Abstract
The Bcr-Abl oncogene, found in Philadelphia chromosome-positive myelogenous leukemia (CML), activates Ras and triggers the stress-activated protein kinase (SAPK or Jun NH2-terminal kinase [JNK]) pathway. Interruption of Ras or SAPK activation dramatically reduces Bcr-Abl–mediated transformation. Here, we report that Bcr-Abl through a Ras-dependent pathway signals the serine/threonine protein kinase GCKR (Germinal Center Kinase Related) leading to SAPK activation. Either an oncogenic form of Ras or Bcr-Abl enhances GCKR catalytic activity and its activation of SAPK, whereas inhibition of GCKR impairs Bcr-Abl–induced SAPK activation. Bcr-Abl mutants that are impaired for GCKR activation are also unable to activate SAPK. Consistent with GCKR being a functional target in CML, GCKR is constitutively active in CML cell lines and found in association with Bcr-Abl. Our results indicate that GCKR is a downstream target of Bcr-Abl and strongly implicate GCKR as a mediator of Bcr-Abl in its transformation of cells.
CHRONIC MYELOGENOUS leukemia (CML) is a human leukemia associated with the t(9,22) Philadelphia translocation, which fuses Bcr gene sequence upstream of the c-abl gene.1 Consistent with a pathogenic role in this leukemia, Bcr-Abl transforms fibroblasts and hematopoietic cells and can recapitulate a CML-like disease in mice.2-5Bcr-Abl encodes, depending on the breakpoint, a constitutively active 210-kD or 185-kD cytoplasmic tyrosine kinase. Although the biochemical mechanisms used byBcr-Abl to transform myeloid cells remain incompletely understood, considerable evidence supports a requirement for the small GTPase Ras. Moreover, multiple and redundant signaling pathways lead from Bcr-Abl to Ras activation.6 7
One of the consequences of Bcr-Abl–induced Ras activation is increased activity of stress-activated protein kinases (SAPK, also referred to as c-Jun NH2-terminal Kinases or JNK) and this activation appears crucial for Bcr-Abl–induced cellular transformation.8,9 The SAPK are the terminal kinases in a three-tiered protein kinase cascade, which also includes a mitogen-activated protein kinase (MAPK) kinase kinase (MAPKKK or MEKK), and a MAPK kinase (SEK1/MKK4 or MEK). Thus, a MEKK activates a MEK that in turn activates SAPK. Among the MEKK that can activate the SAPK pathway are MEKK1, MEKK2, and the mixed-lineage kinase termed MLK-3.10-14 Substrates of the SAPK include the transcription factors AP-1 and Elk-1. These transcription factors regulate the expression of many of the genes involved in the inflammatory response.10 11
As their name indicates, the SAPK are activated by cellular stresses such as heat shock, ultraviolet irradiation, or osmotic shock, and by the inflammatory cytokines tumor necrosis factor (TNF) and interleukin-1.10,11 These cellular stresses trigger intracellular signal transducers that lead to activation of the SAPK cascade. Undoubtedly among them are members of an interesting subfamily of serine/threonine protein kinases. GCK (Germinal Center Kinase),15,16 HPK1 (Hematopoietic Progenitor Kinase),17,18 GCKR (Germinal Center Kinase Related),19 the identical kinase is also termed kinase homologous to SSP1/STE20 (KHS),20 and GLK (germinal center-like kinase)21 all activate the SAPK, but not the related MAPK pathway. Based on the observation that Bcr-Abl preferentially activated the SAPK pathway8 and preliminary experiments that showed Ras to be a potent activator of GCKR, we examined whether GCKR could link Bcr-Abl to SAPK activation.
MATERIALS AND METHODS
Cell lines.
K562, BV173, KCL22, and KY04 are Bcr-Abl+ cell lines whereas U937 cells were derived from a patient with a histiocytic lymphoma and Jurkat cells from a T-cell leukemia. The BV173, KCL22, and KY04 cell lines were provided by Dr John Barrett (Bethesda, MD) whereas the K562, Jurkat, HEK 293, and U937 cells were obtained from the American Type Culture Collection (Rockville, MD). The HEK 293T (embryonal kidney) cell line was obtained following permission from Dr D. Baltimore (Pasadena, CA).
Plasmids and antibodies.
The pMT3-HA-SAPK-p46β plasmid was provided by Dr J. Kyriakis (Boston, MA). Bcr-Abl, Bcr-Abl mutants, and v-Abl expression vectors in pSRαMSVtk Neo were previously described.7,25,26 pcDNA3-Ras-V12, pcDNA3-Ras-N17, pcDNA3-Rac-QL, and pcDNA3-Cdc42-QL were kind gifts of Dr S. Gutkind (Bethesda, MD). The pcDNA-HA-GCKR, pCRIII-GCKR, and pCRIII-GCKR(AS) were described previously.19 The pcDNA-HA-GCKR deletion constructs 1-691, 1-599,1-493, and 1-396 were created by inserting the appropriate polymerase chain reaction product from pcDNA-HA-GCKR into pcDNA-HA. The anti-HA (12CA), anti-pY (4G10), and anti-Abl antibodies were purchased from Boehringer Mannheim (Mannheim, Germany), Upstate Biotechnology (Lake Placid, NY), and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. The GCKR antiserum was generated in rabbits by immunizing with a peptide (RKETEARDEMC) coupled to Keyhole limpet hemocyanin as described.19
In vitro kinase assays, immunoblotting, and coimmunoprecipitations.
The HEK 293T and HEK 293 cells were transiently transfected using a calcium phosphate method. Transfected DNA levels were normalized with control plasmids. Forty-eight to 72 hours after the transfection, HA-immunoprecipitates were subjected to in vitro kinase assays using myelin basic protein or c-jun (79) as substrates for the GCKR and SAPK assays, respectively.19 Before the in vitro kinase assay the HA-immunoprecipitates were washed three times with kinase lysis buffer (20 mmol/L Hepes pH 7.4, 2 mmol/L EGTA, 50 mmol/L β-glycerophosphate, 1% Triton X-100, 1 mmol/L Na3V04, and 10% glycerol) to which a protease inhibitor cocktail tablet was added [Boehringer Mannheim]), three times with a LiCl wash buffer (500 mmol/L LiCl, 100 mmol/L Tris pH 7.4, 0.1% Triton X-100, and 1 mmol/L dithiothreitol), and three times with kinase buffer (20 mmol/L MOPS pH 7.2, 2 mmol/L EGTA, 10 mmol/L MgCl2, and 0.1% Triton X-100). The GCKR (1:300 dilution), HA, and phosphotyrosine immunoblots were performed using standard methodology. The signals were detected by enhanced chemiluminescence (ECL; Amersham, Arlington Heights, IL). The coimmunoprecipitation was performed using lysates (1% digitonin, 50 mmol/L Tris, pH 8.0, 150 mmol/L NaCl, 0.5 mmol/L EDTA, plus protease inhibitors or 20 mmol/L Tris, pH 8.0, 137 mmol/L NaCl, 2 mmol/L EDTA, 1% Triton X-100, 1 mmol/L sodium orthovanadate, plus protease inhibitors) prepared from HEK 293T cells coexpressing HA-GCKR (2 μg) and Bcr-Abl wild type or mutants (2 μg) or from K562 cells. Anti-HA, anti-Abl, anti-GCKR, or the 4G10 monoclonal antibody (MoAb) were added and the immunoprecipitates collected with the appropriate secondary antibody-coupled magnetic beads (Dynal Corp, Oslo, Norway). They were washed three times in lysis buffer, twice in lysis buffer with 1 mol/L NaCl, fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and analyzed by immunoblotting with the appropriate antibody.
RESULTS
Bcr-Abl activates GCKR and the SAPK pathway through a Ras-dependent mechanism.
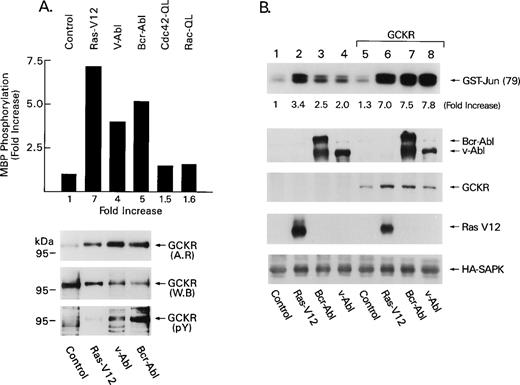
We first examined the effect of Bcr-Abl, v-Abl, or an oncogenic form of Ras on GCKR kinase activity, which we assessed using an in vitro kinase assay that measures the ability of immunoprecipitated GCKR (tagged with a HA epitope) to phosphorylate myelin basic protein (MBP). We observed that HA-GCKR immunoprecipitates prepared from HEK 293T cells transfected with constructs that direct the expression of HA-GCKR and either Bcr-Abl, v-Abl, or Ras-V12 contained elevated levels of GCKR kinase activity (Fig 1A). In contrast, an in vitro kinase assay performed with a HA-GCKR-T178A immunoprecipitate prepared from HEK 293T cells coexpressing HA-GCKR-T178A and Bcr-Abl did not result in a significant phosphorylation of myelin basic protein (data not shown). GCKR-T178A had been previously shown to lack protein kinase activity and to behave as a dominant negative inhibitor of wild-type GCKR activity.19 Expression of activated forms of two other small GTPases (cdc42 and rac), which likely activate the SAPK pathway via a PAK kinase,22 23 did not appreciably activate GCKR. Inspection of the in vitro kinase assay showed a 97 kD phosphoprotein, which HA-immunoblotting identified as GCKR (Fig 1A, top and middle panels). Of note, HA-GCKR migrated slower on SDS-PAGE when immunoprecipitated from the Ras-V12, Bcr-Abl, or v-Abl transfected cells. This result is consistent with GCKR autophosphorylation induced by the activating stimuli and/or phosphorylation by a coimmunoprecipitating protein kinase. However, the wash conditions employed for the in vitro kinase are very stringent and we did not observe any other bands on the autoradiograph besides MBP and GCKR. This would suggest that the phosphorylations, which occurred during the in vitro kinase assays, are predominantly GCKR-mediated MBP phosphorylation and GCKR autophosphorylation. Reimmunoblotting with an antiphosphotyrosine MoAb showed the presence of tyrosine phosphorylation, which was most evident with Bcr-Abl although also observed with v-Abl, but not with Ras-V12 (Fig 1A, bottom panel). Because we did not observe any bands in the in vitro kinase assay that might correspond to Bcr-Abl or v-Abl, the GCKR tyrosine phosphorylation likely occurred in vivo.
GCKR is a downstream target of Bcr-Abl and v-Abl. (A) Bcr-Abl, v-Abl, and Ras-V12 activate GCKR kinase activity. HA-GCKR immunoprecipitates from 1 × 106 HEK 293T cells transfected with control vector (2 μg), Bcr-Abl (2 μg), v-Abl (2 μg), Ras-V12 (2 μg), Cdc42-QL (2 μg), or Rac-QL (2 μg) in the presence of HA-GCKR (1 μg) were assayed for kinase activity using myelin basic protein (MBP) as a substrate. The amount of 32P incorporated into MBP was determined by excising the appropriate band and scintillation counting (results shown in bar graph) and the fold increases compared with control transfections. A portion of the gel was transferred to nitrocellulose and autoradiographed (AR). The same blot was reacted with an anti-HA MoAb (WB), stripped, and then with an antiphosphotyrosine MoAb (pY). Immunoreactivity was detected by enhanced chemiluminescence (ECL). Experiments were performed three times with similar results. (B) Overexpression of GCKR augments the induction of SAPK activity triggered by Bcr-Abl, v-Abl, or Ras-V12. HA-SAPK immunoprecipitates from 1 × 106 HEK 293T cells transfected with Bcr-Abl (2 μg), v-Abl (2 μg), Ras-V12 (2 μg) in the presence of pCR3-GCKR (1 μg) or a control vector (1 μg) along with HA-SAPK (1 μg) were assayed for kinase activity using GST-Jun (79) as a substrate. Bcr-Abl, v-Abl, GCKR, and Ras levels were assessed by immunoblotting (second, third, and fourth panels). The GCKR immunoblot was performed with the GCKR-specific antiserum. HA immunoblotting verified equivalent levels of HA-SAPK (bottom panel). These experiments were performed three times with similar results.
GCKR is a downstream target of Bcr-Abl and v-Abl. (A) Bcr-Abl, v-Abl, and Ras-V12 activate GCKR kinase activity. HA-GCKR immunoprecipitates from 1 × 106 HEK 293T cells transfected with control vector (2 μg), Bcr-Abl (2 μg), v-Abl (2 μg), Ras-V12 (2 μg), Cdc42-QL (2 μg), or Rac-QL (2 μg) in the presence of HA-GCKR (1 μg) were assayed for kinase activity using myelin basic protein (MBP) as a substrate. The amount of 32P incorporated into MBP was determined by excising the appropriate band and scintillation counting (results shown in bar graph) and the fold increases compared with control transfections. A portion of the gel was transferred to nitrocellulose and autoradiographed (AR). The same blot was reacted with an anti-HA MoAb (WB), stripped, and then with an antiphosphotyrosine MoAb (pY). Immunoreactivity was detected by enhanced chemiluminescence (ECL). Experiments were performed three times with similar results. (B) Overexpression of GCKR augments the induction of SAPK activity triggered by Bcr-Abl, v-Abl, or Ras-V12. HA-SAPK immunoprecipitates from 1 × 106 HEK 293T cells transfected with Bcr-Abl (2 μg), v-Abl (2 μg), Ras-V12 (2 μg) in the presence of pCR3-GCKR (1 μg) or a control vector (1 μg) along with HA-SAPK (1 μg) were assayed for kinase activity using GST-Jun (79) as a substrate. Bcr-Abl, v-Abl, GCKR, and Ras levels were assessed by immunoblotting (second, third, and fourth panels). The GCKR immunoblot was performed with the GCKR-specific antiserum. HA immunoblotting verified equivalent levels of HA-SAPK (bottom panel). These experiments were performed three times with similar results.
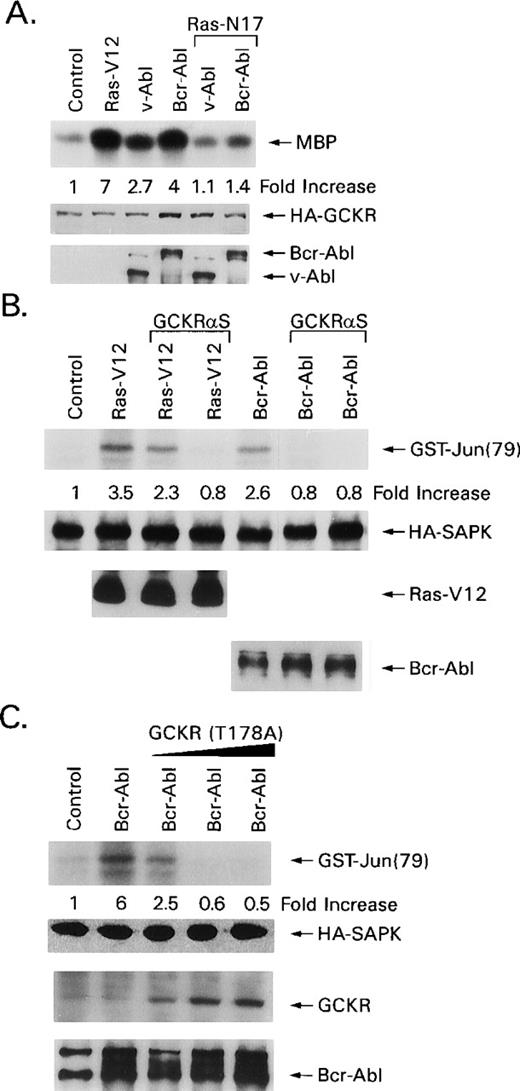
Supporting a role for GCKR in Bcr-Abl–mediated SAPK activation; GCKR-enhanced SAPK activation by Bcr-Abl or v-Abl. HEK 293T cells expressing Ras-V12, Bcr-Abl, or v-Abl had modest increases in SAPK activity (3-, 2.5-, and 2-fold, respectively, in the experiment shown), whereas the addition of GCKR further augmented them (7-, 7.5-, and 7.8-fold, respectively, Fig 1B). Low levels of GCKR were transfected in this experiment to avoid significant SAPK activation by GCKR alone. Coexpression of GCKR did not alter Bcr-Abl, v-Abl, Ras-V12, or SAPK levels. To test whether Ras was involved in Bcr-Abl–induced GCKR activation we used a dominant negative form of Ras, Ras-N17, to inhibit endogenous Ras. Coexpression of Ras-N17 blocked GCKR activation by either Bcr-Abl or v-Abl nearly reducing GCKR activity in the in vitro assay to baseline (Fig 2A). The coexpression Ras-N17 did not alter the expression levels of GCKR, Bcr-Abl, or v-Abl.
Bcr-Abl–induced GCKR activation requires Ras and Bcr-Abl–triggered SAPK activation requires GCKR. (A) A dominant negative Ras inhibits Bcr-Abl–induced GCKR activation. HEK 293T cells (1 × 106) were transfected with HA-GCKR (1 μg) and Ras-V12 (2 μg), v-Abl (4 μg), or Bcr-Abl a (4 μg). The v-Abl and Bcr-Abl transfections were performed in the presence of a control vector (4 μg) or Ras-N17 (4 μg), indicated by bracket. HA-GCKR immunoprecipitates were assayed by an in vitro kinase assay. HA-GCKR and Abl immunoblotting were performed by ECL. These experiments were performed two times with similar results. (B) GCKR antisense expression impairs Ras- and Bcr-Abl–induced SAPK activation. HA-SAPK immunoprecipitates from HEK 293T cells (1 × 106) transfected with Bcr-Abl (4 μg) or Ras-V12 (2 μg) in the presence of pCRIII-GCKR-AS (4 or 8 μg) and HA-SAPK (1 μg) were assayed for kinase activity. A HA-immunoblot verified equivalent levels of HA-SAPK. Ras and Bcr-Abl levels were verified by immunoblotting. Data are reported as increase in SAPK activity compared with basal levels in control transfections. These experiments were performed four times with similar results. (C) A GCKR mutant impairs Bcr-Abl–induced SAPK activation. HA-SAPK immunoprecipitates from HEK 293 cells (1 × 106) transfected with Bcr-Abl (4 μg) and HA-SAPK (1 μg) in the presence or absence of GCKR-T178A (2, 4, or 6 μg) were assessed for kinase activity. Data are reported as in part B. A HA-immunoblot verified equivalent levels of HA-SAPK. Bcr-Abl levels are shown. GCKR (endogenous and the transfected mutant) were detected by immunoblotting with the GCKR-specific antiserum. This experiment was performed twice with similar results.
Bcr-Abl–induced GCKR activation requires Ras and Bcr-Abl–triggered SAPK activation requires GCKR. (A) A dominant negative Ras inhibits Bcr-Abl–induced GCKR activation. HEK 293T cells (1 × 106) were transfected with HA-GCKR (1 μg) and Ras-V12 (2 μg), v-Abl (4 μg), or Bcr-Abl a (4 μg). The v-Abl and Bcr-Abl transfections were performed in the presence of a control vector (4 μg) or Ras-N17 (4 μg), indicated by bracket. HA-GCKR immunoprecipitates were assayed by an in vitro kinase assay. HA-GCKR and Abl immunoblotting were performed by ECL. These experiments were performed two times with similar results. (B) GCKR antisense expression impairs Ras- and Bcr-Abl–induced SAPK activation. HA-SAPK immunoprecipitates from HEK 293T cells (1 × 106) transfected with Bcr-Abl (4 μg) or Ras-V12 (2 μg) in the presence of pCRIII-GCKR-AS (4 or 8 μg) and HA-SAPK (1 μg) were assayed for kinase activity. A HA-immunoblot verified equivalent levels of HA-SAPK. Ras and Bcr-Abl levels were verified by immunoblotting. Data are reported as increase in SAPK activity compared with basal levels in control transfections. These experiments were performed four times with similar results. (C) A GCKR mutant impairs Bcr-Abl–induced SAPK activation. HA-SAPK immunoprecipitates from HEK 293 cells (1 × 106) transfected with Bcr-Abl (4 μg) and HA-SAPK (1 μg) in the presence or absence of GCKR-T178A (2, 4, or 6 μg) were assessed for kinase activity. Data are reported as in part B. A HA-immunoblot verified equivalent levels of HA-SAPK. Bcr-Abl levels are shown. GCKR (endogenous and the transfected mutant) were detected by immunoblotting with the GCKR-specific antiserum. This experiment was performed twice with similar results.
To provide direct evidence that Bcr-Abl signals the SAPK pathway through GCKR, we examined the effects of a GCKR antisense construct and a GCKR mutant on Bcr-Abl–induced SAPK activity. It is known that HEK 293 and HEK 293T cells express GCKR, and that a GCKR antisense construct reduces GCKR levels and inhibits TNF-induced SAPK activation.19 Here, we transfected HEK 293T cells with HA-SAPK along with either Ras-V12 or Bcr-Abl in the presence or absence of the antisense GCKR. The GCKR antisense construct inhibited both Ras- and Bcr-Abl–induced SAPK activation (Fig 2B). Expression of the antisense GCKR construct failed to alter the levels of Bcr-Abl or v-Abl. Not only did the antisense GCKR construct inhibit Bcr-Abl–mediated SAPK activation, but in addition the transfection of a construct that directs expression of GCKR-T178A had a similar result (Fig 2C). The expression of GCKR-T178A was monitored using the GCKR-specific antibody. The low level of endogenous GCKR is observed in the first two lanes of the third panel in the figure. Thus, using two different approaches to target endogenous GCKR, we successfully inhibited Bcr-Abl–induced SAPK activation.
Bcr-Abl and GCKR associate in vivo.
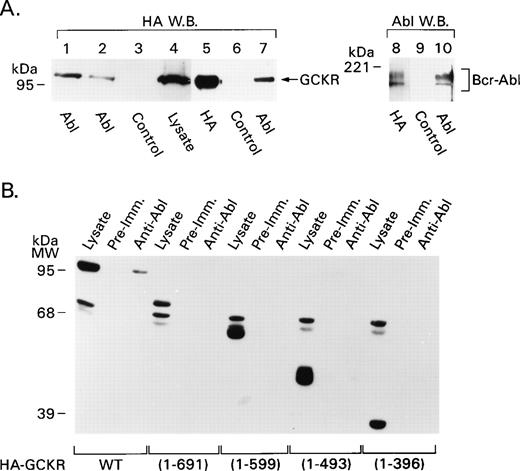
Because we had noted that the coexpression of Bcr-Abl and GCKR resulted in GCKR tyrosine phosphorylation, we sought to determine if GCKR physically associates with Bcr-Abl. Because we had failed to observe a band in the in vitro kinase assay that would correspond to Bcr-Abl, we initially used a digitonin-containing buffer to preserve low-affinity interactions. We transfected HEK 293T cells with Bcr-Abl or v-Abl expression vectors along with HA-GCKR. A HA-immunoblot of anti-Abl immunoprecipitates prepared from cell lysates buffer showed the presence of HA-GCKR (Fig 3A, lanes 1 to 4), a result consistent with an interaction between GCKR and Bcr-Abl as well as between GCKR and v-Abl. Next, we employed a more stringent lysis buffer that contained Triton X-100 rather than digitonin. Again GCKR and Bcr-Abl associated. Bcr-Abl immunoprecipitates contained GCKR (Fig 3A, lanes 5 to 7) and HA-GCKR immunoprecipitates contained Bcr-Abl (Fig 3A, lanes 8 to 10). Thus, Bcr-Abl and v-Abl can associate with GCKR.
GCKR and Bcr-Abl associate in vivo. (A) GCKR and Bcr-Abl coimmunoprecipitate. HEK 293T cells were transfected with constructs expressing HA-GCKR and either Bcr-Abl (lanes 1, 3 to 10), or v-Abl (lane 2). Cell lysates (2 × 106 cells) prepared using a digitonin lysis (lanes 1 to 4) or a Triton X-100 lysis buffer (lanes 5 to 10) were immunoprecipitated with anti-HA, anti-Abl, or a control antibody as indicated underneath. The immunoprecipitates or cell lysates were analyzed by immunoblotting with anti-HA (lanes 1 to 7) or anti-Abl (lanes 8 to 10) antibodies. The signal was detected by ECL. (B) Mapping of the region in GCKR required for interaction with Bcr-Abl. Lysates from HEK 293T cells (2 × 106) transfected with constructs expressing HA-tagged GCKR deletion constructs along with Bcr-Abl were immunoprecipitated with anti-Abl or control antibody. The immunoprecipitates and lysates were analyzed by immunoblotting with an anti-HA antibody. The signal was detected by ECL. A nonspecific doublet in the range of 65 kD is present in each of the lysates. The GCKR truncation mutants migrated with their expected molecular masses and were visualized in the cell lysates.
GCKR and Bcr-Abl associate in vivo. (A) GCKR and Bcr-Abl coimmunoprecipitate. HEK 293T cells were transfected with constructs expressing HA-GCKR and either Bcr-Abl (lanes 1, 3 to 10), or v-Abl (lane 2). Cell lysates (2 × 106 cells) prepared using a digitonin lysis (lanes 1 to 4) or a Triton X-100 lysis buffer (lanes 5 to 10) were immunoprecipitated with anti-HA, anti-Abl, or a control antibody as indicated underneath. The immunoprecipitates or cell lysates were analyzed by immunoblotting with anti-HA (lanes 1 to 7) or anti-Abl (lanes 8 to 10) antibodies. The signal was detected by ECL. (B) Mapping of the region in GCKR required for interaction with Bcr-Abl. Lysates from HEK 293T cells (2 × 106) transfected with constructs expressing HA-tagged GCKR deletion constructs along with Bcr-Abl were immunoprecipitated with anti-Abl or control antibody. The immunoprecipitates and lysates were analyzed by immunoblotting with an anti-HA antibody. The signal was detected by ECL. A nonspecific doublet in the range of 65 kD is present in each of the lysates. The GCKR truncation mutants migrated with their expected molecular masses and were visualized in the cell lysates.
To begin to map the region in GCKR important for this interaction, constructs expressing wild-type GCKR or one of four GCKR truncation mutants were transiently transfected into HEK 293T cells along with Bcr-Abl. Only the wild-type GCKR coimmunoprecipitated with Bcr-Abl (Fig3B). This analysis showed a requirement for the C-terminal 150 amino acids of GCKR to observe the interaction. This region lacks remarkable motifs except for two tyrosine residues, amino acids 836 and 847. Between amino acids 389 and 396 GCKR contains a proline-rich region, a likely src homology (SH) type-3 binding site24; however, whether this site contributes to the interaction with Bcr-Abl remains to be determined.
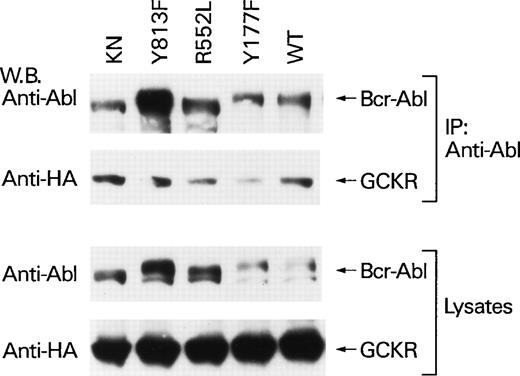
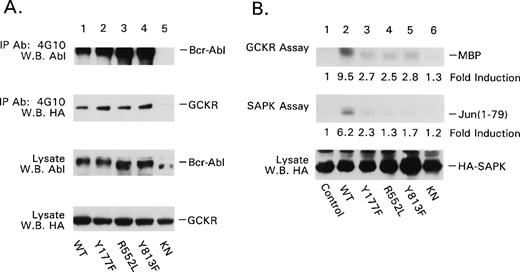
To further characterize the GCKR Bcr-Abl interaction, we examined four Bcr-Abl mutant proteins. We chose p185 Bcr-Abl Y177F, which does not associate with Grb2; p185 Bcr-Abl R552L, which lacks c-Abl’s SH2 domain-dependent interactions25; p185 Bcr-Abl Y813F, which lacks an autophosphorylation site that creates a docking site for interacting proteins26; and p185 Bcr-Abl KN, which is catalytically inactive. Although there was some interexperiment variation in the expression levels of the Bcr-Abl mutants and in the amount of coimmunoprecipitating GCKR, GCKR consistently coimmunoprecipitated with each of the Bcr-Abl mutant proteins (Fig 4). Furthermore, expression of each of the Bcr-Abl mutants with the exception of the p185 KN mutant resulted in the tyrosine phosphorylation of GCKR. This was based on the presence of a 97 kD band detected by phosphotyrosine immunoblotting of GCKR immunoprecipitates of lysates from cotransfected cells (C-S Shi, data not shown). In addition, phosphotyrosine immunoprecipitates prepared from HEK 293T cells coexpressing GCKR and either p185 Bcr-Abl Y177F, R552L, or Y813F contained significant amounts of GCKR (Fig 5A). In contrast, p185 Bcr-Abl KN failed to autophosphorylate and its coexpression with GCKR resulted in a substantial reduction in the amount of GCKR in phosphotyrosine immunoprecipitates. Not surprisingly, the KN mutant failed to activate either GCKR or SAPK kinase activity (Fig 5B). However, the three other mutant Bcr-Abl proteins were also markedly defective in their ability to activate both GCKR and SAPK. Thus, despite the ability of p185 Bcr-Abl Y177F, R552L, and Y813F to associate with GCKR and to result in GCKR tyrosine phosphorylation, only the wild-type Bcr-Abl protein strongly activated GCKR and the SAPK pathway.
P185 Bcr-Abl point mutants all associate with GCKR. p185 Bcr-Abl wild-type and mutant protein expression vectors (4 μg each) were transfected into HEK 293T cells (2 × 106) along with the HA-GCKR expression vector (2 μg). The association of Bcr-Abl point mutants with GCKR was examined by HA-immunoblotting anti-Abl immunoprecipitates. The presence of the p185 Bcr-Abl mutants in the immunoprecipitates was verified by immunoblotting with the immunoprecipitating antibody. The levels of expression of the Bcr-Abl mutants and HA-GCKR in the cell lysates are shown below. All the immunoblots were detected by ECL.
P185 Bcr-Abl point mutants all associate with GCKR. p185 Bcr-Abl wild-type and mutant protein expression vectors (4 μg each) were transfected into HEK 293T cells (2 × 106) along with the HA-GCKR expression vector (2 μg). The association of Bcr-Abl point mutants with GCKR was examined by HA-immunoblotting anti-Abl immunoprecipitates. The presence of the p185 Bcr-Abl mutants in the immunoprecipitates was verified by immunoblotting with the immunoprecipitating antibody. The levels of expression of the Bcr-Abl mutants and HA-GCKR in the cell lysates are shown below. All the immunoblots were detected by ECL.
Analysis of the effects of p185 Bcr-Abl point mutations on GCKR. (A) Effect of p185 Bcr-Abl point mutants on the immunoprecipitation of GCKR by a phosphotyrosine MoAb. p185 Bcr-Abl wild-type and mutant protein expression vectors (4 μg each) were transfected into HEK 293T cells (2 × 106) along with the HA-GCKR expression vector (2 μg). The presence of p185 Bcr-Abl, p185 Bcr-Abl mutant proteins, and HA-GCKR in antiphosphotyrosine immunoprecipitates (MoAb 4G10) was assessed by immunoblotting with either anti-Abl or anti-HA. The levels of the p185 Bcr-Abl point mutants and HA-GCKR in the cell lysates were also assessed. (B) Effect of p185 Bcr-Abl point mutants on GCKR and SAPK activity. The GCKR and SAPK in vitro kinase assays were performed similar to Fig 1. The fold inductions relative to GCKR alone (control) are shown and were assessed by scanning the autoradiographs and quantified using NIH Image. Immunoblotting showed similar levels of HA-GCKR. The kinase assays were performed five times with similar results.
Analysis of the effects of p185 Bcr-Abl point mutations on GCKR. (A) Effect of p185 Bcr-Abl point mutants on the immunoprecipitation of GCKR by a phosphotyrosine MoAb. p185 Bcr-Abl wild-type and mutant protein expression vectors (4 μg each) were transfected into HEK 293T cells (2 × 106) along with the HA-GCKR expression vector (2 μg). The presence of p185 Bcr-Abl, p185 Bcr-Abl mutant proteins, and HA-GCKR in antiphosphotyrosine immunoprecipitates (MoAb 4G10) was assessed by immunoblotting with either anti-Abl or anti-HA. The levels of the p185 Bcr-Abl point mutants and HA-GCKR in the cell lysates were also assessed. (B) Effect of p185 Bcr-Abl point mutants on GCKR and SAPK activity. The GCKR and SAPK in vitro kinase assays were performed similar to Fig 1. The fold inductions relative to GCKR alone (control) are shown and were assessed by scanning the autoradiographs and quantified using NIH Image. Immunoblotting showed similar levels of HA-GCKR. The kinase assays were performed five times with similar results.
GCKR is activated in CML cell lines.
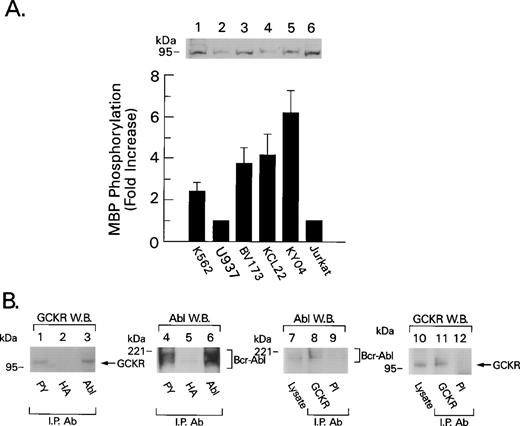
K562, KCL22, BV173, and KY04 are Bcr-Abl+ cell lines derived from CML patients in blast crisis. An immunoblot of cell lysates prepared from these cell lines and two non–Bcr-Abl+ cell lines showed similar levels of GCKR expression. However, GCKR immunoprecipitates from the CML cell lines had twofold to sixfold more kinase activity than did the controls (Fig 6A). GCKR immunoblotting of antiphosphotyrosine immunoprecipitates showed that GCKR is tyrosine phosphorylation or associated with a tyrosine phosphorylated molecule in K562 (Fig 6B), KY04, and KCL22 cells (C. Shi, data not shown). To determine whether endogenous GCKR and Bcr-Abl associate in vivo, we examined GCKR and Abl immunoprecipitates from K562 cells for the presence of Abl or GCKR, respectively. This analysis also showed that GCKR and Bcr-Abl coimmunoprecipitate (Fig 6B).
GCKR is a downstream target of Bcr-Abl in Bcr-Abl+ cell lines. (A) Increased GCKR kinase activity in Bcr-Abl+ cell lines. GCKR immunoprecipitates from lysates prepared from 5 × 106 cells of the indicated cell line were subjected to an in vitro kinase assay. The data were normalized to the results with either U937 cells or Jurkat cells. The data shown summarize the results from four experiments and are presented as mean +/− 2 SD. Above the results of the in vitro kinase assay is an immunoblot showing the levels of GCKR in cell lysates prepared from the different cell lines. (B) Bcr-Abl and GCKR associate in K562 cells. Lysates prepared from 1 × 107K562 cells were immunoprecipitated with an antiphosphotyrosine MoAb (PY), a HA MoAb, the GCKR antiserum, preimmune (PI) antiserum, or an Abl polyclonal antiserum as indicated. The immunoprecipitates were subjected to immunoblotting with the indicated antibodies. The GCKR immunoblot (lanes 1 to 3) was stripped and reblotted for Abl (lanes 4 to 6). The Abl immunoblot (lanes 7 to 9) was stripped and reblotted for GCKR (lanes 10 to 12). Bcr-Abl and GCKR were detected in 50 μg of cell lysate (lanes 7 and 10, respectively). Molecular weight markers and the location of Bcr-Abl and GCKR are indicated.
GCKR is a downstream target of Bcr-Abl in Bcr-Abl+ cell lines. (A) Increased GCKR kinase activity in Bcr-Abl+ cell lines. GCKR immunoprecipitates from lysates prepared from 5 × 106 cells of the indicated cell line were subjected to an in vitro kinase assay. The data were normalized to the results with either U937 cells or Jurkat cells. The data shown summarize the results from four experiments and are presented as mean +/− 2 SD. Above the results of the in vitro kinase assay is an immunoblot showing the levels of GCKR in cell lysates prepared from the different cell lines. (B) Bcr-Abl and GCKR associate in K562 cells. Lysates prepared from 1 × 107K562 cells were immunoprecipitated with an antiphosphotyrosine MoAb (PY), a HA MoAb, the GCKR antiserum, preimmune (PI) antiserum, or an Abl polyclonal antiserum as indicated. The immunoprecipitates were subjected to immunoblotting with the indicated antibodies. The GCKR immunoblot (lanes 1 to 3) was stripped and reblotted for Abl (lanes 4 to 6). The Abl immunoblot (lanes 7 to 9) was stripped and reblotted for GCKR (lanes 10 to 12). Bcr-Abl and GCKR were detected in 50 μg of cell lysate (lanes 7 and 10, respectively). Molecular weight markers and the location of Bcr-Abl and GCKR are indicated.
DISCUSSION
Bcr-Abl activates the SAPK pathway via an ability to recruit and activate GCKR. This conclusion is based on the following evidence. First, Bcr-Abl activates GCKR kinase activity and together they potently activate SAPK activity. Second, either a GCKR antisense construct or a catalytically inactive form of GCKR substantially reduced Bcr-Abl–induced SAPK activation. Third, GCKR and Bcr-Abl physically associate both in transfected cells and in Bcr-Abl+ cells. Fourth, a dominant negative form of Ras blocks both Bcr-Abl–mediated GCKR and SAPK activation. Finally, Bcr-Abl mutant proteins that have an impaired ability to activate GCKR fail to activate SAPK.
The case for Ras involvement in Bcr-Abl–mediated GCKR activation is based on the use of a dominant negative inhibitor of Ras, the result with the p185 Y177F point mutant, and the observation that Ras itself is a potent activator of GCKR kinase activity. Because the cotransfection of Ras-N17 blocked the ability of Bcr-Abl to activate GCKR kinase activity, Bcr-Abl–mediated Ras activation is crucial for the ability of Bcr-Abl to activate GCKR and is consistent with the previously known requirement for Ras in Bcr-Abl–induced SAPK activation.8 The impaired ability of Y177F p185 Bcr-Abl to activate GCKR underscores the essential role for phosphorylated Y177 in Bcr-Abl–mediated Ras activation in fibroblasts.27Unfortunately, the precise signaling defects created in Bcr-Abl by R552L and Y813F are not yet known. Nevertheless, both mutant proteins are defective in fibroblast transformation and for GCKR and SAPK activation in HEK 293 cells. This argues that they do not fully activate Ras in these cells. Because all the Bcr-Abl mutant proteins interacted with GCKR and all but the kinase-dead Bcr-Abl phosphorylated GCKR, we can conclude that the recruitment and/or tyrosine phosphorylation of GCKR is insufficient for its activation. Further support for this hypothesis is provided by experiments in which GCKR (1-699) and GCKR (1-599) were coexpressed with Bcr-Abl. Although these truncated versions of GCKR are not readily associated with GCKR, Bcr-Abl still increases their basal level of kinase activity (C. Shi, unpublished observation). Thus, Bcr-Abl–mediated Ras activation emerges as the key element for Bcr-Abl–mediated GCKR activation and is consistent with the ability of Ras-V12 to activate GCKR. Furthermore, because Ras-activated SAPK activation was markedly impaired by coexpression of a GCKR antisense construct, GCKR appears to be directly involved in Ras-mediated SAPK activation in HEK 293 cells. Overall our results are consistent with a model in which Bcr-Abl assembles a complex of signaling molecules, thereby providing a platform for Ras activation and protein-protein interactions. In myeloid cells the colocalization of GCKR and Bcr-Abl would be expected to localize GCKR to a microenvironment in which activated Ras is present.
How Ras activates GCKR remains enigmatic. Although GCKR lacks the consensus binding site delineated for some direct Ras effectors,28 an intriguing possibility is that GCKR is directly activated by Ras perhaps by a mechanism similar to how Ras activates Raf-1. In support of that possibility, when both proteins were expressed in HEK 293T cells Ras-V12 could be detected in GCKR immunoprecipitates and vice versa (C. Shi, unpublished observation). However, whether this is a direct or an indirect interaction has not been determined. A caveat in interpreting this association data is that both proteins are expressed at high levels perhaps permitting unphysiologic interactions. So far we have been unable to document an interaction between the endogenous proteins.
Consistent with its multiple protein interaction domains, Bcr-Abl binds and/or phosphorylates upwards of 20 proteins.29GCKR joins this group although the mechanism by which the association occurs requires clarification. The C-terminal 150 amino acids of GCKR are necessary for the interaction. Other regions in GCKR may also be important and need to be mapped using N-terminal deletion constructs. Of note, the C-terminal 150 amino acids of GCKR are highly conserved with that of GLK and well conserved with that of GCK and HPK1 suggesting that they also may be recruited by Bcr-Abl. Interestingly, the C-terminal 150 amino acids of GCKR are also required for its interaction with TNF receptor-associated factor 2 (C-S. Shi et al, manuscript submitted for publication).
The hypothesis that Ras activates GCKR, which leads to SAPK activation, is consistent with observations that Ras not only signals the MAPK cascade, but also SAPK. For example, CD3 and CD28 costimulation of T lymphocytes induces Ras-dependent SAPK activation,30insulin stimulates a Ras-dependent increase in SAPK activity in Rat1 fibroblasts,31 and the α1-adrenergic receptor activates SAPK through a pathway that requires Ras and a MEKK.32 In preliminary experiments CD3 and CD28 costimulation of T lymphocytes increased GCKR kinase activity implicating GCKR in T-cell SAPK activation (J. Tuscano, unpublished observations). Thus, Ras-mediated GCKR activation may be an important mechanism by which Ras activates the SAPK pathway in a variety of cell types.
Ras causes cellular transformation by activating multiple signaling pathways. Several studies ascribe a role for SAPK activation. In one study a catalytically inactive form of SEK1/MKK4 selectively inhibited oncogenic Ras-stimulated SAPK activity and transformation, but not MAPK activation.33 Particularly relevant to our findings, a cytoplasmic inhibitor of SAPK signaling blocked the transformation of pre-B lymphocytes by Bcr-Abl.9 Experiments to test whether the catalytically inactive form of GCKR may also impair Bcr-Abl–mediated cellular transformation are in progress. Because of the potential importance of the SAPK pathway in Ras-mediated cellular transformation, GCKR provides an intriguing upstream target in this pathway.
ACKNOWLEDGMENT
The authors thank Mary Rust for her excellent editorial assistance, Dr John Kryiakis for helpful discussions, and Dr Anthony Fauci for his continued support.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal