We have determined the 2905 nucleotide sequence of the rhesus macaque factor IX complementary DNA (cDNA) and found it to be greater than 95% identical to that of the human factor IX cDNA. The cDNA has a large 3′ untranslated region like the human cDNA, but unlike the human cDNA has two polyadenylation sites 224 nucleotides apart that are used for transcription of the messenger RNA. The deduced amino acid sequence is greater than 97% identical to that of human factor IX, differing in only 11 of 461 amino acids in the complete precursor protein. We found a single silent polymorphism in the nucleotide sequence at the third position of the codon for asparagine at position 167 in the secreted protein (AAC/AAT). All residues subject to posttranslational modifications in the human protein are also found in the rhesus factor IX sequence. The high degree of homology between the rhesus and human factor IX proteins suggested the possibility that the human factor IX protein might be nonimmunogenic in the rhesus. We tested the immunogenicity of human factor IX in three rhesus macaques by repeated intravenous injections of monoclonal antibody–purified, plasma-derived human factor IX over the course of more than a year and assessed the recovery and half-life of the infused protein, as well as in vitro indicators of antihuman factor IX antibodies. Human factor IX recovery and half-life remained unchanged over the course of a year in the three animals studied, and aPTT mixing studies showed no evidence for neutralizing antihuman factor IX antibodies. An outbred, nonhuman primate model that permits assessment of the level and duration of factor IX expression as well as vector safety would complement the use of other (mouse and canine) hemophilia B animal models in current use for the development of gene therapy for hemophilia B.
HEMOPHILIA B IS A SEX-LINKED, inherited bleeding disorder caused by deficiency of coagulation factor IX. Factor IX is a serine protease that activates factor X after its own activation by factor XI and/or factor VII as part of the blood coagulation process. Severe disease (<1% of normal factor IX levels) is associated with spontaneous bleeding (particularly hemarthrosis) and excessive bleeding with trauma or surgery.1 Because factor replacement therapy with recombinant or plasma-derived concentrates is expensive and inconvenient, it has been used clinically in a reactive mode rather than the more desirable prophylactic mode. Gene therapy of hemophilia B offers the theoretical advantage of providing a curative approach in which factor IX could be maintained continuously at therapeutic levels (at least 1% of normal) and, based on extensive experience with prophylactic factor administration, would prevent spontaneous hemarthroses and preserve normal joints in these patients.2 3
Gene therapy of hemophilia B using retroviral,4-9adenoviral,10-12 or AAV-based vectors,8,13-15is currently under development, and has relied on animal models for hemophilia B that include knockout mice 16,17 and hemophilia B canines.18,19 These permit in vivo evaluation of vector-mediated gene expression in mammals. The underlying hemophilia makes these animals difficult to maintain, and may pose difficulties for experiments involving surgical procedures that require normal hemostasis. Further, the immune response to human factor IX (in immune competent animals) usually limits the ability to evaluate the level or duration of factor IX expression from the vectors that would be used for treatment of humans.11,20 21
It would be advantageous to be able to test long-term expression of gene transfer vectors that synthesize human factor IX in immune-competent animals that closely resemble humans before their use in clinical trials. We therefore began preliminary investigations of the suitability of the rhesus macaque (Macaca mulatta) for testing of human gene therapy vectors for hemophilia B. We cloned the rhesus factor IX complementary DNA (cDNA) and determined its sequence similarity to be greater than 95% identical with the human factor IX cDNA, and found that intravenously administered human factor IX did not elicit a significant antibody response in three rhesus macaques.
MATERIALS AND METHODS
Animals.
Adult rhesus macaques were housed in a dedicated primate facility under conditions approved by the National Institutes of Health Institutional Animal Care and Use Committee, and all protocols for experiments were approved by the Committee. Rhesus macaques were bred at the National Institutes of Health (Bethesda, MD) for these and other experiments.
Cloning of the rhesus factor IX cDNA.
Fresh liver obtained from a female rhesus after euthanasia for an unrelated study was flash frozen in dry ice and stored under liquid nitrogen before isolation of total RNA for cloning of the factor IX cDNA. Frozen liver was disrupted with an automated tissue homogenizer, and total RNA was prepared by extraction with Trizol (Life Sciences, Bethesda, MD) according to manufacturer’s directions. Total RNA was stored under isopropyl alcohol at −20°C until polyadenylated RNA was purified with the Promega (Madison, WI) Polyattract kit. Polyadenylated RNA was used for first-strand DNA synthesis using reverse transcriptase and second-strand synthesis with Klenow DNA polymerase, after which the double-stranded DNA fragment was ligated to adapter-primers (Clontech, Palo Alto, CA) and subjected to polymerase chain reaction (PCR) amplification using gene-specific oligonucleotide primers and primers specific for the adapter ligated to the double-stranded DNA.
5′ and 3′ rapid amplification of cDNA ends (RACE).
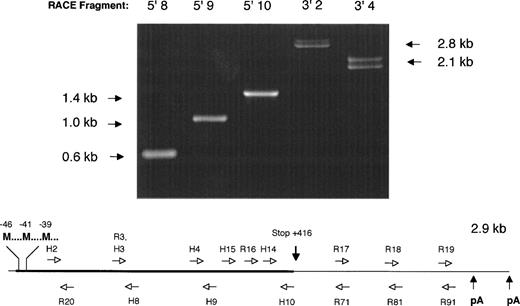
5′ and 3′ RACE PCR was performed using factor IX–specific primers complementary to the human factor IX cDNA sequence. All but one of these proved to be completely conserved after overlapping sequence for the rhesus factor IX cDNA was determined (Fig 1).
Strategy for determination of the nucleic acid sequence of the rhesus macaque factor IX cDNA. (Above) 1% agarose gel electrophoresis of 5′ and 3′ RACE PCR fragments obtained by amplification of adapter-ligated first-strand DNA template with adapter primer AP1 (Clonetech) and various factor IX–specific oligonucleotides. Lane 5′8: amplimer obtained with AP1 and H8 primers; lane 5′9: amplimer obtained with AP1 and H9 primers; lane 5′10: amplimer obtained with AP1 and H10 primers; lane 3′2: amplimer obtained with H2 and AP1 primers; lane 3′4: amplimer obtained with H4 and AP1 primers. Note the double bands observed for 3′ RACE PCR fragments 3′2 and 3′4, due to the two different polyadenylation signal sequences, separated by 224 base pairs in the cDNA. (Below) Schematic diagram of rhesus macaque factor IX cDNA depicting the position of oligonucleotide primers used to amplify overlapping RACE PCR fragments and determine the nucleic acid sequence. H series denotes oligonucleotides completely homologous to human factor IX coding sequence. R series denotes oligonucleotides with sequence unique to the rhesus macaque factor IX cDNA sequence. Not shown is the adapter primer that is ligated to each end of the cDNA, to which oligonucleotide AP1 is complementary. M, Methionine translation initiation start sites; pA, AATAAA polyadenylation signal sequence. Drawing not to scale for clarity in depicting oligonucleotide primer positions.
Strategy for determination of the nucleic acid sequence of the rhesus macaque factor IX cDNA. (Above) 1% agarose gel electrophoresis of 5′ and 3′ RACE PCR fragments obtained by amplification of adapter-ligated first-strand DNA template with adapter primer AP1 (Clonetech) and various factor IX–specific oligonucleotides. Lane 5′8: amplimer obtained with AP1 and H8 primers; lane 5′9: amplimer obtained with AP1 and H9 primers; lane 5′10: amplimer obtained with AP1 and H10 primers; lane 3′2: amplimer obtained with H2 and AP1 primers; lane 3′4: amplimer obtained with H4 and AP1 primers. Note the double bands observed for 3′ RACE PCR fragments 3′2 and 3′4, due to the two different polyadenylation signal sequences, separated by 224 base pairs in the cDNA. (Below) Schematic diagram of rhesus macaque factor IX cDNA depicting the position of oligonucleotide primers used to amplify overlapping RACE PCR fragments and determine the nucleic acid sequence. H series denotes oligonucleotides completely homologous to human factor IX coding sequence. R series denotes oligonucleotides with sequence unique to the rhesus macaque factor IX cDNA sequence. Not shown is the adapter primer that is ligated to each end of the cDNA, to which oligonucleotide AP1 is complementary. M, Methionine translation initiation start sites; pA, AATAAA polyadenylation signal sequence. Drawing not to scale for clarity in depicting oligonucleotide primer positions.
TA cloning.
5′ and 3′ RACE PCR products were subjected to ligation with the PCR 3.1 plasmid (Invitrogen, La Jolla, CA) and the ligation products were used to transfect competent DH5alpha cells (Life Sciences, Gaithersburg, MD) that were selected on LB-ampicillin plates. Colonies were screened for inserts by restriction digests withEcoRI.
Sequence determination.
Automated sequence determination was performed with the ABI Prism model 377 automated fluorescence sequencer (Seqwright, Houston, TX) using the reverse orientation sequencing primer for pCR 3.1 as well as gene-specific primers for factor IX. Because the analysis showed novel sequence data additional primers were designed to “walk” through the 3′ end until both AATAAA polyadenylation sites were located. Sequence data management, alignment, and analysis was performed with DNAstar software (Madison, WI). Sequence data was determined at least twice for each position in the cDNA and in both the sense and antisense orientation for the protein-coding portion.
Factor IX pharmacokinetics.
Monoclonal antibody (MoAb)–purified human factor IX (Mononine, Centeon, Kankakee, IL) was injected intravenously into three rhesus macaques on seven occasions for each animal over the course of a year at a dose of 25 units/kg. Whole blood obtained at sequential time points was anticoagulated with 0.105 mol/L sodium citrate and platelet-poor plasma prepared by centrifugation at 2000g for 20 minutes and frozen until subsequent analysis. The half-life of human factor IX in the rhesus was determined by plotting the natural logarithm of the human factor IX level at T = 1, 2, 4, and 8 hours after injection as a function of time and solving for the time constant k, in which ln [hFIX] = e−kt. The time constant k was determined by curve-fitting with Deltagraph software (Deltapoint, Monterey, CA). The half-life was calculated according to the relationship T1/2 = 0.693/k.
Factor IX immunoassays (enzyme-linked immunosorbent assay [ELISA]).
An ELISA capable of detecting human factor IX in a background of rhesus plasma was developed. 96-well, flat-bottomed Immulon 4 plates (Dynatech, Chantilly,VA) were coated overnight with 0.05 μg of rabbit polyclonal antihuman factor IX (DAKO A300; DAKO, Glastrup, Denmark) in 0.1 mol/L sodium carbonate buffer (pH 8,8) at 4°C. Plates were then washed with phosphate-buffered saline (PBS) with 0.05% Tween 20 (Sigma, St Louis, MO) (PBS/Tween) and blocked with PBS/Tween with 6% bovine serum albumin (BSA) (RIA grade, Sigma; PBS/Tween/BSA) for 1 hour at 37°C, then used immediately or frozen at −20°C before use. Rhesus plasma samples were diluted 1:25 with PBS/Tween/BSA and before loading 50 μL per well. The plate was incubated with samples and standards (diluted in 6% BSA/PBS/0.05% Tween 20) for 1 hour at 37°C. After washing the plate with PBS/Tween, 100 μL of rabbit antihuman factor IX conjugated to horseradish peroxidase (DAKO P380; DAKO) was added and the plate incubated for 1 hour at 37°C. After washing with PBS/Tween the plate was developed with 100 μL 1 mg/mLo-phenylene diamine in 0.1 mol/L sodium citrate buffer (pH 4.5) supplemented with 2 μL 30% hydrogen peroxide per 10 mL solution and stopped by addition of 100 μL of 1 mol/L hydrochloric acid. Absorbance at 492 nm was read with a Thermomax Microplate Reader (Molecular Devices, Menlo Park, CA) to determine the concentration of human factor IX antigen in each sample.
The cross-reactivity of rhesus and human plasma was examined in detail and was found to vary with the dilution of the sample. At 1:25 dilutions (used for the assays reported herein) the cross-reactivity of the sample was about 10%, ie, the amount of factor IX in rhesus plasma diluted 1:25 was only 10% of that to be expected if there were complete cross-reactivity between the human and rhesus proteins and equal concentrations of each in plasma. Background cross-reactivity from endogenous rhesus factor IX in plasma samples was factored out by adding rhesus plasma to the standards and plate-blank at a concentration of 4% (vol:vol), ie, a 1:25 dilution. The ELISA was linear from 30 to 1000 ng/mL of human factor IX when rhesus plasma was spiked with Mononine, then diluted 1:25 as for the assays reported herein.
Sandwich ELISA for rhesus antihuman factor IX antibodies.
Immulon 4 96-well plates (Dynatech) were coated with 0.1 μg of MoAb–purified human factor IX (Mononine) per well in 0.1 mol/L sodium carbonate buffer (pH 8.8) overnight at 4°C, then washed and blocked with BSA/PBS/Tween for 1 hour at 37°C, and frozen before to use. Plates were thawed, washed, and 50 μL samples applied to each well and incubated at 4°C for 1 hour. After washing with PBS/Tween, 100 μL of rabbit antirhesus IgG conjugated to horseradish peroxidase (Sigma) diluted 1:10,000 were applied to each well and incubated for 1 hour at 4°C. After washing with PBS/Tween, the plate was developed with 100 μL of 1 mg/mL o-phenylene diamine in 0.1 mol/L sodium citrate buffer (pH 4.5) supplemented with 2 μL 30% hydrogen peroxide per 10 mL solution and stopped by addition of 100 μL of 1 mol/L hydrochloric acid. Absorbance at 492 nm was read with a Thermomax Microplate Reader (Molecular Devices). Dilutions of plasma from a hemophilia B patient known to have a high-titer antihuman factor IX alloantibody (a gift from Dr Amy Shapiro, Indiana University Medical Center, Indianapolis, IN) were included as positive controls; BSA/PBS/Tween without plasma or serum was included as a negative control. The titer of a sample was defined as the maximum dilution at which the absorbance at 492 nm exceeded that of the preimmune serum by 0.05 absorption units.
RESULTS
Sequence of rhesus FIX cDNA.
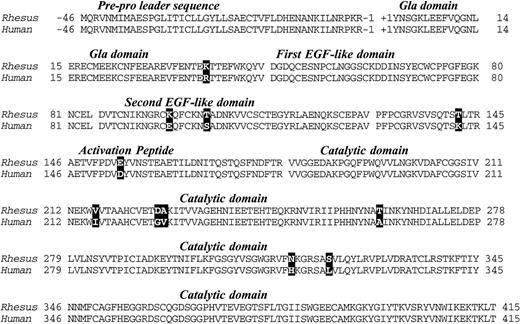
A total of 2.9 kb of cDNA sequence was determined from analysis of the 5′ and 3′ RACE fragments depicted in Fig 1. The sequence began 19 base pairs 5′ to the start of the protein-coding region (Met-46) and extended 1.5 kb past the termination codon at +416. The 5′ sequence overlaps 61 nucleotides of rhesus factor IX promoter sequence reported by Pang et al,22 and is in complete agreement with that data. A 461 amino acid open reading frame encoded a protein with extremely high homology (97% identity) with the human factor IX protein (Fig 2).23 As expected, the classical factor IX protein domain structure (Gla-EGF-EGF-Activation peptide-Catalytic domain) was identical to that of all other factor IX sequences thus far sequenced.
Deduced amino acid sequence of rhesus macaque factor IX cDNA and alignment with the human factor IX protein sequence. Differences are denoted by bold typeface. Numbers denote the amino acid sequence in the translation product and the factor IX domains are indicated in italics and separated from one another by spaces in the sequence.
Deduced amino acid sequence of rhesus macaque factor IX cDNA and alignment with the human factor IX protein sequence. Differences are denoted by bold typeface. Numbers denote the amino acid sequence in the translation product and the factor IX domains are indicated in italics and separated from one another by spaces in the sequence.
The pre-pro leader sequence, which directs posttranslational gamma carboxylation of glutamic acids in the Gla domain, was identical to the human sequence. All 12 glutamic acids in the Gla domain (potential substrates for gamma-glutamyl carboxylation) were present in the rhesus protein. Other key features of the protein that were identical to the human form were the aspartic acid at position 64 (subject to β-hydroxylation), the asparagines at 157 and 167 (subject to N-glycosylation), and serines/threonines at positions 61, 159, 169, and 172, (which in the human protein are subject to O-linked glycosylation) were also present. Serine 158 and threonine 155, which in the human protein are phosphorylated and sulfated, respectively,24were also present in the rhesus protein.
Most of the differences between the human and rhesus factor IX were substitutions of highly homologous amino acids (eg, Lys/Arg at 37, Glu/Asp at 154, Val/Ile at 216, etc.). There was, however, a basic amino acid (lysine) in place of glutamic acid at position 96 in the rhesus that should result in a reversal of charge at that position, though both are hydrophilic residues with similar characteristics with respect to secondary structure formation.25
The rhesus factor IX protein sequence has greater homology to human factor IX than does that of any other known species (Table 1). The rhesus factor IX protein also displayed a similar homology to factor IX from various other species, varying according to phylogenetic relationships.
Homology of Factor IX From Various Mammalian Species With Human Factor IX
| Species . | Region of Comparison . | Identity (%) . |
|---|---|---|
| Human | Complete precursor, 461 amino acids | 100 |
| Rhesus | Complete precursor, 461 amino acids | 97 |
| Canine | Complete precursor, 452 amino acids | 86 |
| Mouse | Complete precursor, 448 amino acids | 83 |
| Bovine | Secreted protein, 415 amino acids | 84 |
| Porcine | Activation peptide/catalytic domain, 265 amino acids | 84 |
| Sheep | Activation peptide/catalytic domain, 272 amino acids | 82 |
| Rabbit | Activation peptide/catalytic domain, 273 amino acids | 79 |
| Rat | Activation peptide/catalytic domain, 282 amino acids | 79 |
| Species . | Region of Comparison . | Identity (%) . |
|---|---|---|
| Human | Complete precursor, 461 amino acids | 100 |
| Rhesus | Complete precursor, 461 amino acids | 97 |
| Canine | Complete precursor, 452 amino acids | 86 |
| Mouse | Complete precursor, 448 amino acids | 83 |
| Bovine | Secreted protein, 415 amino acids | 84 |
| Porcine | Activation peptide/catalytic domain, 265 amino acids | 84 |
| Sheep | Activation peptide/catalytic domain, 272 amino acids | 82 |
| Rabbit | Activation peptide/catalytic domain, 273 amino acids | 79 |
| Rat | Activation peptide/catalytic domain, 282 amino acids | 79 |
In humans, the amino acid at position 148 is polymorphic (Ala/Thr),26 27 whereas in the rhesus there was evidence only for threonine in the animal studied. There is evidence for a nucleic acid polymorphism (in the female we analyzed) at Asn 167 in which different cloned PCR fragments showed either AAC or AAT codons. Direct sequence analysis of multiple uncloned PCR fragments (both in the forward and reverse orientation) confirmed the presence of C and T at the third position of the codon.
Like the human factor IX cDNA, the rhesus factor IX cDNA included a long, 3′ untranslated region of approximately equal length to the protein coding region. The 3′ untranslated region bore a high degree of resemblance (∼95% identity) to the human factor IX 3′ untranslated region. In contrast to the human factor IX cDNA, the rhesus factor IX had two AATAAA polyadenylation signals separated by 224 base pairs at the 3′ end of the cDNA. As can be observed in Fig 1, the 3′ RACE fragments amplified as doublets consistent with two lengths of polyadenylated messenger RNA in approximately equal proportion, differing by 224 base pairs. The presence of multiple polyadenylation signals has previously been described as a feature of the human gamma glutamyl carboxylase cDNA as well.28
Pharmacokinetics and antigenicity of human factor IX in the rhesus macaque.
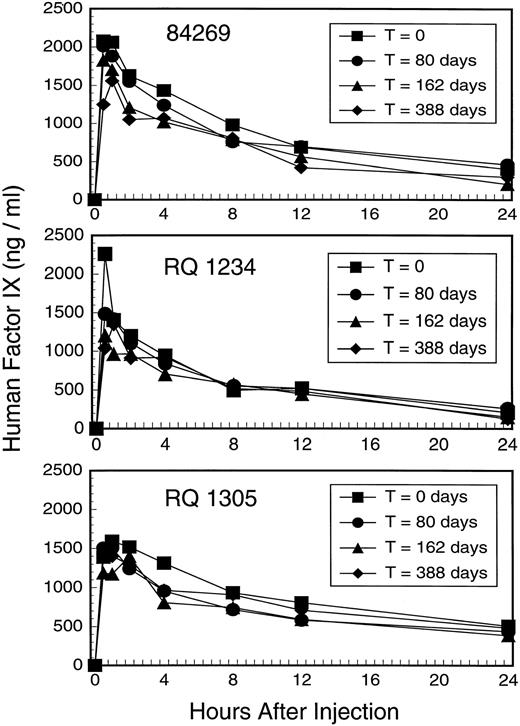
To assess the pharmacokinetics and immunogenicity of human factor IX in the rhesus we repeatedly injected three animals with MoAb–purified, human factor IX derived from plasma (Mononine) over the course of a year. We analyzed human factor IX recovery and half-life of elimination at four different times as shown in Fig 3. The peak recovery at 30 minutes ranged from 83% to 181% of that expected after a dose of 25 units/kg body weight and a volume of distribution of 100 mL per kg body weight, which in the human should result in a peak level of 1250 ng/mL. The half-life of elimination, based on first-order elimination from a single compartment, averaged 6.9 hours (SD = 2.1 hours) for the first falloff and 8.6 hours (SD = 2.7 hours) for the fourth falloff (Table2).
Factor IX falloff studies in three rhesus macaques 84269, RQ 1234, and RQ 1305. Each animal was studied on four occasions by injecting 25 units Mononine/kg body weight followed by immunoassay for human factor IX at the indicated time points. Falloff studies were performed by single injection of factor IX at T = 0, 80 days, and 162 days; three daily injections of factor IX were performed 10 days before a fourth falloff study at T = 388 days to maximize the chance that any immune response might be boosted.
Factor IX falloff studies in three rhesus macaques 84269, RQ 1234, and RQ 1305. Each animal was studied on four occasions by injecting 25 units Mononine/kg body weight followed by immunoassay for human factor IX at the indicated time points. Falloff studies were performed by single injection of factor IX at T = 0, 80 days, and 162 days; three daily injections of factor IX were performed 10 days before a fourth falloff study at T = 388 days to maximize the chance that any immune response might be boosted.
Pharmacokinetic Parameters for Factor IX Falloff Studies 1 to 4
| Animal . | Falloff . | |||
|---|---|---|---|---|
| 1 (T = 0) . | 2 (T = 80 days) . | 3 (T = 162 days) . | 4 (T = 388 days) . | |
| Half-life (hours) | ||||
| 84269 | 7.0 (0.97) | 5.5 (0.99) | 7.1 (0.88) | 9.1 (0.77) |
| RQ1234 | 4.7 (1.00) | 5.4 (0.98) | 8.1 (0.94) | 5.7 (0.90) |
| RQ1305 | 8.9 (0.77) | 6.8 (0.97) | 8.5 (0.48) | 11.1 (0.89) |
| Peak Recovery (% of predicted level) | ||||
| 84269 | 166 | 161 | 146 | 100 |
| RQ1234 | 181 | 118 | 96 | 83 |
| RQ1305 | 111 | 120 | 95 | 111 |
| Animal . | Falloff . | |||
|---|---|---|---|---|
| 1 (T = 0) . | 2 (T = 80 days) . | 3 (T = 162 days) . | 4 (T = 388 days) . | |
| Half-life (hours) | ||||
| 84269 | 7.0 (0.97) | 5.5 (0.99) | 7.1 (0.88) | 9.1 (0.77) |
| RQ1234 | 4.7 (1.00) | 5.4 (0.98) | 8.1 (0.94) | 5.7 (0.90) |
| RQ1305 | 8.9 (0.77) | 6.8 (0.97) | 8.5 (0.48) | 11.1 (0.89) |
| Peak Recovery (% of predicted level) | ||||
| 84269 | 166 | 161 | 146 | 100 |
| RQ1234 | 181 | 118 | 96 | 83 |
| RQ1305 | 111 | 120 | 95 | 111 |
Half-life determinations for each falloff study were calculated from the slope of the fitted equation describing the logarithm of factor IX as a function of time over the interval including time points at 1, 2, 4, and 8 hours. Values listed are the half-life in hours with the coefficient of variance for the fitted curve in parentheses for each value. The peak recovery is the mean of duplicate determinations of the highest factor IX level divided by the theoretical value predicted on the basis of a 25 unit per kg dose (1.25 μg/mL, assuming 5 μg/mL as 100% normal factor IX level).
We sought to maximize our ability to elicit an antibody response to human factor IX protein (without the use of an adjuvant), so 11 days before the fourth falloff study at T = 388 days we gave three daily injections of factor IX (25 units/kg). This series of injections of human factor IX should have boosted any low-titer antibody, and the resulting antibody (if any) should have been readily observed because anamnestic antibody responses to factor VIII or factor IX in humans typically begin to appear 4 to 5 days after re-exposure to antigen and rises rapidly thereafter. Indeed, the fourth falloff curve remained superimposable on the prior three for each animal (Fig 3).
In addition, we performed studies of baseline plasma or plasma from the fourth falloff study mixed with normal human plasma to look for in vitro evidence of inhibitor antibody formation. These studies (Table 3) showed no inhibition of factor IX–dependent coagulation that would have manifested itself as prolongation of the activated partial thromboplastin time. A plate ELISA for rhesus antihuman factor IX antibodies was negative for all postfalloff sera in the three animals studied with the exception of 1:50 titer antibodies observed after the second factor IX injection in animal 84269 and after the third injection in animal RQ1305; these animals were negative for antihuman factor IX antibodies in all subsequent postinjection sera. The positive sera, when heat inactivated for 30 minutes at 56°C, did not prolong the aPTT of normal human plasma significantly when compared with the aPTT of normal human plasma mixed with heat-inactivated negative serum (29.5 seconds, SD = 3.3 seconds for seronegative samples, v 26.5 seconds, SD = 4.5 seconds for 84269 post-second falloff and 30.0 seconds, SD = 2.1 for RQ1305 post-third falloff; P > .05 for both comparisons byt test).
aPTT Mixing Studies, Baseline, and Fourth Falloff Plasma Samples
| Animal . | Falloff 1 Baseline Plasma . | Falloff 4 Baseline Plasma . |
|---|---|---|
| 84269 | 29.3 seconds (2.9) | 26.3 seconds (2.0) |
| RQ1234 | 30.0 seconds (3.0) | 30.2 seconds (2.1) |
| RQ1305 | 32.4 seconds (3.5) | 31.3 seconds (3.3) |
| Animal . | Falloff 1 Baseline Plasma . | Falloff 4 Baseline Plasma . |
|---|---|---|
| 84269 | 29.3 seconds (2.9) | 26.3 seconds (2.0) |
| RQ1234 | 30.0 seconds (3.0) | 30.2 seconds (2.1) |
| RQ1305 | 32.4 seconds (3.5) | 31.3 seconds (3.3) |
aPTT’s were measured on 50:50 mix of heat-inactivated rhesus plasma and pooled normal human plasma using a factor-sensitive aPTT reagent. All values are mean of five determinations with standard deviation in parentheses. aPTT of normal pooled human plasma = 28.6 seconds (4.9), aPTT of 50:50 mix of normal pooled human plasma with Owren’s buffer = 27.3 seconds (1.5). A 1:5 dilution of a human factor IX inhibitor plasma with a Bethesda titer of ∼100 units was mixed 50:50 with normal pooled human plasma resulting in a mean aPTT of 62.3 seconds (average of two determinations, +/−3.7 seconds).
DISCUSSION
We have determined the complete cDNA sequence of the rhesus macaque coagulation factor IX, which is the first complete coagulation factor cDNA sequence reported from a nonhuman primate. The cDNA and the derived amino acid sequence is 97% identical to the human factor IX at the amino acid sequence level. The factor IX protein domain structure and potential sites for post-translation modification are identical to that of the human.
The nucleic acid sequence data and the derived amino acid sequence show the rhesus factor IX protein to be more homologous to the human factor IX protein than either that of the canine or mouse, both of which have been used as models for hemophilia B gene therapy, and both of which are known to make antibodies to human factor IX. It is therefore likely that human factor IX would be less immunogenic in the rhesus than in the canine or mouse models used to test gene therapy vectors.
The immunogenicity of factor IX transgenes in the context of viral (or nonviral) gene transfer is crucial to assess before human trials to detect the development of antihuman factor IX inhibitor antibodies, which have severe adverse consequences in patients with severe hemophilia B. Although inhibitor antibodies are much less frequent in patients with severe hemophilia B (∼3%) than in patients with severe hemophilia A (∼20%), the former are much more serious, having been associated with anaphylaxis and/or nephrotic syndrome when factor IX is repeatedly administered in an effort to induce tolerance for factor IX.29 30
As can be observed in Fig 3, the sequential falloff studies are essentially superimposable for each individual animal. The half-life of human factor IX in the rhesus (5 to 11 hours) is shorter than that observed in humans (18 to 22 hours),1 but is comparable to that observed for human factor IX in other species, particularly rodents.11 The shorter half-life of human factor IX in nonhuman species is perhaps a consequence of differences in posttranslational modifications (eg, glycosylation, sulfation, phosphorylation) or differing affinity for receptors that bind glycosylated proteins in general (eg, the ASOR receptor on the hepatocyte31) or factor IX in particular (factor IX receptors on endothelial cells).32,33 Our studies on the pharmacokinetics of human factor IX in the rhesus show no evidence for inhibitor antibody formation after repeated injections. It must be acknowledged that additional injections of human factor IX might have elicited an antibody response in the animals studied and it is also possible that if a greater number of animals were treated similarly a high-titer antibody response might have been elicited in some. Adjuvants that enhance immunogenicity and antibody formation were not used in this study because our intent was to administer factor IX in the same manner as hemophilia B patients receive it in the clinical setting. It is also possible that repeated administration of a weakly immunogenic protein to an adult animal in a “nondanger” setting may serve to induce tolerance by deleting antihuman factor IX lymphocytes in the absence of “signal 2”.34
There is precedent for a lack of immune response to human factor IX in certain normal (immune competent) animals, despite differences between the factor IX amino acid sequences. The C57Bl/6 strain of (normal) mouse typically does not make antihuman factor IX antibodies even in the context of adenoviral-mediated gene expression, although other normal mouse strains (eg, CD1) readily make antihuman factor IX antibodies.35 In contrast, hemophilia B canines routinely make antibodies to human factor IX after human factor IX infusions36,37 or gene transfer with human factor IX vectors.38
The significance of the transient low-titer antihuman factor IX antibodies in two of our animals is unclear. These are non-neutralizing (by aPTT mixing studies) and do not affect the pharmacokinetics of infused human factor IX protein. Transient, noninhibitory, canine anticanine factor IX antibodies have been detected by Western blotting after AAV-canine factor IX gene transfer into skeletal muscle of hemophilia B canines by Herzog et al,39 indicating that even self-tolerance for factor IX can be broken. The antibodies in the rhesus monkeys suggest at least that there are complementarity-determining region sequences in the rhesus genome necessary for antihuman factor IX antibody production, however an adjuvant (eg, a viral gene transfer vector) would be required to elicit a high-titer antibody response to human factor IX.
If a human factor IX vector causes antihuman factor IX antibodies in the rhesus macaque, the rhesus cDNA could be used to test whether the viral vector or the transgene causes loss of self tolerance (ie, by making and administering a rhesus factor IX vector and looking for autoantibodies to rhesus factor IX). Loss of self-tolerance to erythropoietin has been shown in mice after administration of adenoviral vectors encoding the (human) erythropoietin transgene, despite strong homology between the mouse and human proteins.40 Such studies will permit a better understanding of the potential immunogenicity of endogenous proteins expressed by viral vectors in the future.
Our results show that it should be possible not only to collect vector safety and toxicity data, but also to analyze the level and duration of human factor IX expression in a normal, nonhuman primate animal model, which is available to investigators in the field of gene therapy. Indeed, we have recently been able to show dose-dependent, adenovirus-mediated expression of human factor IX in the three rhesus macaques used in the studies reported herein (data not shown). Studies of human factor IX vectors will be critical to the development of hemophilia B gene therapy protocols, and the rhesus macaque should be considered a potential model for preclinical testing of human factor IX gene transfer vectors.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal