Bitiscetin has recently been shown to induce von Willebrand factor (vWF)-dependent aggregation of fixed platelets (Hamako J, et al,Biochem Biophys Res Commun 226:273, 1996). We have purified bitiscetin from Bitis arietans venom and investigated the mechanism whereby it promotes a form of vWF that is reactive with platelets. In the presence of bitiscetin, vWF binds to platelets in a dose-dependent and saturable manner. The binding of vWF to platelets involves glycoprotein (GP) Ib because it was totally blocked by monoclonal antibody (MoAb) 6D1 directed towards the vWF-binding site of GPIb. The binding also involves the GPIb-binding site of vWF located on the A1 domain because it was inhibited by MoAb to vWF whose epitopes are within this domain and that block binding of vWF to platelets induced by ristocetin or botrocetin. However, in contrast to ristocetin or botrocetin, the binding site of bitiscetin does not reside within the A1 domain but within the A3 domain of vWF. Thus, among a series of vWF fragments, 125I-bitiscetin only binds to those that overlap the A3 domain, ie, SpIII (amino acid [aa] 1-1365), SpI (aa 911-1365), and rvWF-A3 domain (aa 920-1111). It does not bind to SpII corresponding to the C-terminal part of vWF subunit (aa 1366-2050) nor to the 39/34/kD dispase species (aa 480-718) or T116 (aa 449-728) overlapping the A1 domain. In addition, bitiscetin that does not bind to DeltaA3-rvWF (deleted between aa 910-1113) has no binding site ouside the A3 domain. The localization of the binding site of bitiscetin within the A3 domain was further supported by showing that MoAb to vWF, which are specific for this domain and block the interaction between vWF and collagen, are potent inhibitors of the binding of bitiscetin to vWF and consequently of the bitiscetin-induced binding of vWF to platelets. Thus, our data support the hypothesis that an interaction between the A1 and A3 domains exists that may play a role in the function of vWF by regulating the ability of the A1 domain to bind to platelet GPIb.
VON WILLEBRAND FACTOR (vWF) is a multimeric glycoprotein (GP) that plays a key role, under high-shear conditions, in the initial attachment of platelets to the extracellular matrix of the endothelial cells after injury of the vessel wall. In this mechanism, vWF acts as a molecular bridge between constituents of the subendothelium, like collagen, and receptors of the platelet membrane, primarily the GPIb. In vivo, plasma vWF has no affinity for platelet GPIb. The binding of vWF to the latter can be initiated by high-shear conditions1 that contribute to a shape change of the vWF molecule and to its interaction with GPIb.2-6 In vitro, the binding of vWF to GPIb can be promoted by the interaction of vWF with nonphysiological inducers like the antibiotic ristocetin or the snake venom proteins botrocetin or bitiscetin.7
vWF is composed of a linear arrangement of disulfide-linked identical 275 kD subunits. Each subunit possesses 2,050 amino acid (aa) residues and appears as a mosaic protein containing five types of structural domains (A to D and CK), present in a single or multiple copies and showing structural homology with domains identified in several other protein families.8-14 vWF subunit contains three adjacent copies of the A domain. The A1 and A3 domains are essential for the expression of its biological function. Each of them forms a large loop of 185 aa closed by a disulfide bond between Cys 509 and 695 and Cys 923 and 1109. The A1 domain contains the binding sites to GPIb and numerous studies have been helpful in understanding the role of the individual aa or sequences of the loop on the regulation of the binding.15-26 The A1 loop is a positively charged region with negatively charged flanking sequences and the binding of vWF to GPIb appears to involve an electrostatic phenomenon with the interaction of positive charges of vWF with negative charges of GPIb and of botrocetin, whereas ristocetin was shown to interact with the negatively charged flanking regions.
In contrast to the A1 loop, the A3 loop is a negatively charged region. It contains the main site of vWF binding to type-I or type-III collagens.27-30 This site has been localized within the C-terminal part of the loop (aa 1018-1114).30 In addition, the crystal structure of the A3 domain has been determined.31 However, the absence of significant effect of vWF binding to collagen on platelet adhesion under static conditions32 and the fact that high shear stress alone can promote vWF-dependent aggregation of platelets prevent any direct analysis of the role of the vWF-collagen interaction in the capacity of vWF to bind to GPIb.
Bitiscetin, a protein isolated from the venom of the snake Bitis arietans, has recently been identified as a potent inducer of vWF-dependent platelet agglutination.7 This snake venom protein has been shown to act by binding to vWF. However, in contrast to negatively charged botrocetin, bitiscetin appears as a positively charged protein, thus suggesting a distinct pathway for promoting platelet agglutination. To further investigate the mechanism regulating the GPIb-binding activity of vWF we used purified bitiscetin and established that it binds to A3 domain, thereby inducing a conformational change of the A1 loop that allows vWF binding to GPIb.
MATERIALS AND METHODS
Fixed human platelets.
Fixed platelets were prepared from fresh human blood as previously described.19 Blood was collected from healthy volunteers into one-tenth volume of 3.8% sodium citrate. Platelet-rich plasma was prepared by centrifugation of blood for 10 minutes at 100g at 22°C. Platelets were fixed with paraformaldehyde as described.33
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot.
SDS-PAGE was performed according to the method of Laemmli34using vertical slab gels. Molecular weight (MW) markers were low and high MW standards from Pharmacia (Uppsala, Sweden). After migration the proteins were stained with Coomassie blue or electrotransfered onto nitrocellulose paper.
Electrotransfer of proteins from SDS-PAGE was performed using nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany) according to the method of Towbin et al.35 After transfer, the membranes were saturated with 2% skim milk (Merck, Damstadt, Germany) in 25 mmol/L Tris-HCl buffer, 0.15mol/L NaCl, pH 7.4 (TBS), before incubation with the selected ligands in TBS containing 1% skim milk, for 18 hours at 22°C. After extensive washing, the bound radioactivity was displayed by autoradiography.
Purification of bitiscetin.
Bitiscetin was purified from Bitis arietans venom (Sigma, St Louis, MO; batch 13H0760) essentially as described by Hamako et al7 using a series of chromatographies onto Phenyl-Superose columns, Superose columns, and monoS FPLC columns (Pharmacia). Bitiscetin activity was followed by testing the capacity of the eluted fractions to induce binding of purified 125I-vWF to fixed platelets. Bitiscetin was identified on SDS (3.5% to 20% polyacrylamide gradient) gels by comparing Coomassie blue staining of the gels and autoradiography performed after electrotransfer onto nitrocellulose sheets, incubation with purified vWF (10 μg/mL), and staining with immunopurified 125I-polyclonal antibody raised in a goat and directed against vWF.
Purification of botrocetin.
Two-chain botrocetin was purified from Bothrops jararaca venom (Sigma; batch 40H0911) as previously reported.36
Radiolabeling of proteins.
Purified vWF, bitiscetin, antibodies were labeled using Na125I (Amersham, Les Ulis, France) and Iodo-Gen (Pierce Chemical Co, Rockford, IL) according to the method of Fraker and Speck.37 Specific radioactivities varied from 8 to 15 μCi/μg for bitiscetin, 1 to 2 μCi/μg for vWF, and were 4 μCi/μg for IgG.
Recombinant vWF (rvVWF).
Three rvWF were tested in this study. Expression vectors for wild-type rvWF (WT–rvWF) and mutant rvWFLys 875 were constructed and characterized as previously described.38 Both rvWF were expressed in COS-7 cell cultures after electroporation39 of the appropriate plasmid DNA (10 μg) mixed with training DNA (50 μg), using an electric shock (280 V, 1,500 μF, ∞ Ohm). Cells were cultured for 24 hours in MCDB-105 medium (Sigma) containing 10% fetal calf serum and 1% antibiotics, and 48 hours in serum-free medium. rvWF was produced in conditioned medium at the level of ≠0.1 U/mL vWFAg. It was concentrated to ≠1 U/mL vWFAg using Aquacid II (MW 500 000, Calbiochem) and dialyzed against 5 mmol/L Tris-HCl buffer, pH 8. WT-rvWF and rvWFLys875 exhibited a normal multimeric structure and normal binding to platelets in the presence of ristocetin or botrocetin and to purified botrocetin.36
Delta A3-vWF and the A3 domain of vWF were gifts from Professor J.J. Sixma and Dr E.G. Huizinga (Department of Haematology, University Hospital Utrecht, Utrecht, The Netherlands). Delta A3-vWF was expressed in a BHK cell line overexpressing furin. Its characterization was performed by Lankhof et al.29 Delta A3-vWF was deleted of the A3 domain (aa 910-1113). This recombinant vWF also exhibited a normal distribution of multimers, a normal binding to platelets when induced by ristocetin or botrocetin but a strikingly decreased capacity to bind to collagen as compared with WT-vWF. The A3 domain of vWF (aa 920-1111) was expressed in Escherichia coli. Its purification and characterization were performed by Huizinga et al.31
Purification of vWF and its proteolytic fragments.
Human vWF was purified from outdated high-purity vWF concentrates kindly donated by LFB (Les Ulis, France) essentially as previously described.19
The three fragments SpI (monomer, aa 911-1365), SpII (dimer, aa 1366-2050), and SpIII (dimer, aa 1-1365) were produced by digestion of purified vWF with Staphyloccocus aureus V8 protease (Miles, Paris, France) and purified as previously described.40,41The monomeric 39/34 kD species (aa 480-718) overlapping the A1 domain of vWF was prepared by digestion of purified vWF with dispase (Boehringer, Meylan, France) and purified according to the method of Andrews et al.42 The T116 fragment (dimer, aa 449-728) was produced by digestion of purified vWF by trypsin (Sigma) and isolated using affinity chromatography on heparin coupled to Sepharose.
MoAb.
A series of 10 murine MoAb to vWF were used as IgG fractions in this study.43
MoAb 701, 710, and 724 were raised by immunization with the 39/34 kD dispase fragment. MoAb 701 blocks the interaction between GPIb and vWF induced by either ristocetin or botrocetin. MoAb 710, whose epitope is localized between Ser 593 and Ser 678, blocks the vWF-GPIb interaction when induced by ristocetin but not by botrocetin. MoAb 724 is directed against a conformational epitope of the A1 loop.44 It inhibits the binding of sulfatides, botrocetin, and heparin to vWF as well as botrocetin- but not ristocetin-induced binding of vWF to platelets. MoAb 724 does not inhibit vWF binding to collagen but exhibits a strikingly decreased affinity for the vWF-collagen complex in comparison with free vWF. In addition, MoAb 724 promotes shear-dependent platelet aggregation.45
MoAb 322 blocks the binding of vWF to platelets in the presence of ristocetin but not of botrocetin.19 It recognizes an epitope of one of the flanking regions of the A1 loop of vWF.46
MoAbs 201, 400, 505, and 535 are directed against fragment SpI (aa 911-1365) overlapping the A3 domain of vWF. Each of these antibodies blocks the binding of vWF to collagen.27
MoAbs 454 and 487 are directed against fragment SpII (aa 1366-2050). They have no known effect on the functions of vWF.
MoAb 6D1, a gift from Dr B.S. Coller (State University of New York, Stony Brook, NY) directed against GPIb47 and that blocks ristocetin-and botrocetin-induced binding of vWF to platelets, was used as a control of specificity of the interactions.
Synthetic peptides.
Five peptides derived from the sequence of human vWF were synthesized by the method of Merrifield et al48 and purified by high-performance liquid chromatography using a reverse-phase system. Peptides Cys 474 to Pro 488 (CQEPGGLVVPPTDAP) and Lys 569 to Gln 583 (KDRKRPSELRRIASQ) were from Neosystem Laboratoire, Strasbourg, France. Peptides Lys 494 to Arg 511 (LYVEDISEPPLHDFYCSR), Gln 628 to Pro 655 (QRMSRNFVRYVQGLKKKKVIVIPVGIGP) and Ser 692 to Pro 708 (SYLCDLAPEAPPPTLPP) were a gift from Dr J. Diaz (Sanofi Recherche, Montpellier, France). Peptides had free N- and C-terminus. The thiol group of Cys 474, 509, and 695 was S-carboxyaminomethylated.
Binding of 125I-vWF to fixed platelets in the presence of bitiscetin and its inhibition by MoAb.
Binding isotherm of 125I-vWF to fixed platelets was performed essentially as previously described41 using various concentrations of 125I-vWF (0 to 25 μg/mL), 108 cells /mL, and 2 μg/mL of purified bitiscetin as inducer. After 30 minutes at 22°C, aliquots of the reaction mixture (100 μL) were layered at the top of 400 μL conical tubes containing 250 μL of 25% sucrose. Bound and free radioactivity was separated by centrifugation at 12,000g for 3 minutes and cutting the tips of the tubes containing the platelet pellets and was counted. Nonspecific binding was estimated under identical conditions but in the absence of bitiscetin. Binding isotherm and binding parameters (Kd and number of binding sites) were derived from the best fitted Langmuir hyperbole calculated from the experimental data by nonlinear, least-squares, regression analysis using the computer-assisted program Kaleidagraph (Synergy Software, Reading, PA).
Inhibition of bitiscetin-induced binding of 125I-vWF to fixed platelets by MoAb was performed as described19 using 2 μg/mL of purified bitiscetin as inducer, 108 cells/mL, and 125I-vWF (≠1 μg/mL final concentration) premixed with various concentrations of competitor. Nonspecific binding was estimated under the same conditions but in the absence of inducer. After 30 minutes at 22°C the bound and free radioactive material were separated as described above and counted. Results were expressed as the percent of the specific binding estimated in the absence of competitor.
Localization of the bitiscetin-binding domain on vWF subunit.
In a first set of experiments the localization of the vWF binding domain of bitiscetin was performed by SDS-PAGE (3.5% to 20% polyacrylamide gradient gel) and electroblotting of proteolytic or recombinant vWF fragments, followed by binding of125I-bitiscetin (≠106 cpm/mL in TBS containing 1% skim milk and NaN3) to the nitrocellulose membrane. Purified fragments were loaded on the gel at 5 μg/well. In parallel analysis, purified fragments were loaded at 10 μg/well and shown by Coomassie blue staining.
In another set of experiments, purified vWF or its proteolytic or recombinant fragments (10 μg/mL) in TBS were immobilized by coating onto wells of polyvinyl chloride microtiter plates (Dynatech, Marnes-La-Coquette, France) by incubating 200 μL/well overnight at 4°C. Negative control was performed by incubating buffer alone. After postcoating for 30 minutes at 22°C with TBS containing 2% bovine serum albumin (BSA fraction V, Calbiochem, La Jolla, CA), 100 μL/well of various concentrations of purified125I-bitiscetin (specific radioactivity ≠20 × 106 cpm/μg) in TBS containing 1% BSA were incubated for 5 hours at 37°C. After washing with buffer, bound radioactive material was counted.
In some experiments, to compare the material bound to immobilized vWF and the starting material, 125I-bitiscetin bound to coated vWF was extracted after washing by incubating wells with 100 μL of 1% SDS in 0.125 mol/L Tris-HCl buffer, pH 6.8. The radioactive material was compared with the starting labeled material by SDS-15% PAGE followed by autoradiography.
The localization of the binding site of bitiscetin on the vWF subunit was also analyzed by comparing the binding of125I-bitiscetin to recombinant wild type and to recombinant Delta A3-vWF immobilized onto a MoAb to vWF. For this purpose, coating of MoAb 454 (10 μg/mL in 0.05 mol/L sodium carbonate/bicarbonate buffer, pH 9.6) was performed by incubating 200 μL of the solution in wells of polyvinyl chloride microtiter plates for 18 hours at 4°C. After postcoating with 2% BSA in TBS for 30 minutes at 22°C and washing, buffer or serial dilutions (in the range of 0.004 to 0.5 U vWFAg/mL) of WT-rvWF, Delta A3-vWF, or rvWFLys 875 as control, in TBS containing 1% BSA (100 μL/well) were incubated for 18 hours at 37°C. After washing with TBS, a constant amount of125I-bitiscetin in TBS containing 0.1% BSA (≠2 × 105 cpm, 100 μL/well) was incubated for 5 hours at 37°C. After washing, the bound radioactivity was counted. In parallel experiments the amount of immobilized vWF was estimated by incubating 125I-MoAb 487 (≠30,000 cpm, 100 μL/well) instead of 125I-bitiscetin. Results were expressed as bound bitiscetin as a function of bound 125I-MoAb 487. Negative control was performed using dilutions of conditioned medium.
Inhibition of 125I-bitiscetin binding to immobilized vWF by MoAbs, botrocetin, bitiscetin, and peptides.
Purified vWF was immobilized on wells of microtiter plates by coating as described above. After postcoating with BSA, a constant amount of125I-bitiscetin (2 × 105 cpm, 100 μL/well) premixed with various concentrations of competitor was incubated for 5 hours at 37°C. After washing, the bound radioactivity was counted. Nonspecific binding was estimated in the presence of competitor but in the absence of immobilized vWF. Results are expressed as percent of specific binding (100%) obtained in the absence of competitor. Competitors were MoAb anti-vWF, or anti-GpIb in the range of 0 to 30 μg/mL (final concentration), peptides in the range of 0 to 2 mg/mL, purified botrocetin, or purified bitiscetin (0 to 20 μg/mL).
Inhibition of 125I-vWF binding to fibrillar human type-III collagen by bitiscetin or botrocetin.
Human type-III collagen fibrils were prepared from lyophilized human nonfibrillar type-III collagen (Sigma) as previously described27 by dissolving in 0.1 mol/L acetic acid and extensive dialysis against 20 mmol/L Na2HPO4, pH 7.5. Binding of 125I-vWF to type-III collagen and inhibition of binding by bitiscetin was performed by incubating the suspension of fibrillar collagen (1 mg/mL), 125I-vWF (1 μg/mL), and various concentrations of bitiscetin or botrocetin (0 to 100 μg/mL) at 22°C for 30 minutes. Aliquots of the reaction mixture (100 μL) were layered at the top of 400 μL conical tubes containing 250 μL of 25% sucrose. Bound and free radioactivity was separated by centrifugation at 12,000g for 3 minutes and cutting the tips of the tubes containing the collagen pellets and was counted. Nonspecific binding was performed using denatured collagen, heated at 80°C for 1 hour. Results are expressed as the percent of specific binding (100%) obtained in the absence of competitor.
RESULTS
Characterization of purified bitiscetin and vWF-bound 125I- bitiscetin by SDS-PAGE.
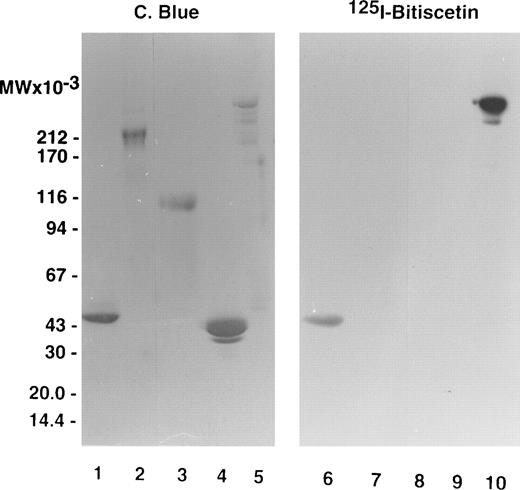
Figure 1 compares labeled purified bitiscetin and bound material extracted from immobilized vWF analyzed by SDS-15% PAGE. When unreduced, purified bitiscetin migrated as a major single band at 29 kD. A faint band was also observed at 18 kD. Analysis of the material bound to immobilized vWF showed that only the major 29 kD band reacted with vWF. Under reducing conditions, labeled purified bitiscetin appears as a major single band at 15 kD that had the same mobility as the single band observed in the bound material after reduction. Thus, in our hands bitiscetin appeared as a dimeric protein composed of chains of identical molecular weight.
Autoradiograph of labeled purified bitiscetin and vWF-bound protein after SDS-(15%) PAGE. Labeled material was treated either nonreduced (lanes 1 and 2) or after reduction (lanes 3 and 4). Purified labeled bitiscetin (≠50,000 cpm/lane) was analyzed unreduced (lane 1) or after reduction (lane 3). 125I-bitiscetin bound to immobilized vWF was extracted by incubating with 1% SDS, 0.125 mol/LTris-HCl buffer, pH 6.8. Recovered radioactivity (200,000 cpm) corresponding to 10 incubation wells were loaded (100,000 cpm/lane) either unreduced (lane 2) or after reduction (lane 4). Each labeled material was mixed with ≠1 μg/lane of cold bitiscetin as carrier. Position of the molecular weight markers is indicated on the left.
Autoradiograph of labeled purified bitiscetin and vWF-bound protein after SDS-(15%) PAGE. Labeled material was treated either nonreduced (lanes 1 and 2) or after reduction (lanes 3 and 4). Purified labeled bitiscetin (≠50,000 cpm/lane) was analyzed unreduced (lane 1) or after reduction (lane 3). 125I-bitiscetin bound to immobilized vWF was extracted by incubating with 1% SDS, 0.125 mol/LTris-HCl buffer, pH 6.8. Recovered radioactivity (200,000 cpm) corresponding to 10 incubation wells were loaded (100,000 cpm/lane) either unreduced (lane 2) or after reduction (lane 4). Each labeled material was mixed with ≠1 μg/lane of cold bitiscetin as carrier. Position of the molecular weight markers is indicated on the left.
Binding isotherm of 125I-vWF to platelets in the presence of bitiscetin.
The concentrations of purified bitiscetin that induced maximum binding of vWF to fixed platelets were determined at room temperature using 108 cells /mL, 1 μg/mL of 125I-vWF, and increasing concentrations of bitiscetin (0 to 30 μg/mL). Maximal binding (B/T ≠ 30%) was reached at 2 μg/mL (not shown). This concentration was chosen for the binding assays of 125I-vWF to fixed platelets.
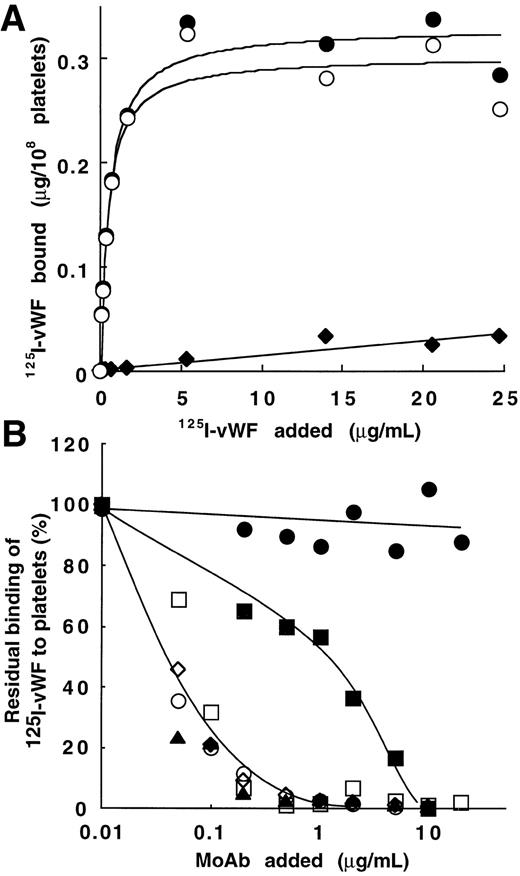
Figure 2A shows binding data for one representative experiment with various concentrations of125I-vWF (1.4 × 106 cpm/μg). Results are interpretable as saturable binding to platelets in the presence of bitiscetin. Total binding increased from 0.055 to 0.33 μg/108 platelets when vWF concentration increased from 0.01 to 25 μg/mL. Nonspecific binding linearly increased to 0.03 μg/108 platelets at the highest concentration of vWF. Parameters of binding were derived from the best-fitted Langmuir hyperbole for the specific binding (correlation coefficient, r = 0.98). The maximal binding was 0.30 μg/108 platelets and the apparent Kd was 0.45 μg/mL (ie, Kd = 1.6 × 10-9mol/L assuming a molecular mass of 275 kD for the vWF subunit).
Bitiscetin-induced binding of 125I-vWF to platelets and its inhibition by MoAb directed against the A1 domain of vWF or against the GPIb-binding site of vWF. (A) The binding of125I-vWF (0.1 to 25 μg/mL) to fixed-platelets (108/mL) in the presence of 2 μg/mL of bitiscetin was quantified as described in Materials and Methods, using a labeled preparation of vWF with a specific radioactivity of 1.4 × 106 cpm/μg. Nonspecific binding estimated in the absence of bitiscetin (⧫) was substracted from total binding in the presence of stimulus (•) to derive the specific binding (○). Binding isotherm and binding parameters were estimated by fitting the data of the specific binding with a nonlinear least-square, regression analysis. (B) Inhibition of the bitiscetin (2 μg/mL)-induced binding of 125I-vWF (1 μg/mL) to fixed platelets (108/mL) by MoAbs (0.01 to 20 μg/mL). Nonspecific binding estimated in the absence of inducer was substracted. Results are expressed as percent of the specific binding measured in the absence of competitor (100%). This value of specific binding represents a bound radioactivity of ≠30% of the total. The added MoAb were MoAb 701 (▴), 710 (◊), 724 (○), 322 (▪) and 6D1 (□). MoAb 454 (•) was used as a control.
Bitiscetin-induced binding of 125I-vWF to platelets and its inhibition by MoAb directed against the A1 domain of vWF or against the GPIb-binding site of vWF. (A) The binding of125I-vWF (0.1 to 25 μg/mL) to fixed-platelets (108/mL) in the presence of 2 μg/mL of bitiscetin was quantified as described in Materials and Methods, using a labeled preparation of vWF with a specific radioactivity of 1.4 × 106 cpm/μg. Nonspecific binding estimated in the absence of bitiscetin (⧫) was substracted from total binding in the presence of stimulus (•) to derive the specific binding (○). Binding isotherm and binding parameters were estimated by fitting the data of the specific binding with a nonlinear least-square, regression analysis. (B) Inhibition of the bitiscetin (2 μg/mL)-induced binding of 125I-vWF (1 μg/mL) to fixed platelets (108/mL) by MoAbs (0.01 to 20 μg/mL). Nonspecific binding estimated in the absence of inducer was substracted. Results are expressed as percent of the specific binding measured in the absence of competitor (100%). This value of specific binding represents a bound radioactivity of ≠30% of the total. The added MoAb were MoAb 701 (▴), 710 (◊), 724 (○), 322 (▪) and 6D1 (□). MoAb 454 (•) was used as a control.
Bitiscetin-induced binding of vWF to platelets involves the interaction of GPIb with the A1 domain.
Figure 2B shows the effect of MoAb 701, 710, 724, and 322 directed against the A1 domain of vWF and of MoAb 6D1 against the vWF-binding domain on GPIb, upon the bitiscetin-induced binding of125I-vWF to platelets. Binding of 125I-vWF to platelets was inhibited in a similar way by MoAbs 701, 710, 724, and 6D1. It was totally inhibited at a concentration of 1 μg/mL and 50% displacement required ≠ 0.05 μg/mL. MoAb 322 appeared as a less-potent inhibitor with a 50% displacement reached at ≠ 1 μg/mL and a total inhibition at ≠ 10 μg/mL. As a control, MoAb 454 directed against the C-terminal part of vWF had no effect on the binding.
Localization of the bitiscetin-binding site on the A3 domain of vWF.
The staining of purified fragments of vWF by125I-bitiscetin after SDS-PAGE and electroblotting is shown on Fig 3. 125I-bitiscetin recognized SpIII and SpI. In contrast, no binding site of bitiscetin was detected on fragments overlapping only the A1 domain, like the 39/34 kD dispase and the T116 species or the C-terminal portion of the vWF subunit (SpII).
SDS-(3.5% to 20% gradient) PAGE of purified fragments produced by proteolysis of vWF. (Left panel) Fragments were loaded at 10 μg/well. After migration the gel was stained using Coomassie blue. (Right panel) The fragments were loaded at 5 μg/well. After migration the proteins were electrotransfered on a nitrocellulose sheet, stained by incubating with 125I-purified bitiscetin, and showed by autoradiography. Fragments were SpI (lanes 1 and 6); SpII (lanes 2 and 7); T116 (lanes 3 and 8); 39/34 kD (lanes 4 and 9); and SpIII (lanes 5 and 10).
SDS-(3.5% to 20% gradient) PAGE of purified fragments produced by proteolysis of vWF. (Left panel) Fragments were loaded at 10 μg/well. After migration the gel was stained using Coomassie blue. (Right panel) The fragments were loaded at 5 μg/well. After migration the proteins were electrotransfered on a nitrocellulose sheet, stained by incubating with 125I-purified bitiscetin, and showed by autoradiography. Fragments were SpI (lanes 1 and 6); SpII (lanes 2 and 7); T116 (lanes 3 and 8); 39/34 kD (lanes 4 and 9); and SpIII (lanes 5 and 10).
These results were confirmed using purified vWF, proteolytic fragments (SpIII, SpII, SpI, 39/34 kD, and T116 fragments) or the recombinant A3 domain immobilized on microtiter plates and incubated with increasing concentrations of 125I-bitiscetin. A specific binding that linearly increased as a function of bitiscetin concentration was observed using immobilized vWF and SpIII fragments (not shown), SpI fragment, or the A3 domain (Fig 4). Binding observed in the presence of the other immobilized fragments was not distinguishable from the nonspecific binding estimated using buffer. However, comparison of the ratio of the bound to total125I-bitiscetin shows that the relative affinity of the ligand for the vWF (B/T ≠ 10%) is significantly higher than for SpIII (B/T ≠ 4%) whereas it is similarly low for SpI or the recombinant A3 domain (≠ 0.5%). In contrast, as shown on Fig 3, SpI was still detectable after SDS denaturation and western blotting whereas the A3 domain was no longer detectable under similar conditions (not shown). Thus, our data suggest an effect of the conformation of the binding site for its recognition by bitiscetin.
Binding of 125I-bitiscetin to SpI (○), SpII (•) and to the recombinant A3 domain (▪) coated on plastic.
Binding of 125I-bitiscetin to SpI (○), SpII (•) and to the recombinant A3 domain (▪) coated on plastic.
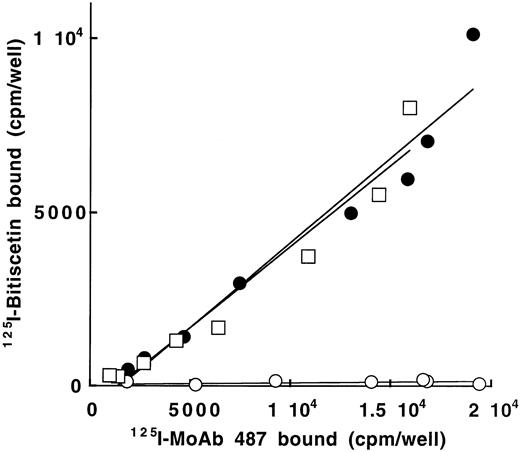
To confirm that bitiscetin recognized a binding site within the A3 domain and to establish that it had no other binding site ouside this domain, we tested the reactivity of 125I-bitiscetin with various concentrations of WT-rvWF or Delta A3-vWF immobilized onto MoAb 454. rvWFLys875 was used as control. In a parallel experiment the amount of immobilized vWF was estimated using 125I-MoAb 487. As shown in Fig 5, even though the bound 125I-MoAb 487 (≠ 1,800 to 19,000 cpm/well) indicated that a similar amount of the three rvWFs was immobilized, the binding of 125I-bitiscetin to Delta A3-vWF was similar to the nonspecific binding (conditioned medium). In contrast, the dose-response curves for binding of biticetin linearly increased with the amount of immobilized WT-rvWF and rvWFLys875, reaching ≠104 cpm/well and 8,000 cpm/well at the highest concentration, respectively.
Binding of 125I-bitiscetin to various concentrations of recombinant vWF immobilized onto MoAb 454. The amount of immobilized rvWF was estimated in parallel experiments using125I-MoAb 487. Recombinant vWF was WT-rvWF (•); rvWFLys 875 (□) and Delta A3-vWF (○).
Binding of 125I-bitiscetin to various concentrations of recombinant vWF immobilized onto MoAb 454. The amount of immobilized rvWF was estimated in parallel experiments using125I-MoAb 487. Recombinant vWF was WT-rvWF (•); rvWFLys 875 (□) and Delta A3-vWF (○).
Inhibition of 125I-bitiscetin binding to immobilized vWF by MoAb, botrocetin, bitiscetin, and peptides.
The localization of the bitiscetin binding site on the A3 domain of vWF was further confirmed by experiments of inhibition of125I-bitiscetin binding to immobilized vWF by MoAb.
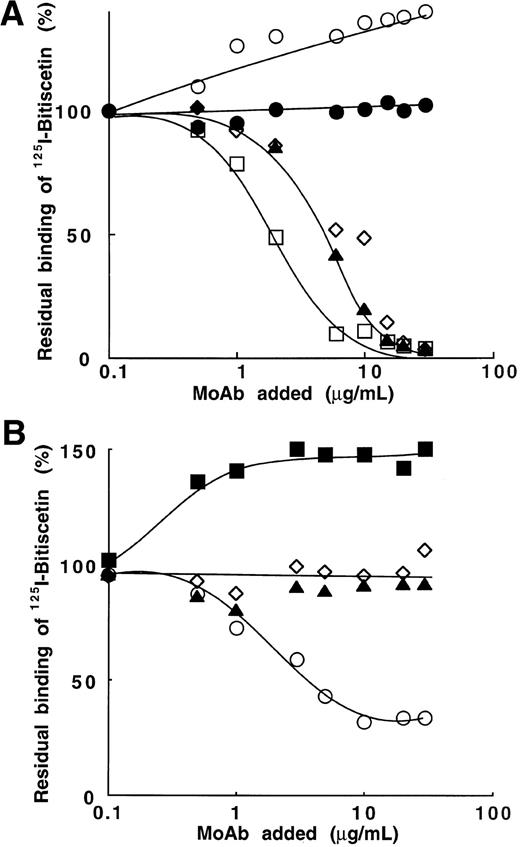
Four MoAbs (201, 400, 505, and 535) directed against the A3 domain (SpI fragment) and that blocked the vWF binding to collagen were tested. Figure 6A shows that three of them (MoAb 201, 400, and 535) totally blocked binding of125I-bitiscetin to vWF at the concentration of ≠10 μg/mL. 50% inhibition was reached at the concentrations of 1.8 μg/mL for MoAb 535 and 4.4 μg/mL for MoAb 201 and 400, respectively. In contrast, the occupancy of epitope 505 appeared to significantly increase (40%) the binding of125I-bitiscetin to vWF. MoAb 454 used as control had no effect on the binding.
Inhibition of the binding of 125I-bitiscetin to immobilized vWF by MoAb directed against vWF. Purified vWF (10 μg/mL) was coated onto wells of microtiter plates.125I-bitiscetin (2 × 105 cpm, 100 μL/well) premixed with various concentrations of competitor was then incubated. After washing, the bound radioactivity was counted. Nonspecific binding was estimated using buffer instead of vWF for coating. Results are expressed as the percent of the specific binding measured in the absence of competitor (100%). This value of specific binding represents a bound radioactivity of ≠15% of the total. (A) MoAb directed against the A3 domain of vWF: MoAb 201 (◊); 400 (▴); 505 (○); 535 (□). MoAb 454 (•) was used as control. (B) MoAb directed against the A1 domain of vWF: MoAb 701(▴); 710 (◊); 724 (○); 322 (▪).
Inhibition of the binding of 125I-bitiscetin to immobilized vWF by MoAb directed against vWF. Purified vWF (10 μg/mL) was coated onto wells of microtiter plates.125I-bitiscetin (2 × 105 cpm, 100 μL/well) premixed with various concentrations of competitor was then incubated. After washing, the bound radioactivity was counted. Nonspecific binding was estimated using buffer instead of vWF for coating. Results are expressed as the percent of the specific binding measured in the absence of competitor (100%). This value of specific binding represents a bound radioactivity of ≠15% of the total. (A) MoAb directed against the A3 domain of vWF: MoAb 201 (◊); 400 (▴); 505 (○); 535 (□). MoAb 454 (•) was used as control. (B) MoAb directed against the A1 domain of vWF: MoAb 701(▴); 710 (◊); 724 (○); 322 (▪).
As a control, the effect of MoAb 201, 400, 505, and 535 (0.01 to 10 μg/mL) was also tested on the bitiscetin-induced binding of125I-vWF to platelets (not shown). As expected this binding was totally abolished by MoAb 201, 400, and 535 but at concentrations much lower than those required to abolish 125I-bitiscetin binding to immobilized vWF (ie, total inhibition at ≠1 μg/mL and 50% inhibition at ≠0.1 μg/mL). It thus appears that the affinity of these MoAb is conformation dependent and higher for vWF in solution than for vWF immobilized on plastic. MoAb 505 did not significantly decrease the binding of 125I-vWF to platelets.
In contrast with the effect observed using MoAb directed to the A3 domain of vWF, MoAb 701 and 710, directed to the A1 domain and blocking bitiscetin-induced binding of 125I-vWF to platelets, had no effect on the interaction of 125I-bitiscetin with vWF (Fig6B). MoAb 322 increased by 50% the binding of125I-bitiscetin. In contrast, MoAb 724 partially blocked this binding. Thus, it appears that an interaction occurred between the A1 and A3 domains of vWF, which can be influenced by modifying the conformation of either domain.
To further investigate the role of selected sequences of the A1 domain on the binding of bitiscetin to the A3 domain we tested the effect of peptides in experiments of inhibition. Among the six peptides tested, Fig 7A shows that only two of them significantly inhibited 125I-bitiscetin binding to vWF: peptide Gln 628 to Pro 655, which belongs to the predominantly basic region of the A1 loop, and peptide Cys 474 to Pro 488, previously involved in the binding of vWF to GPIb. Other peptides (Fig 6A), Lys 569 to Gln 583, Lys 494 to Arg 511, and Ser 692 to Pro 708, had almost no effect on the binding.
Inhibition of the binding of 125I-bitiscetin to immobilized vWF by peptides, bitiscetin, or botrocetin. The method was that described in the legend of Fig 6. (A) peptides aa 474-488 (▴); aa 494-511 (○); aa 569-583 (•); aa 628-655 (▪), and aa 692-708 (◊). (B) botrocetin (▪) and bitiscetin (○).
Inhibition of the binding of 125I-bitiscetin to immobilized vWF by peptides, bitiscetin, or botrocetin. The method was that described in the legend of Fig 6. (A) peptides aa 474-488 (▴); aa 494-511 (○); aa 569-583 (•); aa 628-655 (▪), and aa 692-708 (◊). (B) botrocetin (▪) and bitiscetin (○).
Similarly (Fig 7B), the binding of botrocetin to the A1 domain had only a low effect on the binding of bitiscetin in the range of concentrations tested (0.1 to 20 μg/mL). The residual binding remained higher than 70% of the binding estimated in the absence of competitor. In contrast, bitiscetin totally abolished the binding of125I-bitiscetin at the concentration of 10 μg/mL.
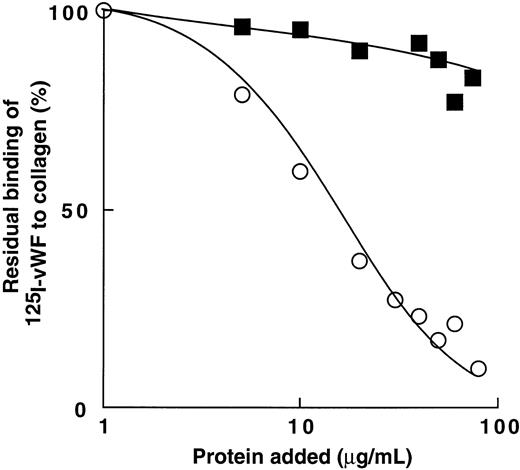
Inhibition of 125I-vWF binding to human type-III fibrillar collagen by bitiscetin or botrocetin.
Figure 8 shows that bitiscetin blocked the interaction of vWF with collagen in a dose-dependent fashion. Binding of 125I-vWF to collagen was totally inhibited by increasing the concentration of bitiscetin to 100 μg/mL and 50% inhibition was reached at the concentration of 42 μg/mL of bitiscetin. In contrast, increasing the concentration of botrocetin had no significant effect on the binding of 125I-vWF to collagen.
Inhibition of binding of 125I-vWF to human fibrillar type-III collagen by bitiscetin (○) or botrocetin (▪). Collagen was used at the final concentration of 1 mg/mL and125I-vWF at 1 μg/mL. Nonspecific binding estimated using denatured heated collagen was substracted from the total binding to obtain specific binding. Results are expressed as the percent of the specific binding estimated in the absence of competitor (100%). This value of specific binding represents a bound radioactivity of ≠40%.
Inhibition of binding of 125I-vWF to human fibrillar type-III collagen by bitiscetin (○) or botrocetin (▪). Collagen was used at the final concentration of 1 mg/mL and125I-vWF at 1 μg/mL. Nonspecific binding estimated using denatured heated collagen was substracted from the total binding to obtain specific binding. Results are expressed as the percent of the specific binding estimated in the absence of competitor (100%). This value of specific binding represents a bound radioactivity of ≠40%.
DISCUSSION
Bitiscetin purified from Bitis arietans venom was recently identified as a new inducer of vWF-dependent platelet agglutination.7 Like botrocetin it was shown to interact with vWF allowing binding of the latter to platelet GPIb. However, bitiscetin was found to be distinct from the negatively charged botrocetin in particular in regard to its highly basic nature suggesting that it acted on vWF through a different mechanism.
Previous studies have established that botrocetin binds to sequences of the A1 domain of vWF.17 18 This binding modifies the structural environment of the A1 domain that promotes its GPIb-binding capacity. In the present paper we have established that bitiscetin induces GPIb-binding of the A1 domain by binding to sequences of the A3 domain. Therefore, we show that a conformational interaction exists between the A1 domain and the bitiscetin-A3 complex and a series of arguments indicate that such an interaction may be modulated by acting on either of the counterparts, ie, on sequences of the A1 or of the A3 domain.
We first showed that bitiscetin-induced binding of vWF to platelets is specific, dose-dependent, and saturable. Inhibition experiments were performed using MoAb directed against the GPIb-binding site of the A1 domain (MoAb 701) or against the vWF-binding site of GPIb (MoAb 6D1). Both antibodies were shown to totally inhibit ristocetin-, botrocetin-, and bitiscetin-induced binding of vWF. Our results thus indicate that the bitiscetin-induced binding involves the same contact sites between the A1 domain and GPIb as those already implicated in the presence of the two other inducers. In addition, our data showing that MoAb 701 had no effect on the binding of bitiscetin to vWF ruled out the possibility that the inhibition of this interaction was responsible for blocking the binding of vWF to GPIb.
On the other hand, several pieces of evidence showed that bitiscetin-induced binding of the A1 domain to GPIb is promoted by the binding of this inducer to the A3 domain. In one set of experiments we tested the reactivity of 125I-bitiscetin with a series of purified proteolytic or recombinant fragments of vWF that were immobilized by either blotting or coating onto wells of microtiter plates. We observed that the two A3 domain-containing fragments SpIII and SpI as well as the recombinant A3 domain, positively reacted with bitiscetin. In contrast, other fragments lacking the A3 domain, ie, SpII, T116, and 39/34 kD dispase fragment, were not recognized by bitiscetin. The absence of potential binding site for bitiscetin outside the A3 loop was further shown by showing that recombinant delta A3-vWF, deleted from the A3 domain and known to normally interfere with ristocetin and botrocetin,29 totally fails to bind to bitiscetin.
The presence of the binding site of bitiscetin within the A3 loop was also shown by inhibition of binding using MoAb directed against the A3 domain. Four MoAb (201, 400, 505, and 535) that all blocked the collagen binding to that region, were analyzed. Three of these MoAb (201, 400, and 535) totally inhibited binding of bitiscetin to vWF and consequently binding of vWF to platelets. Thus, together with results showing the inhibition of vWF binding to collagen by bitiscetin, our data suggest that the two ligands of vWF, collagen and bitiscetin, share at least in part a common binding site on the A3 loop. However, bitiscetin and collagen are not equivalent ligands of vWF because the mechanism by which bitiscetin induces binding of vWF to platelet GPIb is not observed using collagen under static conditions.32 Our results showing that MoAb 505 increases bitiscetin binding but blocks collagen binding to vWF also discriminate between the two ligands and suggest that epitope 505 is a conformational site of the A3 loop that is not directly involved in the ligand-vWF interactions.
More information on the conformational interaction between the A1 domain and the bitiscetin-A3 loop complex comes from experiments performed using selected MoAbs against the A1 region and synthetic peptides. MoAb 322 is directed against one of the flanking regions of the A1 loop46 and MoAb 710 reacts with an epitope located within the A1 loop between Ser 593 and Ser 678.49 Both MoAb were previously shown to block ristocetin- but not botrocetin-induced binding of vWF to GPIb.19 In the present study, both MoAbs also inhibit bitiscetin-induced binding of vWF to platelets suggesting that the mechanisms of the binding of vWF to GPIb in the presence of bitiscetin or ristocetin involved at least in part similar sites within the A1 domain. In addition, the significant increase of the binding of bitiscetin to vWF observed in the presence of MoAb 322 discriminates its epitope from that of MoAb 710 and strongly supports its involvement in the potential interaction between A1 and A3 domains. Experiments using MoAb 724 also suggested the involvement of the corresponding epitope in the A1-A3 loop interaction. Epitope of MoAb 724 was previously identified as a conformational site of the A1 loop playing a key role in the interactions of vWF with GPIb in the presence or absence of ristocetin.45 In addition, this antibody was shown to inhibit the binding of heparin, sulfatides, and botrocetin to vWF, suggesting that its epitope overlaps the common binding site (aa 569-584) for the three ligands. In the present study we observed that MoAb 724 totally inhibits bitiscetin-induced binding of vWF to platelets and partially blocks binding of bitiscetin to vWF in a way similar to that observed using botrocetin.44 However, we observed that addition of botrocetin has no significant effect on the binding of bitiscetin to vWF. Thus, because it is unlikely that the occupancy of the same site on the A1 domain by either botrocetin or MoAb 724 is not responsible for the same effect on the bitiscetin binding to the A3 loop, we assume that epitope 724 is distinct from the common binding site for heparin, botrocetin, and sulfatides. It is likely that MoAb 724 acts in all cases by disrupting the folding of the A1 and A3 regions through a conformational change of the A1 loop that blocks bitiscetin- and botrocetin-induced binding of vWF to GPIb as well as heparin, sulfatides, and botrocetin binding to the A1 domain; and a conformational change of the A3 domain that at least in part prevents the binding of bitiscetin to vWF.
Interaction of the A1 domain with the A3 loop was further shown by testing the effect of synthetic peptides corresponding to sequences of the A1 domain on the binding of bitiscetin to vWF. Sequences corresponding to vWF residues Cys 474-Pro 488, Leu 494-Arg 511, and Ser 692-Pro 708 were shown to play a role in modulating vWF binding to GPIb. Peptides 474-488 and 692-708 blocked ristocetin-induced binding of vWF to GPIb and the spontaneous binding of asialo-vWF20but they had no effect on botrocetin-induced binding of vWF to platelets.17, 19 Both peptides have been proposed to be ristocetin binding sites on vWF.17, 50 In addition, experiments using deleted recombinant fragments of vWF18 or mutated recombinant vWF15 as well as studies of the natural mutations of vWF leading to the 2B subtype of vWD16, 51-54suggest that sequences 494-511 and 692-708 play a role on the conformation of the A1 loop by limiting exposure of the GPIb-binding site. In the present study only peptide 474-488 blocked the binding of bitiscetin to the A3 domain suggesting that the interaction of the sequence Cys 474-Pro 488 with the A3 loop also regulates the binding of vWF to platelet GPIb. Binding of vWF to GPIb was proposed to be regulated by both the amino acid sequences of contact sites and by an electrostatic mechanism involving positively charged regions of the A1 domain and negatively charged domains of GPIb.55 Two positively charged sequences of the A1 domain, ie, aa 569-583 and 628-655, appeared to play an important role in the regulation of the interaction. Both are involved in the binding sites of vWF to its negatively charged ligands like botrocetin,15,18sulfatides,22,25 or heparin.23,26 In addition, the sequence 628-655 of vWF was shown to contain two segments (aa 629-632 and 642-645) that belong to the contact site with GPIb.15 Because it is likely that the modulation of the interaction of GPIb with the A1 domain involving the negatively charged A3 domain also requires an electrostatic mechanism, we tested the effect of the corresponding synthetic peptides on the binding of bitiscetin to vWF. Our results showed that peptide 628-655 totally blocks bitiscetin binding whereas peptide 569-583 has no effect. Thus, our data are in agreement with the hypothesis that the electrostatic charges are an important feature of the interaction between the A1 and A3 loops. However, the discrepancy that we observed in the effect produced by each peptide also suggests an important role of the amino acid sequence.
In conclusion, we report that the conformation of the A1 domain is modulated by binding of bitiscetin to the A3 loop. Several hypothesis can be raised to explain the mechanism: (1) the binding of bitiscetin to the A3 loop may change or disrupt an interaction preexisting in native vWF between the positively charged A1 loop and the negatively charged A3 domain. This hypothesis is in agreement with the fact that the 39/34 kD monomeric dispase fragment overlapping the A1 domain and free of A3 domain spontaneously interacts with GPIb42; (2) the complex formation between bitiscetin and the A3 domain could facilitate a new interaction with the A1 loop leading to the exposure of the GPIb-binding site of vWF. On the one hand such an interaction may result from the binding of sequences of the A3 domain to the A1 region. On the other hand, bitiscetin could change its own conformation after its binding to the A3 loop, exposing a binding site for the A1 domain that in turn could activate that domain in a way similar to other inducers. Our data showing that the binding of bitiscetin occurs on purified A3 loop-containing fragments but can be inhibited by specifically acting on the A1 domain, together with the distribution of the electrostatic charges among the various reagents, are in favor of the hypothesis involving the modulation of a direct interaction between sequences of the A1 and A3 domains.
ACKNOWLEDGMENT
We thank Professor J.J. Sixma and Dr E.G. Huizinga from the Department of Haematology, University Hospital Utrecht, Utrecht, The Netherlands, for the gift of recombinant delta A3-vWF and of the A3 domain of vWF and Dr B.S. Coller, State University of New York, Stony Brook, NY, for the gift of MoAb 6D1. We are grateful to the Laboratoire Français du Fractionnement et des Biotechnologies, Les Ulis, France, for providing high-purity vWF concentrates, to Dr J. Diaz, Sanofi Recherche, Montpellier, France, for the gift of synthetic peptides, and to Dr A.S. Ribba from the same Institution for the gift of the expression vectors for wild-type rvWF and mutant rvWFLys 875.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal