transient low inhibitory antibodies, the nature of which is not clear. Of the patients who did have higher levels of in vitro inhibition, the majority did not have this inhibitory activity correlate with deleterious effects on clinical efficacy. These patients may have developed a polyclonal response that clearly inhibited the molecule’s in vitro enzymatic activity, yet were clinically irrelevant. Because the enzyme must be active within the lysosomes of macrophages, it may be that serum binding is not relevant. Antibodies may, in fact, enhance the uptake of the enzyme via Fc receptors. Very few patients had their clinical response deleteriously effected by the development of antibodies to GCR and clearly developed neutralizing antibodies. One of these patients stopped therapy, while the other three patients22 23 were treated with immunosuppressive regimens, but continued receiving enzyme therapy due to deterioration of their condition. This very low incidence of clinically relevant neutralization stands noticeably different from the experience with Factor VIII.
An interesting trend described in our previous study was the development of humoral tolerance with continued treatment. Our current study confirmed this finding, that with time, the antibody response to GCR is not sustained. The serologic response became abrogated despite the fact these patients continue to be infused with enzyme at the same dose and frequency. These findings suggest that patients developed a state of B-cell unresponsiveness toward the therapeutic molecule. This observation has also been reported with other therapies. Immunologic tolerization was reported for interferon alpha treatment in hairy cell leukemia.30 After a median of 14.5 months, nonneutralizing antibody patients became seronegative. Within 10 months of the development of neutralizing antibodies, 50% of those patients became nonneutralizing and another 30% became seronegative.30With alglucerase treatment, 80% of the patients developed humoral tolerance to the molecule within 2 years of treatment and 93% within 30 months. In addition, most of the antibodies detected were not neutralizing, differentiating the reactivity to GCR from that reported generally for other protein therapies.
In conclusion, this evaluation demonstrated that alglucerase therapy does not cause major immunological consequences for Gaucher patients. The molecule is not immunogenic in the majority of the patients. In a small percentage (12.8%) of the treated population, patients seroconvert, but became tolerized with time. In general, the presence of antibodies does not affect the efficacy of the therapy. Of the 74 patients experiencing putative immune-related adverse events, only 34 were found to have developed IgG specific to GCR, and only one patient has developed IgE antibodies to GCR. This incidence was considerably lower than what has been reported for many other protein therapies. The mechanism by which nonseroconverted patients manifest immune-mediated symptoms is unclear, but could be related to release of other factors such as cytokines. This study supports the rationale that continued treatment of patients who have developed antibodies is warranted.
ACKNOWLEDGMENT
The authors wish to thank N. Moore and M. Hesselton for their excellent technical assistance and J. Angell for statistical analysis. We are also grateful to the participating ICGG registry patients and physicians as well as the staff for patient information required to analyze this retrospective study and to J. McPherson and R. Moscicki for critically reviewing this manuscript.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Mireille Rosenberg, PhD, Genzyme Corp, 1 Mountain Rd, Framingham MA 01701-9322.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal