Abstract
We determined the effects of the potent bisphosphonate ibandronate in a murine model of human myeloma bone disease. In this model, bone lesions typical of the human disease develop in mice following inoculation of myeloma cells via the tail vein. Treatment with ibandronate (4 μg per mouse per day) significantly reduced the occurrence of osteolytic bone lesions in myeloma-bearing mice. However, ibandronate did not prevent the mice from developing hindlimb paralysis and did not produce a detectable effect on survival. There was no significant effect of ibandronate on total myeloma cell burden, as assessed by morphometric measurements of myeloma cells in the bone marrow, liver, and spleen, or by measurement of serum IgG2b levels. These results support clinical findings that bisphosphonates may be useful for the treatment of myeloma-associated bone destruction, but suggest that other therapies are also required to reduce tumor growth.
PATIENTS WITH multiple myeloma frequently have extensive bone involvement, which is manifested as osteolytic lesions. These osteolytic bone lesions are responsible for some of the most distressing symptoms of the disease, such as intractable bone pain, increased susceptibility to fractures, hypercalcemia, and nerve compression syndromes such as spinal cord compression. An agent that could prevent or reduce the osteolysis associated with multiple myeloma would be of major therapeutic benefit for patients with this disease.
Recent studies have shown that specific treatment of the bone disease associated with myeloma using inhibitors of bone resorption can be beneficial to the patient (for reviews see1-4). Berenson et al5 showed that in patients treated with the bisphosphonate pamidronate, there was a decrease in the number of skeletal-related events including the need for analgesics, courses of radiation therapy, episodes of hypercalcemia, and pathologic fractures. Similar data were published by Lahtinen et al,6 Laakso et al,7 and McCloskey et al8 using clodronate. These findings have led to the Federal Drug Administration (FDA) recently releasing pamidronate for the treatment of not just hypercalcemic patients but also nonhypercalcemic patients with osteolytic bone disease due to myeloma.
An important question is whether treatment with bisphosphonates will influence tumor burden, as shown by Sasaki et al using experimental models of human breast cancer cell metastasis.9 In these studies, bisphosphonates were found to reduce tumor burden, specifically in the skeleton, with no effect on the growth of breast cancer metastases in soft tissues. This suggested that the breast cancer cells may be dependent on factors released during bone resorption for their growth in the bone microenvironment. It is not known currently whether myeloma cells are similarly dependent on the products of bone resorption for their growth in the bone marrow cavity and whether inhibitors of bone resorption, such as bisphosphonates, will inhibit tumor growth. Shipman et al10 and Aparicio et al11 have recently reported that some bisphosphonates induce apoptosis of myeloma cells in vitro. However, it is not yet clear whether bisphosphonates have a beneficial effect on survival in multiple myeloma patients. McCloskey et al8 reported no significant improvement in survival in myeloma patients treated with oral clodronate. Similarly, Brincker et al12 reported no improvement in survival of myeloma patients treated with oral pamidronate. However, this may have been due to poor uptake of pamidronate given orally. In the studies of Berenson et al,5 13 pamidronate treatment, administered by intravenous infusion, did not improve overall survival in myeloma patients. However, in a subgroup of patients on salvage therapy, a significant improvement in survival was observed. Unfortunately, the issues raised by these studies are difficult to answer definitively in patients because (1) bisphosphonates are rarely administered in the absence of other treatments such as chemotherapy and radiation; (2) there are many confounding variables in all patients with advanced malignant disease, including other non–bone-related complications; (3) it is difficult to assess responses to specific therapies in patients with advanced bone disease; and (4) patient studies often take many years to complete.
One practical way of overcoming some of these problems is with the use of an appropriate animal model of myeloma bone disease. Such an animal model is the 5T murine model of myeloma in which myelomas arise spontaneously in an inbred substrain of C57 black mice (C57BL/KaLwRij substrain).14-16 The myelomas can be propagated from mouse to mouse in this inbred strain by marrow transfer. Several of the 5T myeloma lines closely mimic myeloma disease in humans, with monoclonal gammopathy, marrow replacement, focal osteolytic bone lesions, hindlimb paralysis, and occasionally hypercalcemia. Cell lines have been established from this myeloma model, which also mimic the human disease.17 18
In the present study, we used the 5T murine myeloma model to examine the effects of a potent bisphosphonate on myeloma-associated bone destruction. We found that ibandronate, an amino bisphosphonate,19 markedly inhibited myeloma-associated bone resorption in this model. Ibandronate has potential advantages over pamidronate because it can be used orally and is more potent. Treatment with ibandronate (4 μg per mouse per day) significantly reduced the development of osteolytic lesions in myeloma-bearing mice. Ibandronate was not effective in preventing animals from developing hindlimb paralysis and did not prolong survival of myeloma-bearing animals. Consistent with its lack of effect on survival, ibandronate did not reduce the total tumor burden, as assessed by serum IgG2b levels, or the tumor burden in the bone marrow, liver, and spleen. These results suggest that ibandronate may be a useful adjunctive therapy for the treatment of myeloma to specifically inhibit the increased bone resorption that typically occurs in this disease.
MATERIALS AND METHODS
Cell Culture
Unless stated otherwise, all tissue culture reagents were obtained from Life Technologies Inc (Gaithersburg, MD) or JRH Biosciences (Kansas City, MO).
5TGM1 myeloma cells.
5TGM1 myeloma cells were derived from a myeloma, designated 5T33, which arose spontaneously in an aged C57BL/KaLwRij mouse.14 The characterization of these cells is described below. These cells were propagated by marrow transfer in the inbred C57BL/KaLwRij strain of mice and reliably produced disease exhibiting many of the features of human myeloma. In our hands, the 5TGM1 variant of 5T33 grows more avidly and causes more bone destruction in vivo. Additionally, this variant grows well in culture without supplementation with IL-6 or stromal cell–conditioned media. For preparation of myeloma cells for inoculation, marrow was flushed from femurs and tibias of 5TGM1 myeloma-bearing mice that exhibited increased serum IgG2b monoclonal protein using a syringe and 27-gauge needle. The marrow cells were then centrifuged and resuspended in 20 mL Iscove’s modified Dulbecco’s media (IMDM) supplemented with 10% fetal bovine serum (FBS), 2 mmol/L L-glutamine (LG), 100 U/mL penicillin-streptomycin (P/S). After overnight culture in a 90-mm Petri dish, nonadherent cells were recovered and replated in 75-cm2 tissue culture flasks in 20 mL IMDM supplemented with 10% FBS, 2 mmol/L LG, and 100 U/mL PS. These nonadherent cells were expanded in culture for 7 days and then prepared for injection into the tail vein of recipient mice as described below.
5T33 myeloma cell line.
We have also recently established a clonal myeloma cell line (5T33) that can be maintained in long-term culture and that, when injected into C57BL/KaLwRij mice, produces myeloma disease exhibiting most of the features of human myeloma as described above. The isolation and characterization of this IgG2b-producing clonal cell line has been described previously.18
Preparation of myeloma cells for intravenous injection.
All cultures for inoculation were harvested at subconfluency and re-fed with fresh culture medium 24 hours before use. Cells were centrifuged, washed twice in 50 mL phosphate-buffered saline (PBS), and then resuspended at 5 × 106 cells per mL of PBS. Two hundred microliters of this cell suspension (ie, 106 cells) was inoculated into experimental mice via tail vein injection using a 27-gauge needle. Control animals received injections of PBS alone.
Characterization of 5TGM1 Myeloma Cells
5TGM1 myeloma cells were characterized by histological examination of affected bones and soft tissues in both the founder animal from which the myeloma variant was isolated and in mice inoculated with 5TGM1 myeloma cells. Besides routine histology, cytocentrifuge preparations of cells isolated from the bone marrow of 5TGM1 myeloma-bearing mice were also stained by the May-Grünwald-Giemsa method for examination. Radiographs of the myeloma-bearing animals were taken to confirm the presence of osteolytic lesions in the founder animal and in mice inoculated with 5TGM1 myeloma. Serum IgG2b levels were measured by ELISA as described below and were found to be elevated to >30 mg/mL in the founder animal and to increase to similar levels after 4 weeks in mice inoculated with 5TGM1 myeloma cells. The increase in serum IgG2b in 5TGM1 myeloma-bearing mice was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Details of these methods are described below.
Animals
Animal studies were conducted using 8- to 10-week-old female C57BL/KaLwRij mice in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Whole blood samples were collected at baseline and then weekly after tumor inoculation by retroorbital puncture under methoxyflurane inhaled anesthesia. Body weights of animals were also determined at baseline and weekly thereafter.
Ibandronate Administration
Ibandronate, a potent bisphosphonate,19 was kindly donated by Boehringer Mannheim (Mannheim, Germany). Ibandronate was diluted in sterile PBS at a concentration of 20 μg/mL for injection into animals. Two hundred microliters of this solution was administered daily into experimental animals by subcutaneous injection (the dosage used, expressed as free acid equivalents of the monosodium salt monohydrate, was 4 μg per mouse per day). Control animals received injections of vehicle (PBS).
The dose of ibandronate used (0.16 mg/kg) was at the highest end of the range of doses tested in various animal models of normal and pathologic bone resorption, including: ovariectomized rats and dogs, thyroparathyroidectomized rats with and without treatment with parathyroid hormone-related protein, and rats bearing the Walker 256 carcinoma.20 In these experiments the doses used ranged between 0.001 to 0.1 mg/kg. The dose used in the present study (0.16 mg/kg) is approximately 100-fold higher than the doses required to inhibit bone resorption in these animal models. This dose was selected based on the above studies and also based on our previous results showing this dose to be effective in reducing both osteolytic lesions and tumor burden in bone using a murine model of breast cancer metastasis to bone.21
Analytical Methods
Measurement of serum IgG2b levels.
Serum IgG2b levels were measured using a specific two-site ELISA. The capture antibody (rat anti-mouse IgG2b; Zymed Laboratories Inc, San Francisco, CA) was coated onto EIA/RIA plates (Costar, Cambridge, MA) overnight at 4°C at a concentration of 2 μg/mL in PBS. The plates were washed three times in PBS + 0.05% tween 20 and blocked in PBS + 5% bovine serum albumin (BSA) for 3 hours at room temperature. The plates were then incubated for 1 hour at 37°C with IgG2b standard (mouse IgG2b kappa; Cappel Research Products, West Chester, PA) or serum samples which had been diluted in PBS + 0.5% BSA. The plates were washed six times in PBS + 0.05% tween 20 and then incubated for 1 hour at 37°C with detection antibody (peroxidase-conjugated rat anti-mouse IgG antibody; Biodesign International, Kennebunk, ME), used at a 1:5000 dilution in PBS + 0.5% BSA. The plates were washed 10 times in PBS + 0.05% tween 20, and then O-phenylenediamine tablets were used as the reaction substrate according to manufacturer’s instructions (Sigma Chemical Co, St Louis, MO). The reaction was stopped by addition of 3 mol/L HCl (25 μL/100 μL reaction volume). The absorbance at 492 nm was read on an EIA plate reader. IgG2b concentrations were calculated by linear regression using the Immunofit EIA/RIA program, version 2.00 (Beckman Instruments Inc). This ELISA has a linear detection range of approximately 1 to 100 ng/mL.
Quantitation of osteolytic bone lesions from radiographs.
After death, whole animal radiographs were obtained as described previously22 using a Faxitron radiographic inspection unit (Field Emission Corporation Inc, McMinnville, OR). Similar x-rays were also taken of the limbs, spine, and calvaria after dissection of the tissues, removal of the skin, and fixation in 10% buffered formalin. Quantitation of lesions visible on radiographs was performed by computerized image analysis as described previously.22Using this technique, lesions as small as 0.1 mm2 can be visualized as radiolucent areas in the bones. The number and area of these lesions was quantified by an individual who was without knowledge of the experimental protocols.
Histology and morphometric analysis.
After fixation in 10% buffered formalin, skeletal tissues were decalcified in 14% EDTA and embedded in paraffin by standard techniques as described previously.9,23 Soft tissues were fixed in 10% buffered formalin and embedded in paraffin without prior decalcification. Nonconsecutive sections were cut longitudinally through the sagittal plane of the lumbar vertebrae and through blocks of liver and spleen of each animal for histomorphometric analysis using a standard microtome. The sections were then placed on poly-L-lysine-coated glass slides and stained with hematoxylin and eosin.9 23
Histomorphometric analysis of vertebral trabecular bone volume (bone volume/tissue volume; BV/TV, %) and the percentage of vertebral bone marrow replaced by tumor was performed stereologically in two representative nonconsecutive sections of lumbar vertebrae L2 through L7 using point counting and a Zeiss Integrationsplatte II eyepiece graticule along with an Olympus BHS microscope and a ×20 magnification objective lens. Measurements were made in a minimum of three vertebrae to obtain a mean value for each animal. Trabecular bone volume was measured in two fields (0.314 mm2) in the center of each vertebral body at a standard location (0.056 mm) from each growth plate. The percentage of marrow replaced by tumor was measured in five fields (0.078 mm2) chosen randomly within the marrow cavity of each vertebra. The mean thickness of the cortices of lumbar vertebra was measured in two nonconsecutive longitudinal sections of lumbar vertebrae L2 through L7 using an Olympus BX40 microscope fitted with a drawing tube, together with the Osteomeasure computerized histomorphometry system (Osteometrics, Atlanta, GA). A total of 20 individual measurements of cortical bone thickness was made in each vertebra on each section using a ×10 objective lens. The percentage of liver and spleen replaced by tumor was measured in 10 randomly chosen fields (0.168 mm2) in each of two nonconsecutive sections from each organ using the Osteomeasure histomorphometry system and a ×20 objective lens.
SDS-PAGE and Western blotting.
For Western blotting analysis, 0.25 μL serum samples from control and tumor-bearing animals were separated on duplicate 7% SDS-PAGE gels under nonreducing conditions. Coomassie blue staining was performed by standard techniques. For Western blotting analysis, proteins were transferred onto a nitrocellulose membrane and immunoblotting was performed as described previously.24 The primary antibody was a rat anti-mouse IgG2b (Zymed Laboratories Inc, San Francisco, CA) used at 2 μg/mL in TBS + 1% BSA and the secondary antibody was an HRP-conjugated donkey anti-rat (Jackson Immunoresearch, Westgrove, PA) used at a 1:2,500 dilution in 5% skimmed milk. Immunostained proteins were detected using an enhanced chemiluminescence (ECL) kit according to manufacturer’s instructions (Amersham International PLC, Arlington Heights, IL).
In Vitro Growth and Cell Viability Assays
To examine the effects of bisphosphonates on in vitro growth and viability of 5TGM1 myeloma cells a dye exclusion assay was used. 5TGM1 myeloma cells were plated in 24 well plates at 105 cells per milliliter in IMDM supplemented with 5% FBS in the presence or absence of 1 to 100 μmol/L ibandronate or risedronate (kindly donated by Rhone-Poulenc-Rorer, Collegeville, PA). After incubation for 24 to 72 hours, trypan blue was added to 0.04% and the plates incubated at 37°C for 30 minutes. The total number of cells and the number of trypan blue-positive cells was then counted using a Neubauer chamber under brightfield illumination. Percentage viability was calculated by subtracting the number of trypan blue-positive (ie, nonviable) cells from the total cell number and expressing this figure as a percentage of the total cell number.
Statistical Analysis
Student’s t-test was used for comparisons made between two groups of data. In experiments where comparisons were made between more than two treatment groups, analysis of variance was used followed by the Student Newman-Keuls method of multiple comparisons. Statistical differences in the survival rate of the animals and the number of animals developing hindlimb paralysis were analyzed by the generalized Wilcoxon test.25 In all cases, data were accepted as significantly different with a probability of .05 or less.
RESULTS
Characterization of 5TGM1 Myeloma Cells
Histological analysis of affected bones from the founder animal from which the 5TGM1 myeloma variant was isolated revealed myeloma cells that had almost completely replaced the normal marrow and had caused bone destruction. An example of this is shown in Fig1A, which shows a lumbar vertebra from the founder animal. Note the loss of cortical bone (arrowheads), and the reduced amounts of trabecular bone at the endochondral growth plates. Myeloma cells (MY) can also be seen to have invaded the surrounding soft tissues. The spinal cord (SP) is surrounded by myeloma cells, which has resulted in spinal cord compression (the animal was paraplegic at the time of death). A similar pattern of tumor growth occurred after 4 weeks in mice that were inoculated with 106 5TGM1 myeloma cells (for examples refer to Fig 5). The myeloma-bearing mice also showed osteolytic lesions that were visible on radiographs (see Fig 2). Giemsa staining of cytocentrifuge preparations of 5TGM1 cells isolated from the marrow of myeloma-bearing mice revealed a typical myeloma morphology (see Fig 1B). ELISA measurements revealed that the serum IgG2b levels were elevated approximately 10- to 50-fold in myeloma-bearing animals compared with non–tumor-bearing controls (for examples refer to Fig 6). SDS-PAGE and Western blotting analysis confirmed increased serum monoclonal protein in myeloma-bearing animals, which co-migrated with a purified IgG2b standard and was recognized by an anti-IgG2b antibody by Western blotting (see Fig 1C and D).
Characterization of 5TGM1 myeloma cells. (A) Hematoxylin and eosin (H&E) stained section of a lumbar vertebra from the 5TGM1 founder animal, showing myeloma cells (MY), which have almost completely replaced the normal bone marrow and are associated with osteolysis. Note that trabecular bone is absent from the growth plate shown at left and greatly reduced in the growth plate shown at right. Arrowheads indicate areas where cortical bone has been destroyed, allowing tumor cells to invade the surrounding tissue. This animal was paraplegic at the time of death and in this section, the spinal cord (SP) can be seen to be surrounded by myeloma cells, which has resulted in spinal compression (bar = 200 μm). (B) Giemsa stained cytocentrifuge preparation of cultured 5TGM1 myeloma cells, exhibiting a characteristic myeloma morphology (bar = 100 μm). (C) Coomassie blue-stained SDS-PAGE analysis of serum from control mice (lanes 1, 2), 5TGM1 myeloma-bearing mice (lanes 3, 4), and the 5TGM1 founder animal (lane 5), lane 6 shows 1 μg of IgG2b standard for comparison. The arrow indicates the IgG2b band. (D) Identical gel to C, which was analyzed by Western blotting using antibodies against mouse IgG2b. Lanes 1 through 6 are the same as for C.
Characterization of 5TGM1 myeloma cells. (A) Hematoxylin and eosin (H&E) stained section of a lumbar vertebra from the 5TGM1 founder animal, showing myeloma cells (MY), which have almost completely replaced the normal bone marrow and are associated with osteolysis. Note that trabecular bone is absent from the growth plate shown at left and greatly reduced in the growth plate shown at right. Arrowheads indicate areas where cortical bone has been destroyed, allowing tumor cells to invade the surrounding tissue. This animal was paraplegic at the time of death and in this section, the spinal cord (SP) can be seen to be surrounded by myeloma cells, which has resulted in spinal compression (bar = 200 μm). (B) Giemsa stained cytocentrifuge preparation of cultured 5TGM1 myeloma cells, exhibiting a characteristic myeloma morphology (bar = 100 μm). (C) Coomassie blue-stained SDS-PAGE analysis of serum from control mice (lanes 1, 2), 5TGM1 myeloma-bearing mice (lanes 3, 4), and the 5TGM1 founder animal (lane 5), lane 6 shows 1 μg of IgG2b standard for comparison. The arrow indicates the IgG2b band. (D) Identical gel to C, which was analyzed by Western blotting using antibodies against mouse IgG2b. Lanes 1 through 6 are the same as for C.
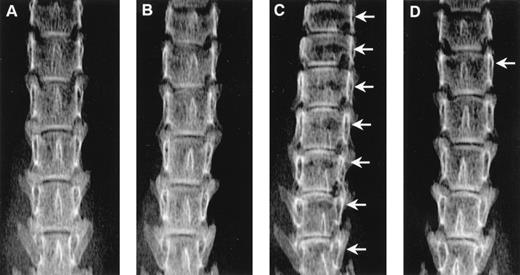
Radiographs showing the effect of ibandronate (4 μg per mouse per day for 28 days) on osteolytic lesions (arrows) in the lumbar vertebrae of 5TGM1 myeloma-bearing mice. (A) Non–tumor-bearing control treated with PBS. (B) Non–tumor-bearing control treated with ibandronate. (C) 5TGM1 myeloma-bearing mouse treated with PBS. (D) 5TGM1 myeloma-bearing mouse treated with ibandronate. Note the large number of lesions visible in myeloma-bearing mice treated with PBS (C), which are prevented by treatment with ibandronate (D). Note also the reduction in height of the lumbar vertebrae in myeloma-bearing mice treated with PBS (C) as compared with controls (A), (B), and myeloma-bearing mice treated with ibandronate (D). There are seven vertebrae visible in the field in (C), as compared with six in (A), (B), and (D).
Radiographs showing the effect of ibandronate (4 μg per mouse per day for 28 days) on osteolytic lesions (arrows) in the lumbar vertebrae of 5TGM1 myeloma-bearing mice. (A) Non–tumor-bearing control treated with PBS. (B) Non–tumor-bearing control treated with ibandronate. (C) 5TGM1 myeloma-bearing mouse treated with PBS. (D) 5TGM1 myeloma-bearing mouse treated with ibandronate. Note the large number of lesions visible in myeloma-bearing mice treated with PBS (C), which are prevented by treatment with ibandronate (D). Note also the reduction in height of the lumbar vertebrae in myeloma-bearing mice treated with PBS (C) as compared with controls (A), (B), and myeloma-bearing mice treated with ibandronate (D). There are seven vertebrae visible in the field in (C), as compared with six in (A), (B), and (D).
Effects of Ibandronate Treatment in 5TGM1 Myeloma-Bearing Mice
A number of different experimental protocols were designed using the 5TGM1 model of multiple myeloma to assess the effects of ibandronate on myeloma bone disease. Initial experiments were designed to determine the effect of continuous (prophylactic) treatment with ibandronate on 5TGM1 myeloma bone disease and also to determine the effects of ibandronate in non–tumor-bearing control animals. Ibandronate (4 μg per mouse per day) was administered at the time of tumor inoculation and daily thereafter for the duration of the experiment. Four experimental groups (n = 6) were used: group A received myeloma cells and daily injections of ibandronate, group B received myeloma cells and daily injections of vehicle (PBS), groups C and D did not receive myeloma cells, but received daily injections of ibandronate and vehicle (PBS), respectively. The experiment was terminated when the first animal developed hindlimb paralysis in either of the tumor-bearing groups.
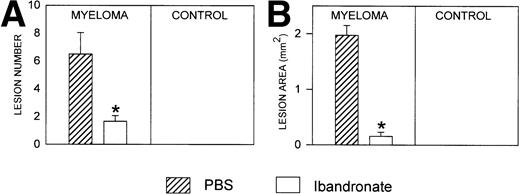
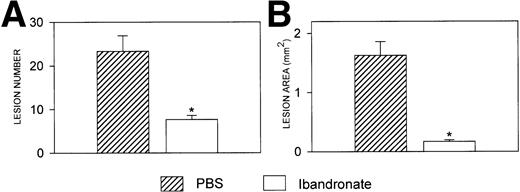
Mice injected with 5TGM1 myeloma cells showed osteolytic bone lesions by 4 weeks after tumor inoculation and experiments were terminated on day 28. Lesions in the vertebrae were readily detectable in 5TGM1 myeloma-bearing animals that were treated with PBS (representative radiographs are shown in Fig 2). In contrast, 5TGM1 myeloma-bearing animals that were treated with daily ibandronate showed a dramatic reduction in the number of osteolytic lesions visible in their vertebrae. Results from quantitation by computerized image analysis of the osteolytic lesions in the vertebrae of 5TGM1 myeloma-bearing mice with and without ibandronate treatment are shown in Fig3A and B. Ibandronate (4 μg per mouse per day) significantly reduced both the number and area of vertebral osteolytic lesions in 5TGM1 myeloma-bearing mice. Moreover, a significant reduction in the mean height of the lumbar vertebrae was noted in 5TGM1 myeloma-bearing mice compared with non–tumor-bearing controls, presumably due to crush fractures (see Figs 2 and 3C). This reduction in height was less severe in myeloma-bearing mice that were treated with ibandronate compared with myeloma-bearing mice treated with PBS. Myeloma-induced osteolytic lesions in the long bones were also significantly reduced by ibandronate treatment (see Fig 4A and B).
Histograms showing (A) the number and (B) the area of osteolytic lesions in the lumbar vertebrae of 5TGM1 myeloma-bearing and control mice with and without ibandronate treatment (4 μg per mouse per day for 28 days). Lesions were quantified by computerized image analysis from radiographs taken at sacrifice. Data are mean ± SEM (n = 6). ∗, Significant reduction compared with myeloma + PBS. (C) Histogram showing the effect of ibandronate treatment (4 μg per mouse per day for 28 days) on the mean height of the lumbar vertebrae in 5TGM1 myeloma-bearing and control mice. The height of each lumbar vertebra was measured by image analysis from radiographs taken at sacrifice and the mean vertebral height calculated for each animal. Data are mean ± SEM (n = 6). +, Significant decrease compared with PBS treated non–tumor-bearing control. ∗, Significant increase compared with myeloma + PBS.
Histograms showing (A) the number and (B) the area of osteolytic lesions in the lumbar vertebrae of 5TGM1 myeloma-bearing and control mice with and without ibandronate treatment (4 μg per mouse per day for 28 days). Lesions were quantified by computerized image analysis from radiographs taken at sacrifice. Data are mean ± SEM (n = 6). ∗, Significant reduction compared with myeloma + PBS. (C) Histogram showing the effect of ibandronate treatment (4 μg per mouse per day for 28 days) on the mean height of the lumbar vertebrae in 5TGM1 myeloma-bearing and control mice. The height of each lumbar vertebra was measured by image analysis from radiographs taken at sacrifice and the mean vertebral height calculated for each animal. Data are mean ± SEM (n = 6). +, Significant decrease compared with PBS treated non–tumor-bearing control. ∗, Significant increase compared with myeloma + PBS.
Histograms showing (A) the number and (B) the area of osteolytic lesions in the long bones of 5TGM1 myeloma-bearing and control mice with and without ibandronate treatment. Lesions were quantified by computerized image analysis from radiographs taken at death. Data are mean ± SEM (n = 6). ∗, Significant reduction compared with myeloma + PBS.
Histograms showing (A) the number and (B) the area of osteolytic lesions in the long bones of 5TGM1 myeloma-bearing and control mice with and without ibandronate treatment. Lesions were quantified by computerized image analysis from radiographs taken at death. Data are mean ± SEM (n = 6). ∗, Significant reduction compared with myeloma + PBS.
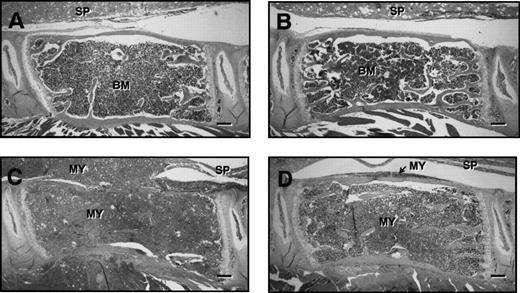
Figure 5 shows representative histological sections of lumbar vertebrae from control and myeloma-bearing mice treated with PBS or ibandronate. In myeloma-bearing mice treated with PBS (Fig 5C) a reduction in trabecular bone as well as occasional thinning and destruction of cortical bone was observed when compared with non–tumor-bearing control mice (Fig 5A and B). This loss of bone was prevented by treatment with ibandronate in myeloma-bearing animals (Fig 5D). Histomorphometric analysis confirmed that mean values for trabecular bone volume in the vertebrae of 5TGM1 myeloma-bearing mice treated with PBS were significantly lower than those in non–tumor-bearing controls (see Table 1). This reduction in bone volume was prevented by treatment with ibandronate. Mean values for trabecular bone volume in the vertebrae of both control and tumor-bearing animals treated with ibandronate were higher than those in the PBS-treated controls, but these differences failed to reach statistical significance.
H & E stained sections of lumbar vertebrae from control and 5TGM1 myeloma-bearing mice treated with PBS or ibandronate (4 μg per mouse per day for 28 days). (A) Non–tumor-bearing control treated with PBS, (B) non–tumor-bearing control treated with ibandronate, (C) 5TGM1 myeloma-bearing mouse treated with PBS, (D) 5TGM1 myeloma-bearing mouse treated with ibandronate. The spinal cord (SP) is indicated in each figure. Note that in the non–tumor-bearing animals, (A) and (B), the marrow cavity is filled with normal marrow (BM). In contrast in myeloma-bearing animals (C) and (D), the normal bone marrow has been replaced by myeloma cells (MY), which have also invaded the surrounding tissues. In the myeloma-bearing animal treated with PBS (C), there is a clear loss of trabecular bone and also some loss of cortical bone. This bone loss is prevented by ibandronate treatment (D). Bar = 200 μm.
H & E stained sections of lumbar vertebrae from control and 5TGM1 myeloma-bearing mice treated with PBS or ibandronate (4 μg per mouse per day for 28 days). (A) Non–tumor-bearing control treated with PBS, (B) non–tumor-bearing control treated with ibandronate, (C) 5TGM1 myeloma-bearing mouse treated with PBS, (D) 5TGM1 myeloma-bearing mouse treated with ibandronate. The spinal cord (SP) is indicated in each figure. Note that in the non–tumor-bearing animals, (A) and (B), the marrow cavity is filled with normal marrow (BM). In contrast in myeloma-bearing animals (C) and (D), the normal bone marrow has been replaced by myeloma cells (MY), which have also invaded the surrounding tissues. In the myeloma-bearing animal treated with PBS (C), there is a clear loss of trabecular bone and also some loss of cortical bone. This bone loss is prevented by ibandronate treatment (D). Bar = 200 μm.
Histomorphometric Analysis of Lumbar Vertebrae From Control and 5TGM1 Myeloma-Bearing Mice With or Without Ibandronate Treatment
| Treatment Group . | Percent Trabecular Bone Volume . | Cortical Bone Thickness (μm) . | Percent Marrow Replacement by Tumor . |
|---|---|---|---|
| Control + PBS | 18.1 ± 0.8 (n = 6) | 48.8 ± 1.4 (n = 6)† | None |
| Control + IBN | 21.0 ± 1.0 (n = 6) | 63.9 ± 3.4 (n = 6)* | None |
| Myeloma + PBS | 12.9 ± 1.2 (n = 6)*,† | 42.9 ± 2.5 (n = 4)† | 71.7 ± 12.2 (n = 6) |
| Myeloma + IBN | 21.3 ± 1.2 (n = 6) | 59.0 ± 2.9 (n = 5)* | 70.6 ± 4.9 (n = 6) |
| Treatment Group . | Percent Trabecular Bone Volume . | Cortical Bone Thickness (μm) . | Percent Marrow Replacement by Tumor . |
|---|---|---|---|
| Control + PBS | 18.1 ± 0.8 (n = 6) | 48.8 ± 1.4 (n = 6)† | None |
| Control + IBN | 21.0 ± 1.0 (n = 6) | 63.9 ± 3.4 (n = 6)* | None |
| Myeloma + PBS | 12.9 ± 1.2 (n = 6)*,† | 42.9 ± 2.5 (n = 4)† | 71.7 ± 12.2 (n = 6) |
| Myeloma + IBN | 21.3 ± 1.2 (n = 6) | 59.0 ± 2.9 (n = 5)* | 70.6 ± 4.9 (n = 6) |
The effect of ibandronate treatment (4 μg per mouse per day for 28 days) on percent trabecular bone volume, cortical bone thickness, and percent marrow replacement by tumor. Data are mean ± SEM. Each data point represents a mean calculated from measurements from at least three lumbar vertebrae for each animal.
Significantly different from control + PBS.
Significantly different from myeloma + ibandronate.
In myeloma-bearing mice treated with PBS, cortical bone loss was observed only in vertebrae that had a relatively high degree of marrow replacement by tumor. Histomorphometric analysis of cortical bone thickness in myeloma-bearing mice was therefore restricted to vertebrae in which the marrow replacement by tumor was shown to be greater than 70% (see Table 1). The mean cortical bone thickness was significantly lower in myeloma-bearing mice treated with PBS compared with myeloma-bearing and non–tumor-bearing mice treated with ibandronate, consistent with the anti-resorptive effects of this drug. Although myeloma-bearing animals treated with PBS showed a lower mean cortical bone thickness compared with non–tumor-bearing controls treated with PBS, this difference did not reach statistical significance.
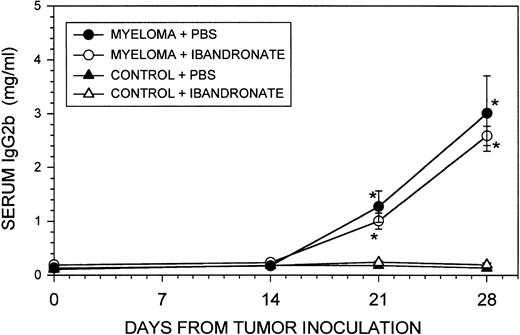
In contrast to its effect on osteolytic lesions and trabecular bone volume, ibandronate treatment did not significantly affect the percentage of bone marrow replaced by tumor cells (see Table 1). The liver and spleen are the two major sites other than bone where myeloma growth occurs in the 5T33 and 5TGM1 myeloma models. Ibandronate treatment had no significant effect on the 5TGM1 tumor volume in the liver and spleen, as reflected in the organ weights at death and by histomorphometric analysis (see Table 2). Ibandronate also did not significantly reduce the total tumor burden, as assessed by serum IgG2b concentrations (see Fig 6). Body weights of the animals were similar in all groups throughout the experiment and showed a modest reduction in tumor-bearing animals between days 21 and 28. However, this reduction was not significantly different from control non–tumor-bearing animals (data not shown).
Effect of Ibandronate on Tumor Burden to the Liver and Spleen in 5TGM1 Myeloma-Bearing Mice
| Treatment Group . | Organ Weights (g) . | Percent Tumor Volume . | ||
|---|---|---|---|---|
| Spleen . | Liver . | Spleen . | Liver . | |
| Control + PBS (n = 6) | 0.066 ± 0.003 | 1.029 ± 0.051 | None | None |
| Control + IBN (n = 6) | 0.073 ± 0.006 | 1.060 ± 0.020 | None | None |
| Myeloma + PBS (n = 6) | 0.191 ± 0.027* | 1.778 ± 0.089* | 47.0 ± 9.40* | 2.58 ± 0.66* |
| Myeloma + IBN (n = 6) | 0.194 ± 0.015* | 1.246 ± 0.029 | 59.4 ± 5.23* | 3.29 ± 1.22* |
| Treatment Group . | Organ Weights (g) . | Percent Tumor Volume . | ||
|---|---|---|---|---|
| Spleen . | Liver . | Spleen . | Liver . | |
| Control + PBS (n = 6) | 0.066 ± 0.003 | 1.029 ± 0.051 | None | None |
| Control + IBN (n = 6) | 0.073 ± 0.006 | 1.060 ± 0.020 | None | None |
| Myeloma + PBS (n = 6) | 0.191 ± 0.027* | 1.778 ± 0.089* | 47.0 ± 9.40* | 2.58 ± 0.66* |
| Myeloma + IBN (n = 6) | 0.194 ± 0.015* | 1.246 ± 0.029 | 59.4 ± 5.23* | 3.29 ± 1.22* |
The effect of ibandronate treatment (4 μg per mouse per day for 28 days) on tumor burden to liver and spleen, as assessed by organ weights and percent tumor volume as measured by histomorphometry. Data are mean ± SEM.
Significantly different from control + PBS.
Graph showing the effect of ibandronate treatment (4 μg per mouse per day) on serum IgG2b concentrations in 5TGM1 myeloma-bearing and control mice. Serum IgG2b levels were measured by ELISA. Data are mean ± SEM (n = 6). ∗, Significantly different from control + PBS. No significant differences were observed between myeloma-bearing animals treated with PBS or with ibandronate.
Graph showing the effect of ibandronate treatment (4 μg per mouse per day) on serum IgG2b concentrations in 5TGM1 myeloma-bearing and control mice. Serum IgG2b levels were measured by ELISA. Data are mean ± SEM (n = 6). ∗, Significantly different from control + PBS. No significant differences were observed between myeloma-bearing animals treated with PBS or with ibandronate.
Effect of Ibandronate on Hindlimb Paralysis and Survival in 5TGM1 Myeloma-Bearing Animals
Further experiments were designed to determine whether ibandronate treatment of 5TGM1 myeloma-bearing animals had any beneficial effect on their survival or the onset of hindlimb paralysis, which occurs frequently in the murine 5T myeloma model. Two experimental groups were used (n = 10). Group A received myeloma cells and daily injections of vehicle (PBS) beginning at the time of tumor inoculation and continuing until the death of each animal. Group B received daily injections of ibandronate (4 μg per mouse per day) beginning at the time of tumor inoculation and continuing until the death of each animal.
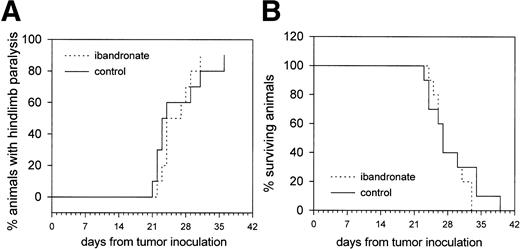
Treatment with ibandronate did not significantly reduce the occurrence of hindlimb paralysis or prolong survival in 5TGM1 myeloma-bearing mice (see Fig 7). At necropsy, both PBS and ibandronate-treated mice showed enlargement of the liver and spleen, due to growth of the myeloma in these sites. It is therefore assumed that the animals died from the consequences of large tumor burdens in these organs because of the large amount of circulating monoclonal protein and/or opportunistic infections or from some other illness due to the immunocompromization resulting from replacement of marrow with myeloma cells.
Graphs showing the effect of ibandronate treatment (4 μg per mouse per day) on (A) hindlimb paralysis and (B) survival in 5TGM1 myeloma-bearing mice. Significant differences were not observed between myeloma-bearing mice treated with PBS or with ibandronate.
Graphs showing the effect of ibandronate treatment (4 μg per mouse per day) on (A) hindlimb paralysis and (B) survival in 5TGM1 myeloma-bearing mice. Significant differences were not observed between myeloma-bearing mice treated with PBS or with ibandronate.
Effect of Ibandronate Treatment in 5T33 Myeloma-Bearing Mice
The effects of continuous (prophylactic) treatment with ibandronate were also assessed in another murine model of myeloma, which uses the 5T33 clonal myeloma cell line. Ibandronate (4 μg per mouse per day) was administered at the time of tumor inoculation and thereafter administered daily for the duration of the experiment. Two experimental groups (n = 6) were used: group A received myeloma cells and daily injections of ibandronate, group B received myeloma cells and daily injections of vehicle (PBS). The experiment was terminated when the first animal developed hindlimb paralysis in either of the tumor-bearing groups.
Mice injected with the clonal 5T33 myeloma cell line showed osteolytic bone lesions similar to the 5TGM1 myeloma, by 10 weeks after tumor inoculation. These lesions occurred predominantly in the long bones. Figure 8 shows results from quantitation by image analysis of the area and number of osteolytic lesions seen on radiographs of the long bones of 5T33 myeloma-bearing mice at death. Treatment of myeloma-bearing mice with ibandronate (4 μg per mouse per day) significantly reduced both the number and area of osteolytic lesions. Similar to the results obtained with the 5TGM1 myeloma, ibandronate treatment did not reduce the total tumor burden, as assessed by the serum IgG2b concentrations and the liver and spleen weights (data not shown). Body weights of the animals were similar in all groups (data not shown).
Histograms showing (A) the number and (B) the area of osteolytic lesions in the long bones of 5T33 myeloma-bearing mice with and without ibandronate treatment. Lesions were quantified by computerized image analysis from radiographs taken at death. Data are mean ± SEM (n = 6). ∗, Significant reduction compared with myeloma + PBS.
Histograms showing (A) the number and (B) the area of osteolytic lesions in the long bones of 5T33 myeloma-bearing mice with and without ibandronate treatment. Lesions were quantified by computerized image analysis from radiographs taken at death. Data are mean ± SEM (n = 6). ∗, Significant reduction compared with myeloma + PBS.
Effect of Bisphosphonate Treatment on 5TGM1 Cell Growth In Vitro
The above data suggested that ibandronate inhibited bone destruction without reducing tumor burden in the 5TGM1 mouse myeloma model, however, bisphosphonates have recently been reported to induce apoptosis in myeloma cell lines in vitro.10,11 To further investigate the lack of effect of ibandronate on tumor burden in the 5TGM1 in vivo model of myeloma, we examined its effects on in vitro growth and apoptosis of the 5TGM1 myeloma cell line. The bisphosphonate risedronate was also tested for comparison, as it is a non-amino bisphosphonate with similar potency.26 In the present study, using a dose of 4 μg ibandronate per mouse, we estimate the peak serum concentration of ibandronate to be approximately 5 μmol/L (assuming a blood volume of approximately 2 mL for a 20-g mouse). Initial experiments to examine the effects of bisphosphonates on growth of 5TGM1 cells in vitro were therefore performed using a dose range of 1 to 5 μmol/L ibandronate or risedronate. Using this dose range, no significant effect was seen with ibandronate or risedronate on the total number of myeloma cells or on the percentage of viable cells in the cultures, even in prolonged cultures of up to 72 hours (data not shown). An extended dose range of 10 to 100 μmol/L ibandronate was then tested (see Table 3). Treatment for 72 hours with 10 μmol/L ibandronate produced a slight but significant reduction in the total number of myeloma cells with no significant effect on the percentage of viable cells. Treatment with 50 μmol/L caused a modest reduction in both cell number and cell viability, and a more dramatic effect was seen with 100 μmol/L, where there was an almost complete loss of cell viability.
Effects of Ibandronate on In Vitro Growth and Viability of 5TGM1 Cells
| Treatment Group . | Total Cell Number (×103) . | Percent Viability . |
|---|---|---|
| Control | 233.5 ± 8.9 | 93.9 ± 1.8 |
| IBN (10 μmol/L) | 199.8 ± 11.63-150 | 87.3 ± 3.0 |
| IBN (50 μmol/L) | 166.5 ± 13.03-150 | 69.0 ± 1.83-150 |
| IBN (100 μmol/L) | 78.0 ± 8.63-150 | 11.6 ± 5.03-150 |
| Treatment Group . | Total Cell Number (×103) . | Percent Viability . |
|---|---|---|
| Control | 233.5 ± 8.9 | 93.9 ± 1.8 |
| IBN (10 μmol/L) | 199.8 ± 11.63-150 | 87.3 ± 3.0 |
| IBN (50 μmol/L) | 166.5 ± 13.03-150 | 69.0 ± 1.83-150 |
| IBN (100 μmol/L) | 78.0 ± 8.63-150 | 11.6 ± 5.03-150 |
The effect of ibandronate (IBN) treatment for 72 hours on cell number and percent viability in cultures of 5TGM1 myeloma cells. Data are mean ± SEM (n = 4).
Significantly different from control.
DISCUSSION
We have used the murine 5T in vivo model of myeloma to study the effects of the bisphosphonate ibandronate, which is a powerful inhibitor of osteoclastic bone resorption, on the osteolytic bone disease associated with this cancer. The advantage of this in vivo animal model is that the effects of the bisphosphonate can be quantified in the complete absence of other cancer therapies, which often compound the results of studies in human patients.5-8,12 13 We have found that ibandronate markedly inhibits myeloma-associated bone destruction without reducing the total tumor burden in this animal model, using two different myeloma cell lines.
In contrast to the data reported here, we have previously shown that ibandronate and other bisphosphonates reduce tumor burden to the skeleton in models of osteolysis induced by breast cancer cells.9,21,27 In those studies, although the bisphosphonates had no beneficial effect on growth of breast cancer metastases in soft tissue organs, they dramatically reduced tumor burden in the bone and bone marrow. We hypothesized that factors released by the breast cancer cells stimulate osteoclastic resorption. The actively resorbing osteoclasts then in turn release factors such as transforming growth factor beta (TGFβ) and insulin-like growth factors (IGFs), which change the local bone microenvironment so that it is more favorable for growth of the breast cancer cells. This may lead to a vicious cycle between growth of the breast cancer cells and pathologic bone resorption.4 Myeloma cells, in contrast to breast cancer cells, are derived from hematopoietic cell lineages, which normally reside in the bone marrow. Thus, the marrow microenvironment may already be favorable for growth of the myeloma cells independent of local rates of bone resorption, possibly by the production by marrow stroma of cytokines such as interleukin-6.28-30 This may explain why myeloma cells, unlike breast cancer cells, appear to be able to grow in the marrow cavity and completely replace the normal marrow cells even when osteoclastic activity is inhibited by bisphosphonates such as ibandronate. Thus myeloma cells may be less dependent than breast cancer cells on factors released by resorbing osteoclasts for their growth in the bone marrow cavity.
Our data showing that bisphosphonates reduce skeletal lesions in the 5T model of myeloma bone disease confirms and extends an earlier study using pamidronate.31 In this study, pamidronate was effective in reducing osteolysis in mice bearing the 5T2 myeloma line. Similar to the present study, no effect of pamidronate on the total tumor burden or tumor burden to the skeleton was observed. However, the effect of pamidronate on tumor burden in non-bone sites was not assessed. In the present study, ibandronate treatment was effective in reducing myeloma-associated osteolytic lesions when administered at the time of tumor inoculation and continually for the duration of the experiment. Future studies are required using therapeutic regimens in which treatment is initiated after establishment of the myeloma or is given in conjunction with other anti-cancer therapies. This may more closely model the clinical situation, in which treatment is initiated after clinical diagnosis of multiple myeloma, at which stage the patient frequently already has extensive bone involvement. Our results provide support for recent clinical studies using bisphosphonates, which report that they are effective in reducing the occurrence of skeletal-related events (ie, pathologic fractures, radiation therapy to bone, surgery to bone, spinal cord compression), reducing progression of osteolytic lesions on x-ray, reducing pain scores, and improving quality of life assessments in myeloma patients.5,7 13
In the present study ibandronate treatment was not effective in preventing the development of hindlimb paralysis in tumor-bearing mice. Hindlimb paralysis is a frequent complication of disease in 5T myeloma-bearing mice and probably occurs due to spinal cord compression by myeloma cells. However, at present it is not clear whether these cells gain access to the spinal cord by escaping from the vertebral marrow cavity through resorption-induced cavities or by direct invasion of the spinal canal through pre-existing vascular channels independent of bone resorption.
Although ibandronate had beneficial effects on the osteolytic component of the myeloma disease, it did not prolong survival of 5TGM1 myeloma-bearing mice. Consistent with this observation, ibandronate did not reduce the total tumor burden, as assessed by serum IgG2b levels, or the tumor burden in the bone marrow cavities, liver, and spleen. However, the natural history of the disease in these mice runs a rapid course over a few weeks, and thus effects on survival are not as easy to discern as they may be in humans, where the median survival is several years. Animals probably died of other complications of the myeloma disease, such as liver involvement, renal failure, and/or infections due to compromised immunity. Interestingly, in a follow-up to the study of Radl et al,31 Croese et al reported a significant prolongation of survival in 5T2 myeloma-bearing mice treated with pamidronate, although no significant effect on tumor growth was observed.32 The beneficial effect of pamidronate in this model might be due to differences in the modes of action of different bisphosphonates or to the longer times (months as opposed to weeks) required for the 5T2 myeloma disease to run its course.
Our results support those of a number of human clinical trials in which the effects of bisphosphonates on survival have been reported. Lahtinen et al,6 Laakso et al,7 and McCloskey et al8 showed no improvement in survival in myeloma patients treated with clodronate. Similar results were reported by Brincker et al12 using oral pamidronate. In a randomized double-blind, placebo-controlled study of intravenous pamidronate treatment for multiple myeloma, no significant effect of pamidronate on overall patient survival was observed; however, in a subgroup of patients who entered the trial receiving salvage chemotherapy, a significant improvement in survival was observed.5 13
Recent in vitro studies have raised the intriguing possibility that bisphosphonates may exhibit anti-tumor activity by directly stimulating apoptosis in myeloma cells.10,11 However, in these studies the concentrations of bisphosphonates used were generally over 10 μmol/L, even for the most potent bisphosphonate presently available, Zoledronate (Novaritis, Basel, Switzerland).11 These doses are high relative to the peak serum concentrations achieved in patients undergoing treatment with bisphosphonates. Thus, it remains to be determined whether sufficiently high levels of bisphosphonates could readily be attained in patients for this anti-tumor activity to manifest. We estimate that the peak serum concentrations of ibandronate achieved in the present study were approximately 5 μmol/L. This concentration of ibandronate did not affect the growth or viability of 5TGM1 cells in vitro, and cytotoxic effects were seen only at doses of 50 μmol/L and higher. This cytotoxic effect is probably nonspecific, as a similar loss of viability was observed in normal mouse bone marrow cultures and in a murine osteoblast cell line, 2T3, using similar concentrations of ibandronate (data not shown). In contrast, the doses of bisphosphonates effective in inducing apoptosis in osteoclasts are several orders of magnitude lower,33 suggesting a specific effect of bisphosphonates on osteoclast apoptosis. Although we report no significant effect of ibandronate on growth and viability of 5TGM1 myeloma cells in vivo or in vitro, except at high doses that produced nonspecific effects on other cell types, the possibility remains that bisphosphonates with different chemical structures may have different effects on tumor cell growth and apoptosis. Thus, the cytotoxic effects of bisphosphonates on myeloma cells may be limited to particular bisphosphonates with specific structural features. Future studies are clearly warranted to address this important question. The 5T mouse myeloma model may be an ideal system in which to compare the effects of bisphosphonates with different chemical side groups for their effects on both tumor-associated bone resorption and on growth and apoptosis of the tumor cells.
In summary, our results suggest that although not useful as a direct therapy to reduce tumor burden in myeloma patients, ibandronate may be extremely useful as an adjunctive therapy for the treatment of the osteolytic component of myeloma disease. Since it is this bone destructive component that causes the most distressing and painful symptoms for the patient, the use of bisphosphonates such as ibandronate in myeloma disease may improve dramatically the quality of life of the patient. However, other treatments, such as chemotherapy, radiotherapy, and marrow transplantation should continue to be the main therapies directed at preventing growth of the myeloma cells.
ACKNOWLEDGMENT
We gratefully acknowledge the technical assistance provided by Arlene Farias and secretarial assistance provided by Nancy Garrett.
Supported by Grant No. P01-CA40035 (from the National Institutes of Health) and by Boehringer Mannheim, GmbH, D-68305 Mannheim, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Sarah L. Dallas, PhD, Department of Medicine/Endocrinology, University of Texas Health Science Center, San Antonio, TX 78284-7877; e-mail: dallas@uthscsa.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal