Abstract
Idiopathic thrombotic thrombocytopenic purpura (TTP) is a thrombotic microangiopathy of obscure etiology. The fundamental pathologic lesion is a hyaline thrombus composed of platelets and some fibrin accompanied by endothelial cell proliferation and detachment, in the absence of an inflammatory response. We have previously demonstrated that plasmas from patients with both idiopathic TTP and a related disorder, sporadic hemolytic-uremic syndrome (HUS), induce apoptosis and expression of the apoptosis-associated molecule Fas (CD95) in vitro in those lineages of microvascular endothelial cells (MVECs) that are affected pathologically. We now demonstrate the presence of enhanced MVEC apoptosis in splenic tissues from patients with TTP, documented by terminal deoxynucleotidyl-transferase–mediated dUTP nick-end labeling (TUNEL) and morphology. This is accompanied by elevated Fas expression. It contrasts with the absence of apoptosis in splenic tissues obtained after splenectomy for trauma or immune thrombocytopenic purpura. TUNEL-positive cells, identified by immunohistochemistry as MVECs or macrophages, presumably engulfing apoptotic ECs, are noted in numerous areas, including those apart from microthrombi. Thus, it is unlikely that EC apoptosis is simply a sequela of thrombus formation. Based on these data, we propose that MVEC apoptosis is of pathophysiologic significance in idiopathic TTP/sporadic HUS.
THROMBOTIC thrombocytopenic purpura (TTP) is classically a pentad of thrombocytopenia, microangiopathic hemolytic anemia, neurologic abnormalities, renal dysfunction, and fever.1 Hemolytic-uremic syndrome (HUS) is part of the spectrum of TTP-related disorders, defined by a triad of thrombocytopenia, microangiopathic hemolytic anemia, and renal dysfunction.2 Both of these entities are thrombotic angiopathies. Idiopathic TTP and sporadic HUS, but not diarrhea, malignancy, or most drug-associated forms of TTP/HUS, are closely related in terms of response to therapeutic plasmapheresis and distribution of pathologic lesions.2 The fundamental pathologic lesion in idiopathic TTP/sporadic HUS is a hyaline thrombus, composed of platelets and some fibrin, that is accompanied by localized endothelial cell (EC) proliferation and detachment in the distinct absence of inflammation.1,3-5 These thrombi are limited to the microvasculature and found in all tissues, with the notable exception of the lungs.5
Several observations suggest that EC damage may be the initial event in TTP/sporadic HUS, mediated by components in the plasma of these patients. Electron microscopy studies of early lesions have shown multiple cytoplasmic vacuoles, lysosomes, and swollen mitochondria in the microvascular endothelial cells (MVECs).1,6 These changes, classically associated with early apoptotic events, have been noted even in the absence of overt thrombi in these vessels, indicating that they are not simply sequelae of thrombosis. Morphologic evidence of overt apoptosis has also been recognized in renal glomerular cells of probable MVEC origin in one HUS patient who has been extensively studied.7 In addition, Lefevre et al8 have demonstrated the presence of ECs in the whole blood of patients with TTP, consistent with the detachment of apoptotic ECs and their entry into the circulation. Finally, our group has documented the ability of plasmas from TTP and sporadic HUS patients to induce apoptosis of cultured MVECs, an activity that resolves after therapeutic plasmapheresis.9-11
We now document the presence of enhanced apoptosis and expression of the activation and apoptosis-associated molecule Fas (CD95) in MVECs in sinusoidal areas of splenic tissues from patients with TTP. We compare the incidence of these changes with that in control spleens, removed after trauma or as a therapeutic intervention in immune thrombocytopenic purpura (ITP). In conjunction with our prior in vitro data, this suggests that MVEC apoptosis is a fundamental event in idiopathic TTP/sporadic HUS and that the use of apoptotic inhibitors should be considered in the experimental treatment of the thrombotic microangiopathies.
PATIENTS, MATERIALS, AND METHODS
Patients.
Cases of TTP were selected by screening surgical pathology records from New York Hospital-Cornell University Medical Center (New York, NY) for the period of 1975 through 1997. Paraffin blocks of splenic tissues were accessible from 8 splenectomized patients with TTP. Patient charts were reviewed for the validity of the diagnosis, duration of disease, treatment, and outcome. The diagnosis of TTP was made according to the following criteria: fever, neurologic dysfunction, renal dysfunction with serum creatinine level of greater than 1.5 mg/dL or greater than 50% of the previous baseline value, thrombocytopenia with platelet count less than 150,000/μL, and evidence of microangiopathic hemolytic anemia on peripheral blood smear.
Control paraffin blocks of splenic tissues were obtained from 2 patients with ITP, 3 patients with splenic trauma, and 1 patient with littoral cell angioma.
Tissues.
All specimens, fixed in formalin and embedded in paraffin, were cut in 5-μm sections. Selected slides, stained with hematoxylin and eosin (H&E) and periodic-acid-Schiff (PAS), were examined for the presence of hyaline subendothelial deposits (SED), endothelial cell reactive changes, arteriolar thrombi, periarteriolar concentric fibrosis (PAF) or “onion-skinning,” and germinal centers. These changes have all been described in TTP, but none is considered pathognomonic for the disease.12
TUNEL.
A TUNEL (terminal deoxynucleotidyl-transferase–mediated dUTP nick-end labeling) assay was used to identify double stranded DNA fragmentation, characteristic of DNA degradation by apoptosis. An ApopTag in situ apoptosis detection kit (Oncor, Gaithersburg, MD) was used according to the manufacturer’s directions. Briefly, tissue slides were deparaffinized, treated with proteinase K (20 μg/mL) for 15 minutes at room temperature, and then quenched in 2% hydrogen peroxide. After rinsing in phosphate-buffered saline (PBS), pH 7.4, specimens were incubated in 1× Equilibration Buffer (Oncor) for 10 to 15 seconds. Slides were next incubated with terminal deoxynucleotidyl transferase (Tdt) for 1 hour at 37°C, blocked with Stop/Wash Buffer (Oncor), and then incubated with peroxidase-conjugated antidigoxigenin antibody for 30 minutes at room temperature. Finally, slides were developed using diaminobenzidine (DAB; Sigma, St Louis, MO) and counterstained with methyl green.
Immunohistochemical analysis.
An immunohistochemical analysis for CD34- (endothelial cell, stem cell marker) and CD68- (macrophage-specific marker) expressing cells was performed on all 4 TUNEL-positive slides using the Chem Mate Secondary Detection Kit Alkaline Phosphatase (Ventana Medical Systems, Inc, Tucson, AZ). These samples were stained initially via TUNEL assay, as described above, without the methyl green counterstaining.
One set of slides was heated in 10 mmol/L sodium citrate buffer, pH 6.0, in a pressure cooker (Prestige; Gebrauchsanweisung Beachten, London, UK) for 10 minutes and then allowed to cool in cold water. Slides were then washed in distilled water 4 times and in PBS 3 times. Next, samples were quenched in Blocking Antibodies, a mixture of sodium azide, and proprietary ingredients (Ventana Medical Systems, Inc) and then incubated in mouse anti-CD34 MoAb (Biogenex, San Ramon, CA) at 1:100 dilution for 15 minutes at room temperature.
Another set of slides was incubated in 0.2% trypsin for 14 minutes at 37°C, washed in distilled water and PBS, and then quenched in Blocking Antibodies before incubation with mouse anti-CD68 MoAb (DAKO, Carpinteria, CA) at 1:300 dilution for 15 minutes at room temperature.
Slides were washed in PBS and then incubated in a mixture of biotinylated goat antimouse IgG and IgM and goat antirabbit IgG (3Ab-AB2 Biotin; Ventana Medical Systems, Inc). Slides were next incubated in Avidin-Alkaline Phosphatase complex (Ventana Medical Systems, Inc) for 30 minutes, washed in PBS, developed with BT Red chromogen components (Ventana Medical Systems, Inc) for 10 minutes, and then counterstained with Mayers hematoxylin.
Fas expression.
Slides from 7 of 8 TTP patients and 5 of 6 control patients were available to compare Fas (CD95) expression in splenic tissues. The Immunocruz Staining System FAS (C-20) K:sc-715-K (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) was used according to the manufacturer’s directions. Slides were deparaffinized, treated with proteinase K (20 μg/mL) for 30 minutes at 37°C, heated in 10 mmol/L sodium citrate buffer, pH 6.0, for 5 minutes at 95°C, and then cooled for 20 minutes. Specimens were washed with distilled water, incubated in peroxidase for 5 minutes and prediluted goat serum block for 20 minutes, and then incubated in prediluted anti-Fas rabbit polyclonal Ig for 2 hours. Samples were washed in PBS and incubated in biotinylated goat antirabbit polyclonal Ig for 30 minutes and in horseradish peroxidase (HRP)-streptavidin complex for 30 minutes. Specimens were then washed in PBS, developed with HRP substrate DAB for 10 minutes, and then counterstained with methyl green.
Quantitation of apoptotic cells and Fas expression.
The percentage of apoptotic cells on each slide was determined with a Cell Analysis System (CAS) 100 (Cell Analysis Systems, Inc, Lombard, IL), a software-controlled analyzer.13 Images from each slide were screened at 400× and stored, permitting measurements of the number of cells per image and analysis of the percentage of apoptosis in any given population of cells. For each slide, eight random images of the splenic red pulp and eight random images of arterioles were analyzed and averaged for the presence of apoptosis. All cell types were examined within these fields. The significance of the difference in apoptosis between TTP and control samples was measured using two-tailed variance analysis.
The same principle was used to detect for the percentage of Fas expression in the splenic red pulp of available slides. For trauma-related splenectomy samples, areas away from lacerations were studied.
RESULTS
Patients.
The clinical findings of all patients are summarized in Table 1. There were 7 females and 1 male with TTP, ranging from 10 to 48 years of age. Only 2 of 8 patients presented with the full pentad of fever, thrombocytopenia, microangiopathic hemolytic anemia, neurologic abnormality, and renal dysfunction. However, all individuals had moderate to severe schistocytosis on peripheral blood smear, thrombocytopenia, and fever. Renal abnormalities were the least common finding, present in only 2 of 8 patients. Patients’ platelet counts ranged from 9,000 to 83,000/μL, with an average of 31,000/μL (Table 1). The lactate dehydrogenase (LDH) levels ranged from 834 to 2,000 IU/L, with a mean of 1,312 IU/L (Table 1). Neurologic abnormalities ranged from headache to confusion, hemiparesis, seizures, and coma. Treatment consisted of plasma infusion and plasma exchange in 5 patients (Table 1). Other treatments included aspirin, dipyridamole, steroids, and vincristine (Table 1). All patients eventually underwent splenectomy due to a relapsing course or refractory TTP. Remission was achieved in 7 of 8 patients. One patient died on hospital day 59 of cardiopulmonary arrest.
Clinical Patient Profile
| Patient No. . | Diagnosis . | Sex/ Age (yr) . | Initial Platelet/LDH* . | Medical Therapy . | Splenectomy . | Spleen Weight (g) . | Outcome . |
|---|---|---|---|---|---|---|---|
| TTP-1 | TTP | F/48 | 20/930 | asp, pyr, ster | 1975 | 265 | Remission |
| TTP-2 | TTP | F/29 | 83/1,569 | asp, pyr, ster | 1976 | 100 | Expired |
| TTP-3 | TTP | F/10 | 18/932 | asp, pyr, ster | 1976 | 180 | Remission |
| TTP-4 | TTP | F/38 | 27/1,650 | asp, pyr, ster, FFP | 1988 | 146 | Remission |
| TTP-5 | TTP | F/23 | 15/2,000 | pyr, ster, FFP, pph | 1989 | 185 | Remission |
| TTP-6 | TTP | F/45 | 68/834 | ster, FFP, pph, vcr | 1990 | 108 | Remission |
| TTP-7 | TTP | M/19 | 10/1,600 | FFP, pph, ster, vcr | 1991 | 289 | Remission |
| TTP-8 | TTP | F/41 | 9/982 | FFP, pph, ster, vcr | 1997 | 170 | Remission |
| Control-1 | Splenic trauma | M/30 | NC | None | 1981 | 160 | Alive |
| Control-2 | Splenic trauma | M/35 | NC | None | 1989 | 180 | Alive |
| Control-3 | ITP | F/41 | 20/NC | ster, IVIG | 1989 | 81 | Remission |
| Control-4 | Splenic trauma | F/60 | NC | None | 1990 | 260 | Alive |
| Control-5 | ITP | F/14 | 75/NC | ster, IVIG | 1993 | 156 | Remission |
| Control-6 | Splenic littoral cell angioma | F/27 | NC | None | 1993 | 1,087 | Alive |
| Patient No. . | Diagnosis . | Sex/ Age (yr) . | Initial Platelet/LDH* . | Medical Therapy . | Splenectomy . | Spleen Weight (g) . | Outcome . |
|---|---|---|---|---|---|---|---|
| TTP-1 | TTP | F/48 | 20/930 | asp, pyr, ster | 1975 | 265 | Remission |
| TTP-2 | TTP | F/29 | 83/1,569 | asp, pyr, ster | 1976 | 100 | Expired |
| TTP-3 | TTP | F/10 | 18/932 | asp, pyr, ster | 1976 | 180 | Remission |
| TTP-4 | TTP | F/38 | 27/1,650 | asp, pyr, ster, FFP | 1988 | 146 | Remission |
| TTP-5 | TTP | F/23 | 15/2,000 | pyr, ster, FFP, pph | 1989 | 185 | Remission |
| TTP-6 | TTP | F/45 | 68/834 | ster, FFP, pph, vcr | 1990 | 108 | Remission |
| TTP-7 | TTP | M/19 | 10/1,600 | FFP, pph, ster, vcr | 1991 | 289 | Remission |
| TTP-8 | TTP | F/41 | 9/982 | FFP, pph, ster, vcr | 1997 | 170 | Remission |
| Control-1 | Splenic trauma | M/30 | NC | None | 1981 | 160 | Alive |
| Control-2 | Splenic trauma | M/35 | NC | None | 1989 | 180 | Alive |
| Control-3 | ITP | F/41 | 20/NC | ster, IVIG | 1989 | 81 | Remission |
| Control-4 | Splenic trauma | F/60 | NC | None | 1990 | 260 | Alive |
| Control-5 | ITP | F/14 | 75/NC | ster, IVIG | 1993 | 156 | Remission |
| Control-6 | Splenic littoral cell angioma | F/27 | NC | None | 1993 | 1,087 | Alive |
Abbreviations: asp, aspirin; FFP, fresh frozen plasma; IVIG, intravenous Ig; pph, plasmapheresis; pyr, dipyridamole; ster, steroid; vcr, vincristine; NC, not contributory.
Platelet count is expressed as ×109/μL. LDH is expressed as international units per liter.
Control patients included 2 females with ITP (14 and 41 years of age), 2 males and 1 female with splenic trauma (30, 35, and 60 years of age), and 1 female with littoral cell angioma (27 years of age).
Gross examination of the spleens showed weights of 100 to 289 g in TTP patients. There was no splenomegaly in the ITP and trauma patients. Gross splenomegaly was present in the patient with littoral cell angioma, with a spleen weighing 1,087 g (Table 1).
Histology.
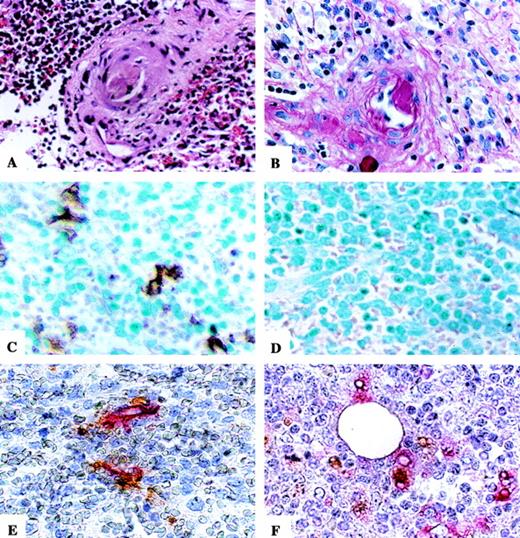
The histologic findings in TTP and control splenic tissues are summarized in Table 2. In our review of the selected pathology slides, we identified 2 TTP spleens with evidence of arteriolar thrombi (Fig 1A). In a review of the accompanying surgical pathology reports, we found additionally 3 more TTP spleens with arteriolar thrombi. No thrombi were noted in the control samples. All 8 TTP splenic tissues had evidence of subendothelial deposits associated with the presence of focally reactive ECs with hyperchromatic nuclei (Fig 1B). However, subendothelial deposits were also seen in 2 patients with splenic trauma and the patient with littoral cell angioma. Only 1 TTP patient and 1 ITP patient had periarteriolar concentric fibrosis. Variable numbers of germinal centers were present in 3 TTP spleens but also in all control samples, including 2 ITP spleens. There was no evidence of vasculitis in any of the specimens.
Pathologic Findings and CAS 100 Analysis of Degree of Apoptosis in the Splenic Red Pulp and Arterioles and Fas Expression in Splenic Red Pulp of TTP and Control Patients
| Patient No. . | Arteriolar Thrombi . | Histopathology . | Apoptosis (% TUNEL positivity) . | Fas Expression . | |||
|---|---|---|---|---|---|---|---|
| SED . | PAF . | Germinal Centers . | Red Pulp . | Arterioles . | Red Pulp . | ||
| TTP-1 | + | + | − | − | 7.3% | 0.0% | 9.2% |
| TTP-2 | + | + | − | − | 1.2% | 0.0% | 18.7% |
| TTP-3 | + | + | − | − | 0.0% | 0.0% | 3.7% |
| TTP-4 | − | + | − | +++ | 4.3% | 4.1% | NA |
| TTP-5 | + | + | − | + | 0.1% | 0.0% | 8.6% |
| TTP-6 | + | + | − | − | 4.8% | 0.0% | 16.5% |
| TTP-7 | − | + | + | +++ | 0.0% | 0.0% | 1.5% |
| TTP-8 | − | + | − | − | 12.8% | 2.0% | 5.3% |
| Control-1 | − | + | − | + | 0.0% | 0.0% | 5.4% |
| Control-2 | − | + | − | + | 0.0% | 0.0% | NA |
| Control-3* | − | − | + | ++ | 1.0% | 0.0% | 3.6% |
| Control-4 | − | − | − | + | 0.5% | 0.0% | 4.2% |
| Control-5* | − | − | − | +++ | 0.0% | 0.0% | 14.4% |
| Control-6 | − | + | − | +++ | 0.0% | 0.0% | 2.2% |
| Patient No. . | Arteriolar Thrombi . | Histopathology . | Apoptosis (% TUNEL positivity) . | Fas Expression . | |||
|---|---|---|---|---|---|---|---|
| SED . | PAF . | Germinal Centers . | Red Pulp . | Arterioles . | Red Pulp . | ||
| TTP-1 | + | + | − | − | 7.3% | 0.0% | 9.2% |
| TTP-2 | + | + | − | − | 1.2% | 0.0% | 18.7% |
| TTP-3 | + | + | − | − | 0.0% | 0.0% | 3.7% |
| TTP-4 | − | + | − | +++ | 4.3% | 4.1% | NA |
| TTP-5 | + | + | − | + | 0.1% | 0.0% | 8.6% |
| TTP-6 | + | + | − | − | 4.8% | 0.0% | 16.5% |
| TTP-7 | − | + | + | +++ | 0.0% | 0.0% | 1.5% |
| TTP-8 | − | + | − | − | 12.8% | 2.0% | 5.3% |
| Control-1 | − | + | − | + | 0.0% | 0.0% | 5.4% |
| Control-2 | − | + | − | + | 0.0% | 0.0% | NA |
| Control-3* | − | − | + | ++ | 1.0% | 0.0% | 3.6% |
| Control-4 | − | − | − | + | 0.5% | 0.0% | 4.2% |
| Control-5* | − | − | − | +++ | 0.0% | 0.0% | 14.4% |
| Control-6 | − | + | − | +++ | 0.0% | 0.0% | 2.2% |
Germinal centers are graded from rare (+) to occasional (++) to many (+++).
Abbreviations: SED, subendothelial deposit; PAF, periarterial concentric fibrosis; NA, not available.
Control-3 and Control-5 are ITP specimens.
Photomicrographs of microthrombus, SED, TUNEL staining, and immunohistochemical staining for CD34- and CD68-expressing cells on TUNEL-positive slides. Microthrombus (A) and SED with associated reactive endothelial cells (B) are seen in the splenic section of a representative TTP patient. H&E stain and PAS stain, respectively. TUNEL is positive in the splenic red pulp of a TTP patient (C) and negative in a control patient (D). Methyl green counterstain. Double staining for CD34- or CD68-expressing cells on TUNEL-positive TTP slides demonstrates the presence of apoptotic ECs (E) and apoptotic bodies in the cytoplasm of macrophages (F). Hematoxylin counterstain. Original magnification × 400 for all sections.
Photomicrographs of microthrombus, SED, TUNEL staining, and immunohistochemical staining for CD34- and CD68-expressing cells on TUNEL-positive slides. Microthrombus (A) and SED with associated reactive endothelial cells (B) are seen in the splenic section of a representative TTP patient. H&E stain and PAS stain, respectively. TUNEL is positive in the splenic red pulp of a TTP patient (C) and negative in a control patient (D). Methyl green counterstain. Double staining for CD34- or CD68-expressing cells on TUNEL-positive TTP slides demonstrates the presence of apoptotic ECs (E) and apoptotic bodies in the cytoplasm of macrophages (F). Hematoxylin counterstain. Original magnification × 400 for all sections.
Identification of apoptotic cells.
TUNEL assay demonstrated apoptosis in the splenic red pulp in 4 of 8 TTP patients (representative section, Fig 1C). In marked contrast there was virtual absence of detectable apoptosis (≤1%) in all control samples (representative section, Fig 1D). CAS 100 (Table 2) showed an apoptotic range of 4.3% to 12.8% (average, 7.3%) in the splenic red pulp of the 4 TTP patients with apoptosis versus 0.0% to 1.0% in all of the control samples (P < .0001). Furthermore, 2 of the 4 TTP patients with apoptosis in the red pulp had apoptosis in their arterioles (2.0% and 4.1%, respectively), whereas no control patient demonstrated any measurable apoptosis of their arterioles.
Many ECs in sinusoidal regions of the red pulp of TTP, but not control, spleens were TUNEL positive. The ECs were identified initially based on their location and structure. These cells had flattened nuclei and lined the lumen of capillaries and sinuses. Their identity was further confirmed by immunohistochemical analysis. Concurrent TUNEL and immunohistochemical stainings demonstrated the colocalization of TUNEL positivity and either CD34 (EC) or CD68 (macrophage) expression in the 4 TTP samples. This confirmed the presence of apoptotic ECs (Fig 1E) and showed apoptotic bodies, presumably of endothelial origin, within neighboring macrophages (Fig 1F).
Fas expression.
All 7 TTP and all 5 control samples available for analysis showed some Fas expression in the splenic red pulp (Table 2 and Fig 2). CAS 100 showed Fas expression in TTP samples ranging from 1.5% to 18.7% of the cells, with a mean of 9.1%. The other 3 non-ITP control specimens demonstrated Fas expression from 2.2% to 5.4%, with a mean of 3.9%. In addition, the intensity of staining was much greater in the TTP versus control specimens (Fig 2). One of the two ITP control spleens showed levels of Fas staining comparable to that seen in TTP (Table 2).
Photomicrographs of Fas expression in representative TTP and control slides. Fas expression of TTP-8 (A) is enhanced and more intense in appearance than that of Control-6 (B). Original magnifications × 400 for all sections.
Photomicrographs of Fas expression in representative TTP and control slides. Fas expression of TTP-8 (A) is enhanced and more intense in appearance than that of Control-6 (B). Original magnifications × 400 for all sections.
DISCUSSION
The pathogenesis of TTP is unknown. Previously, our laboratory has demonstrated that plasmas from idiopathic TTP and sporadic HUS patients induce apoptosis in cultured human MVECs of dermal, renal, cerebral, and tonsillar, but not in those of pulmonary origin or in ECs of large vessels.9-11 This finding is consistent with restriction of in vivo pathologic change to the microvessels of certain tissues5 and raises the possibility that enhanced EC apoptosis, initiated by plasma factors, is etiologic in idiopathic TTP/sporadic HUS. Using the TUNEL assay, we now demonstrate apoptosis in the splenic red pulp in 4 of 8 TTP patients and in arterioles of 2 of these patients but not in control samples. Using immunohistochemical analysis, we have identified the TUNEL-positive cells in the splenic red pulp of TTP patients as either ECs or macrophages. These TUNEL-positive macrophages, which are in the perivascular distribution, are presumed to have engulfed apoptotic bodies, most of which are likely to be endothelial in origin.
Although only half of our TTP specimens show evidence of apoptosis, we believe that this is a significant finding and that the extent of apoptosis in these specimens, an average of 7.3%, is of pathologic importance. There are several explanations for the presence of EC apoptosis in only 50% of the TTP specimens. First, a minor population of scattered apoptotic cells would not be detectable in situ. Such cells are rapidly phagocytosed by macrophages and by “nonprofessional” phagocytes, obviating an inflammatory response.14,15 Thus, a significant number of cells must be undergoing an accelerated apoptotic process to see any evidence in a tissue section at one point in time. This phenomenon is consistent with other disorders associated with apoptosis in vitro and in vivo, including human immunodeficiency virus (HIV) and CD4+T-lymphocyte apoptosis.16-18 Thus, the rapid process of removal of apoptotic cells may account for the presence of apoptosis seen in only one half of our TTP specimens. Secondly, MVEC apoptosis may not occur at all stages of TTP/sporadic HUS, or the syndrome may involve more than one pathologic mechanism. Indeed, plasma-based MVEC apoptosis has been previously recognized in vitro with only about 75% of the TTP/sporadic HUS plasmas that we have screened.10 11Thirdly, the TUNEL assay cannot demonstrate the early phase of cellular apoptosis, such as cell shrinkage and membrane blebbing. Thus, some evidence of apoptosis will not be recognized by the TUNEL system.
There are certain precautions that must be taken when using the TUNEL technique to detect apoptosis. In a variety of experimental systems, it has been demonstrated that internucleosomal cleavage of DNA is a late event in the apoptotic process, whereas chromatin condensation and reduction in cell volume occur much earlier.19,20Furthermore, DNA fragmentation, similar to that seen in apoptosis, can be present in necrosis.21 The fact that our tissues were obtained at surgery and fixed quickly obviate much of the later problems reported in autopsy series using the TUNEL assay.
Additionally, in agreement with our previous in vitro data of enhanced expression of the apoptosis-associated molecule Fas in restricted lineages of MVECs exposed to plasmas from TTP/sporadic HUS patients,10 we are able to demonstrate increased Fas expression in available splenic tissues of TTP (mean, 9.1%) versus 3 non-ITP control patients (mean, 3.9%). Because of the autoimmune process in ITP, we suspect that Fas expression would be elevated in ITP, but the small number of specimens available is insufficient to reach a conclusion about Fas expression in the setting of ITP. We have found no correlation between levels of Fas expression and degree of apoptosis in the current pathologic specimens or in our in vitro MVEC model.10,11 This finding is consistent with our in vitro data that MVEC apoptosis is only partially inhibited by soluble anti-Fas MoAb.9 One possible explanation for the discordance between the degree of TUNEL positivity and Fas expression in some of our tissue samples is that enhanced EC apoptosis in TTP may occur via a Fas-independent mechanism, and enhanced Fas expression is simply a marker for activated cells in TTP.
Classic pathologic examinations of TTP/sporadic HUS lesions have been unable to establish whether EC damage with exposure of subendothelial surfaces is a primary event, preceding deposition of platelets and fibrin within the vessel lumen, or if EC damage surrounding these thrombi, including apoptosis, is a secondary response to platelet aggregation resulting in microvascular occlusion.22However, some electron micrograph-based studies of TTP have demonstrated that MVECs develop such preapoptotic changes as multiple cytoplasmic vacuoles and lysosomes, swollen mitochondria, and other evidence for intense EC activation before EC detachment and platelet plugging.1,6 This finding is consistent with our hypothesis that apoptosis is an initiating event in TTP. Similarly, we have noted TUNEL-positive cells in areas apart from microthrombi (Fig 1C), making it unlikely that apoptosis is simply an event of prior MVEC thrombus formation. Indeed, apoptotic ECs have been demonstrated by our group10 and others23,24 to be procoagulant and thus more likely to predispose to platelet aggregation and fibrin formation.25,26 Finally, Karpman et al27 have demonstrated marked apoptosis of epithelial, but not endothelial, cells from tubuli and glomeruli from diarrhea-associated HUS patients and from mice inoculated with Shiga-like toxin-2, which is thought to be the causative factor of this disorder. The fact that EC apoptosis is not a prominent feature of this form of HUS in humans further suggests that EC apoptosis is not simply a response to thrombotic events. This finding is consistent with our in vitro work, which fails to demonstrate plasma-induced EC apoptosis using samples from patients with diarrhea-associated HUS.10 11
To our knowledge, this is the first reported series of TTP patients with evidence of significantly enhanced apoptosis of ECs in an involved tissue. Based on our results with the TUNEL assay and immunohistochemistry, we propose that EC apoptosis plays an important role in the pathophysiology of idiopathic TTP and its related disorder, sporadic HUS. The cause of enhanced EC apoptosis in idiopathic TTP/sporadic HUS is unknown. One possible explanation is the loss of extracellular matrix (ECM) proteins, which are important to cell survival. This process is known as anoikis or “without a home.” Hynes28 has demonstrated that ECM-cell interactions are mediated largely by integrins, and Meredith et al29 have shown that the loss of ECM proteins may block integrin-mediated signals, resulting in EC apoptosis in vitro. Based on this background, we are currently studying the expression of several ECM proteins, such as thrombospondin-1 and fibronectin, in splenic tissues of TTP and control patients. Further work pertaining to the pathophysiology of enhanced EC apoptosis in idiopathic TTP/sporadic HUS may provide clues to the treatment of this syndrome. Finally, recent reports of the use of apoptosis inhibitors in our in vitro TTP model30 and in in vivo murine models for other apoptotic disorders31 also suggest possible avenues for the experimental therapeutics of disorders linked to accelerated programmed cell death, perhaps including idiopathic TTP/sporadic HUS.
ACKNOWLEDGMENT
The authors thank Liang Ying for excellent technical support.
Supported by National Institutes of Health Grants No. HL55646, AI41327, and DE11348 to J.L.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Jeffrey Laurence, MD, Cornell University Medical College, 411 E 69th St, New York, NY 10021.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal