Abstract
One hundred seven adult patients with thalassemia aged from 17 through 35 years and transplanted from HLA-identical siblings between November 1988 and September 1996 were evaluated on December 31, 1997. The outcome experience of 20 consecutive patients transplanted between November 13, 1988 and January 10, 1991 and reported in September 1992 is updated after 5 additional years. The experience on 87 patients transplanted between May 1991 and September 1996 is described and evaluated as of the end of December 1997. Of 107 patients, 69 survive between 1.5 and 9 years after transplantation. Sixty-six of these patients do not have thalassemia and are identified as ex-thalassemic after bone marrow transplantation. The youngest survivor is 20 years old, 6 are older than 30 years, and the oldest is 37 years of age. Patients with chronic active hepatitis at the time of transplant were significantly more likely to die than patients without (P = .05; relative risk, 2.05). Marrow transplantation is a valid treatment option for older patients with thalassemia who have suitable donors and show deterioration with conventional therapy.
BONE MARROW transplantation provides radical cure of the hematopoietic disorder of thalassemia,1,2 with the best results in young patients who have been regularly transfused and chelated with deferoxamine.3 Examination of the results of transplantation in patients less than 17 years of age treated with a regimen of busulfan (BU) at 14 mg/kg and cyclophosphamide (CY) at 200 mg/kg showed three risk categories predicting the outcome of transplantation.3 In that study, the probabilities of survival, of rejection, and of event-free survival were 94%, 0%, and 94% in class 1; 80%, 9%, and 77% in class 2; and 61%, 16%, and 53% in class 3 patients. Subsequently, a regimen with a smaller dose of CY (120 or 140 mg/kg) reduced the incidence of transplant-related toxicity and mortality for class 3 patients in this age group.4
Early experience suggested that the results of transplantation for thalassemia were particularly poor for patients older than 16 years. However, when the categorization described above was retrospectively applied to the adult experience, it became clear that these poor results were associated with a large proportion of older patients being in class 3. When the revised regimen for class 3 pediatric patients was used for older class 3 patients, the results were much improved, and in 1992 we reported the results of marrow transplantation in 20 adult thalassemic patients aged 17 through 26 years with the regimen determined by risk class.5
Through 1996, a further 87 patients aged 17 through 35 years have been transplanted in Pesaro, Italy and we now report the results of this experience together with an update on the results in the 20 patients previously reported.
MATERIALS AND METHODS
Patients
Between November 13, 1988 and January 10, 1991, 20 consecutive patients older than 16 years were transplanted from HLA-identical and MLC nonreactive sibling donors, and this experience, evaluated as of September 1991, was reported in September 1992.5 The treatment of these patients was described at that time and is not described in this report. They have now been observed for an additional 5 years and they constitute group A in this report, which updates their outcomes.
Between May 1991 and September 1996, 87 consecutive patients aged from 17 through 35 years were transplanted from HLA-identical and MLC nonreactive sibling donors. These patients constitute group B in this report, and their treatment and outcomes evaluated as of the end of December 1997 are described below.
Categorization Into Risk Groups
Disease status was categorized according to established criteria5 in which three risk factors were identified. These were defined as hepatomegaly greater than 2 cm, portal fibrosis of any degree, and inadequate compliance with the chelation regimen. Class 2 patients were those who had two of these factors and class 3 patients had all three risk factors. Of the 87 new patients, 13 were class 2 and 74 were class 3.
The quality of chelation was characterized as regular when deferoxamine therapy was initiated within 18 months of the first transfusion and administered subcutaneously for 8 to 10 hours continuously for at least 5 days each week. The chelation variable was defined as irregular for any deviation from this requirement. The age in months when the patient first received regular chelation was recorded. A chelation index was calculated describing the number of months each patient received regular chelation as a percentage of the number of months the patient should have received chelation by the definition given above. With this index, a completely satisfactory chelation history is represented by 100% and a completely unsatisfactory one by 0%.4
Conditioning Regimens
Marrow ablation.
All patients were prepared for transplantation with regimens containing BU and CY. All class 2 patients received BU at 3.5 mg/kg administered orally on each of 4 consecutive days (total dose, 14 mg/kg), followed by CY at 50 mg/kg administered intravenously on each of 4 consecutive days. Of the class 3 patients, 31 received BU at 4.0 mg/kg administered orally on each of 4 consecutive days (total dose, 16 mg/kg) and 43 received BU at 3.5 mg/kg administered orally on each of 4 consecutive days (total dose, 14 mg/kg). For 13 class 3 patients, the BU was followed by CY at 30 mg/kg on each of 4 consecutive days (total dose, 120 mg/kg) and the other 61 class 3 patients received CY at 40 mg/kg on each of 4 days (total dose, 160 mg/kg). Thus, of the class 3 patients, 13 received BU at 16 mg/kg and CY at 120 mg/kg, 18 received BU at 16 mg/kg and CY at 160 mg/kg, and 56 received BU at 14 mg/kg and CY at 160 mg/kg.
Prophylaxis against acute graft-versus-host disease (GVHD).
All patients received cyclosporine (CSP) at 5 mg/kg/d intravenously from day −3 to day 5 and then at 3 mg/kg/d until day 21. This schedule was changed to 12.5 mg/kg/d orally as soon as oral administration could be tolerated. In addition, all class 3 patients received CY at 7.5 mg/kg intravenously on day 1 and then methotrexate (MTX) at 10 mg/m2 on days 3 and 6 after transplantation. In addition, 15 of the class 3 patients received equine antilymphocyte globulin (Lymphoglobuline Merieux) at 10 mg/kg/d intravenously from day −5 to day +5 as a continuous infusion over 12 hours.
Marrow Infusion
Marrow was infused 36 hours after the last dose of CY and the date of marrow infusion was designated day 0.
Supportive Care
All patients were treated in positive-pressure isolation rooms and received nonabsorbable oral antibiotics. Acyclovir at 15 mg/kg administered intravenously in three divided doses and prednisolone at 0.5 mg/kg administered intravenously were initiated on day −2 and administered daily through the transplant period. Amphotericin B at 0.3 mg/kg was initiated prophylactically on day +9 and administered daily until day +21, with reduced or suspended doses when levels of creatinine and of blood urea nitrogen were elevated. Acute and chronic GVHD were graded according to the Seattle criteria.6-8 The first choice drug for treatment of acute GVHD was prednisolone at escalating doses up to 10 mg/kg, depending on the degree and duration of acute GVHD.
Evaluation of Graft Status
Engraftment was documented by in situ Y chromosome hybridization of bone marrow or blood samples in sex-mismatched donor-recipient pairs and by analysis of variable number tandem repeat (VNTR) polymorphism in bone marrow and/or blood samples in the case of sex-matched pairs. Globin-chain synthesis of marrow and peripheral-blood reticulocytes was examined by incorporation of 3H-leucine followed by column or high-pressure liquid chromatography (HPLC). Analyses of engraftment and the patterns of hemoglobin synthesis were first performed on peripheral blood and on bone marrow aspirates on day 21 posttransplant and repeated at biweekly intervals until day 60 to demonstrate engraftment.4
Other Evaluations
Liver biopsies were performed on all patients before transplantation. Liver hemosiderosis, portal fibrosis, chronic aggressive hepatitis, and chronic persistent hepatitis were evaluated according to an established grading system and recorded as absent, mild, moderate, or severe.9 Liver iron concentrations were determined by atomic absorption spectroscopy10 on frozen deparaffinized liver specimens.
Statistical Evaluations
Regression analysis of survival data with death as the endpoint was performed using the SAS PHREG procedure. The number of rejection events was insufficient to support regression analysis of rejection and rejection-free survival. Variables examined were age, class, age at diagnosis, age at first transfusion, number of transfusions, chelation index, serum ferritin, aspartatate aminotransferase (AST), alanine aminotransferase (ALT), liver and spleen size, liver hemosiderosis, portal fibrosis, and chronic aggressive hepatitis. In estimating rejection-free survival, rejection with recurrence of thalassemia and deaths were identified as events. Deaths in the absence of rejection were categorized as nonrejection mortality. Survival distributions were estimated by the product-limit method of Kaplan and Meier.11 These statistics were calculated with censoring on the date of last contact. In estimating rejection, competing risks were accommodated by using the cumulative incidence statistic.12
All statistical analyses were performed using SAS for Windows version 6.11 (SAS Institute Inc, Cary, NC). The data set was locked for recording events on December 31, 1997.
RESULTS
Group A
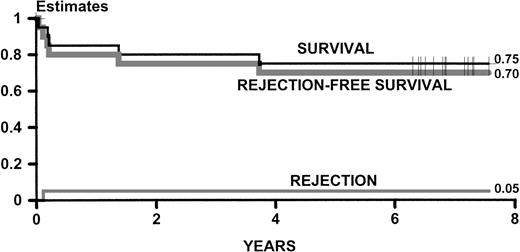
Figure 1 describes the estimates of survival and rejection of the group A patients updated for this report. Since the original report on these patients,5 there have been no further rejections and 2 patients have died (1 from mushroom poisoning on day 502 and 1 from septic shock on day 1,363). Fifteen of the original patients survive between 6.5 and 8.5 years from transplant, 14 of them without thalassemia.
Kaplan-Meier estimates of survival and rejection-free survival and cumulative incidence estimates of rejection for 20 adult patients originally reported in September 1992.5 The statistics are updated as of December 1997.
Kaplan-Meier estimates of survival and rejection-free survival and cumulative incidence estimates of rejection for 20 adult patients originally reported in September 1992.5 The statistics are updated as of December 1997.
Group B
Patient characteristics at the time of transplantation potentially influential on transplant outcome are shown in Table 1. Three class 3 patients had noteworthy values. One patient, diagnosed as having β-thalassemia at 24 months of age and not transfused until 27 years, was transplanted at 35 years after receiving 140 red blood cell transfusions without regular chelation. Another, who was transplanted at 21 years and had received 58 red blood cell transfusions at the time of the transplant, had a serum ferritin of 5,825 μg/L and a liver iron concentration of 41.90 mg/g at the time of transplant. The third patient, who was transplanted at 24 years of age and who had received 372 red blood cell transfusions before transplant, received intensive intravenous chelation continuously during the 18 months before transplantation, with a serum ferritin of 322 μg/L and a liver iron concentration of 1.4 mg/g. At the time of transplant, 2 of 13 class 2 (15%) and 33 of 74 class 3 (45%) patients had evidence of chronic active hepatitis.
Group B Patients: Pretransplant Characteristics
| Characteristic . | Value . |
|---|---|
| Age (yr) | |
| Range | 17-35 |
| Mean (standard deviation) | 21.5 (3.8) |
| Gender female | 39 |
| Previous red blood cell transfusions (no.) | |
| Range | 58-900 |
| Mean (standard deviation) | 362.0 (132.4) |
| Age at first transfusion (mo) | |
| Range | 2-324 |
| Mean (standard deviation) | 28.5 (49.3) |
| Age at first chelation (mo) | |
| Range | 3-324 |
| Mean (standard deviation) | 107.0 (73.7) |
| Chelation index | |
| Range | 0-97 |
| Mean (standard deviation) | 55.0 (33.0) |
| No. of patients with index of 0 | 20 |
| Serum ferritin (μg/L) | |
| Range | 322-8,106 |
| Mean (standard deviation) | 2241 (1620) |
| Chronic active hepatitis (CAH) | |
| No. of patients with CAH | 35 |
| Unbound iron-binding capacity (μg/dL) (values greater than 0 were available for 37 patients*) | |
| Range | 1-147 |
| Mean (standard deviation) | 30.0 (34.4) |
| Liver iron concentration (mg/g) (normal values are <1.6 mg/g) (values were not available for 10 patients) | |
| Range | 1.4-41.9 |
| Mean (standard deviation) | 14.4 (10.2) |
| Characteristic . | Value . |
|---|---|
| Age (yr) | |
| Range | 17-35 |
| Mean (standard deviation) | 21.5 (3.8) |
| Gender female | 39 |
| Previous red blood cell transfusions (no.) | |
| Range | 58-900 |
| Mean (standard deviation) | 362.0 (132.4) |
| Age at first transfusion (mo) | |
| Range | 2-324 |
| Mean (standard deviation) | 28.5 (49.3) |
| Age at first chelation (mo) | |
| Range | 3-324 |
| Mean (standard deviation) | 107.0 (73.7) |
| Chelation index | |
| Range | 0-97 |
| Mean (standard deviation) | 55.0 (33.0) |
| No. of patients with index of 0 | 20 |
| Serum ferritin (μg/L) | |
| Range | 322-8,106 |
| Mean (standard deviation) | 2241 (1620) |
| Chronic active hepatitis (CAH) | |
| No. of patients with CAH | 35 |
| Unbound iron-binding capacity (μg/dL) (values greater than 0 were available for 37 patients*) | |
| Range | 1-147 |
| Mean (standard deviation) | 30.0 (34.4) |
| Liver iron concentration (mg/g) (normal values are <1.6 mg/g) (values were not available for 10 patients) | |
| Range | 1.4-41.9 |
| Mean (standard deviation) | 14.4 (10.2) |
The iron-binding capacity of 38 patients was completely saturated.
Values were not available for 12 patients.
The pretransplant characteristics age, age at diagnosis, age at first transfusion, number of transfusions, chelation index, class, liver and spleen size, liver hemosiderosis, portal fibrosis, and levels of serum ferritin, aspartate and alanine aminotransferase were not significantly influential on survival in univariate regression analysis. However, patients with chronic active hepatitis at the time of transplantation were significantly more likely to die than patients without (P= .05; relative risk, 2.05).
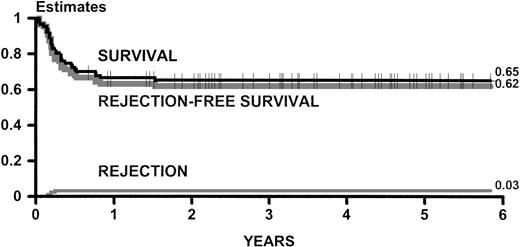
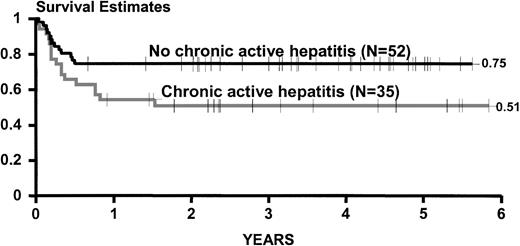
Figure 2 shows estimates of survival, rejection-free survival, and rejection for all group B patients. Figure 3 shows estimates of survival for group B patients categorized according to the presence of chronic active hepatitis at the time of transplant.
Kaplan-Meier estimates of survival and rejection-free survival and cumulative incidence estimates of rejection for 87 adult patients transplanted between May 1991 and September 1996. This experience is updated as of December 1997.
Kaplan-Meier estimates of survival and rejection-free survival and cumulative incidence estimates of rejection for 87 adult patients transplanted between May 1991 and September 1996. This experience is updated as of December 1997.
Kaplan-Meier estimates of survival for 87 adult patients transplanted between May 1991 and September 1996 and categorized on the basis of the presence of chronic active hepatitis at the time of transplantation. This experience is updated as of December 1997.
Kaplan-Meier estimates of survival for 87 adult patients transplanted between May 1991 and September 1996 and categorized on the basis of the presence of chronic active hepatitis at the time of transplantation. This experience is updated as of December 1997.
Three patients died without evidence of engraftment on days 13, 16, and 19 (2 from hemorrhage and 1 from veno-occlusive disease of the liver). All other patients engrafted successfully, but 3 patients rejected their grafts on days 56, 70, and 91, with a full return of host type myelopoiesis in each case. These patients are currently alive with thalassemia at 8 months, 2 years, and 5 years after transplantation, respectively. One patient developed severe marrow hypoplasia at 5 months after transplantation with evidence of persistent donor-type hematopoiesis. A second transplant from the same donor after conditioning with CY and total lymphoid irradiation resulted in successful engraftment, but the patient died from aspergillus infection and pneumonia on day 279 after the first and on day 90 after the second transplant. One patient, untransfused since the transplant with hemoglobin level stable at 11 g/dL, survives with persistent mixed chimerism 2,863 days after transplantation. A total of 30 patients died, 17 (49%) of the 35 patients who had chronic active hepatitis at the time of transplant and 13 (25%) from the group of 51 patients who did not. Table 2 shows the principal causes of death.
Causes of Death
| Cause . | No. of Patients . |
|---|---|
| Infections and/or pneumonia | 14 |
| Acute GVHD | 4 |
| Cardiac tamponade | 1 |
| Interstitial pneumonia (Pneumocystis) | 1 |
| Veno-occlusive disease of liver | 1 |
| Liver failure | 7 |
| Hemorrhage | 2 |
| Cause . | No. of Patients . |
|---|---|
| Infections and/or pneumonia | 14 |
| Acute GVHD | 4 |
| Cardiac tamponade | 1 |
| Interstitial pneumonia (Pneumocystis) | 1 |
| Veno-occlusive disease of liver | 1 |
| Liver failure | 7 |
| Hemorrhage | 2 |
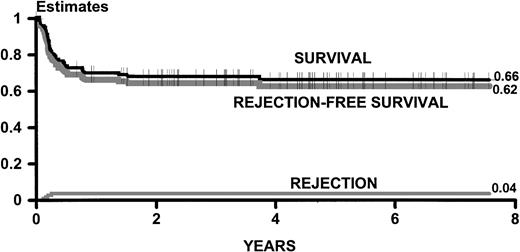
Of the total experience in transplanting patients with thalassemia who were older than 16 years at the time of transplant, 72 of 107 patients survived at least 1 year after the transplant, with 3 deaths subsequent to 1 year (Fig 4). Sixty-nine patients survive between 1 year and 8.5 years after transplantation, and 66 of these patients do not have thalassemia and have Karnofsky scores of 100.
Kaplan-Meier estimates of survival and thalassemia-free survival and cumulative incidence estimates of rejection for 107 adult patients transplanted between November 1988 and September 1996. The statistics are updated as of December 1997.
Kaplan-Meier estimates of survival and thalassemia-free survival and cumulative incidence estimates of rejection for 107 adult patients transplanted between November 1988 and September 1996. The statistics are updated as of December 1997.
As of December 1997, the youngest survivor is 20 years old, 6 are older than 30 years, and the oldest is 37 years of age.
DISCUSSION
The proportion of patients with thalassemia surviving beyond the age of 16 years is increasing, and this is a tribute to the effectiveness of transfusion/chelation therapy.13 Data on disease status and iron burden of populations of older patients are not readily available, but, almost certainly, patients who have already achieved class 2 or 3 transplant-risk status have substantial morbidity and face significant clinical problems associated with iron overload.
We have demonstrated that regimens with lower doses of CY are associated with reduced mortality in class 3 patients less than 17 years old.4 However, this did not result in improved thalassemia-free survival because of an increase in rejection (with associated disease recurrence). In a previous study, we applied this approach to the treatment of older patients (most of whom are class 3) by using treatment regimens selected on the basis of risk-group.5 The probabilities of survival, disease-free survival, and rejection were reported as 85%, 80%, and 5%, but the number of patients in that study was too small to permit useful statistical analysis.
In the present study, we could not demonstrate any influence of risk class or the factors that determine risk class upon outcome, and this is not surprising considering that the regimens used were designed to compensate for extra risk associated with class 3. As in the previous report, we did not see an increase in rejection associated with the less aggressive CY regimen such as we have reported in younger patients4 and we cannot account for this. In the younger patients, this increase was most pronounced in patients receiving less than 100 red blood cell transfusions before transplantation. In the present study, only 1 patient had received less than 100 transfusions, and transfusion exposure was much greater than that seen in younger patients. However, it is unlikely that this is the only influential factor, because the rejection incidence in younger patients receiving lower CY dosages was high even in patients with a heavy transfusion history.
The only factor significantly associated with outcome was the presence of chronic active hepatitis at the time of transplantation. The implication of this finding on the decision to transplant older patients is unclear, because it is not known how the presence of chronic active hepatitis influences the survival of adults with thalassemia in the absence of transplantation.
The findings reported here confirm that allogeneic marrow transplantation from an HLA-identical sibling donor offers a reasonable prospect of success for adult patients with homozygous thalassemia. The probability of 5-year cure for patients in risk classes 2 or 3 is 62%. We do not know what proportion of patients surviving to adulthood will be in classes 2 or 3 or what would be the prospects of success for adult patients in risk class 1. Transplantation for such patients could be deferred until progression to class 2, but we have reported elsewhere that reversal of iron overload induced organ damage incurred before transplantation is particularly difficult in class 3 patients.9 14
Supported by The Berloni Foundation against Thalassemia and A.I.L. of Pesaro.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Guido Lucarelli, MD, Divisione Ematologica, Ospedale di Pesaro, 61100 Pesaro, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal