Abstract
The mechanism underlying the cytotoxicity mediated by a human CD4+ cytotoxic T-lymphocyte (CTL) clone directed against a peptide derived from the acute myelogenous leukemia-associated fusion protein, DEK-CAN, was investigated. A DEK-CAN fusion peptide-specific CD4+ Th0 CTL clone, designated HO-1, was established from the peripheral blood lymphocytes of a healthy individual. HO-1 exerted direct but not “innocent bystander” cytotoxicity within 2 hours. The cytotoxicity mediated by HO-1 was completely Ca2+-dependent. Because HO-1 lysed peptide-loaded Fas-deficient target cells derived from a patient with a homozygousFas gene mutation, its cytotoxicity appeared to be mediated by a Fas-independent pathway. In addition, its cytotoxicity was only partially inhibited by treatment with concanamycin A and strontium ions, which are inhibitors of the perforin-based cytotoxic pathway. Although membrane-bound type of tumor necrosis factor- (TNF-) was expressed on HO-1, an anti–TNF- antibody had no effect on HO-1–mediated cytotoxicity. HO-1 expressed mRNA for apoptosis-inducing mediators, including perforin, granzyme B, Fas ligand, TNF-, and lymphotoxin; however, no DNA fragmentation was detected in target cells incubated with HO-1 by 5-[125I]Iodo-2′-deoxyuridine release assay and agarose gel electrophoresis of DNA. Although it has been suggested that the Fas/Fas ligand system is the main pathway by which CD4+ CTL-mediated cytotoxicity is exerted in murine systems, HO-1 produced peptide-specific and HLA-restricted cytotoxicity via a Fas-independent and nonapoptotic pathway. The present study thus describes a novel mechanism of cytotoxicity mediated by CD4+ CTL.
FUSION PROTEINS RESULTING from chromosomal translocations can be detected in certain types of leukemia, for example BCR-ABL in chronic myelogenous leukemia, AML1-MTG8/ETO in acute myelogenous leukemia (AML) (M2), and PML-RARα in acute promyelocytic leukemia (M3). The DEK-CAN fusion protein is produced by the translocation (6;9)(p23;q34) and is associated with a specific subtype of AML.1 It is well known that patients with t(6;9) AML treated with conventional chemotherapy have a poor prognosis2; therefore, new therapies based on novel concepts are being actively sought. Because fusion proteins are produced only in leukemia cells and not in normal cells, the area of fusion between DEK and CAN is a potential target for immunotherapy in this kind of leukemia. On the basis of this concept, we attempted to establish human T-cell clones, which specifically react to the DEK-CAN fusion peptide. In the present study, we succeeded in establishing a DEK-CAN fusion peptide-specific CD4+ human cytotoxic T-lymphocyte (CTL) clone and examined the mechanism underlying its cytotoxic effect.
Various mediators of CTL-mediated cytotoxicity have been identified. Previous studies have shown that two main pathways account for the cytotoxicity mediated by CTLs.3-10 The first is granule exocytosis, which is mediated by two types of molecules released into the extracellular space after contact between CTLs and their target cells: the pore-forming perforin and the lymphocyte-specific granule serine esterase granzyme B. The second pathway is a nonsecretory one, involving interaction of the Fas ligand with the Fas molecule expressed on the target cells. Both of these cytotoxic pathways result in apoptosis of the target cells.3,4 In addition, residual cytotoxic activity observed in mice deficient in both Fas ligand and perforin suggests the presence of other cytotoxic pathways.11,12 Recent studies have demonstrated that membrane-bound tumor necrosis factor-α (TNF-α) and lymphotoxin (LT) may be mediators of a third cytotoxic pathway.13-15 When mediated by membrane-bound TNF-α and LT, the cytotoxic activity of CTLs against target cells needs a longer incubation period to become apparent than when mediated by the granule exocytosis and Fas/Fas ligand systems.12 Among these cytolytic mediators, the interaction between Fas and Fas ligand is thought to be the main pathway by which CD4+ CTLs exert their cytotoxicity16-18; however, the precise mechanism underlying the cytotoxicity mediated by CD4+ CTLs is still obscure.
We have established a B-lymphoblastoid cell line (B-LCL) from a patient with a homozygous Fas-gene mutation.19 Because this cell line completely lacks Fas expression, it is useful in studying the Fas dependency of CTL-mediated cytotoxicity. In the present study, we clearly show that a DEK-CAN–specific CD4+ CTL clone exerted antigen-specific and HLA-restricted cytotoxicity via a Fas-independent pathway, using this Fas-deficient cell line as a target. In addition, the results of various other experiments using potent inhibitors of the granule exocytosis pathway, DNA fragmentation assay, and transmission electron microscopy demonstrated that the cytotoxicity mediated by CD4+ T cells involves a novel mechanism. That is, the DEK-CAN peptide-specific and HLA-DR–restricted cytotoxicity of a human CD4+ CTL clone was mediated by Fas-independent and nonapoptotic mechanisms. The potential application of this kind of CTL clone to peptide-based cancer immunotherapy is also discussed.
MATERIALS AND METHODS
Generation of DEK-CAN fusion peptide-specific T-cell clones.
Peptides were synthesized to a minimum of 90% purity using an automated peptide synthesizer (Model 432A Synergy; Applied Biosystems Inc, Foster City, CA) with the Fmoc procedure. The sequences of the 16-mer amino acid peptides synthesized were as follows, where “-” indicates the breakpoint of the DEK-CAN fusion protein1: DEK-CAN, TMKQICKK-EIRRLHQY; DEK, TMKQICKKVYENYPTY; and CAN, LDPKSQLQEIRRLHQY. Peripheral blood mononuclear cells (PBMCs) from a healthy individual, whose HLA-DR was HLA-DRB1*0405/1502, HLA-DRB4*0103/-, were suspended in RPMI 1640 medium supplemented with 10% heat-inactivated human AB serum (referred to hereafter as the culture medium). These were seeded into round-bottom microtiter plate wells at a concentration of 1 × 105 cells/0.2 mL together with DEK-CAN fusion peptide at a concentration of 10 μg/mL. After 7 days in culture, half of the medium was exchanged for fresh culture medium and a second stimulation was performed by adding 1 × 105 autologous PBMCs treated with mitomycin C (MMC) and DEK-CAN peptide at a concentration of 10 μg/mL. After a further 7 days, a third stimulation was performed as for the second stimulation, 4 days after the third stimulation with peptide (day 18 of culture), recombinant human interleukin-2 (IL-2) (Boehringer Mannheim, Mannheim, Germany) was added to each well at a concentration of 10 U/mL. The growing cells were then transferred to 16-mm diameter wells, and their proliferative response was examined. The bulk of the cells, which showed a proliferative response on stimulation with DEK-CAN fusion peptide, were cloned by a limiting dilution method as described previously.20 T-cell clones were continuously cultured in IL-2–containing culture medium, and MMC-treated autologous PBMCs and DEK-CAN peptide were added to the wells every 2 weeks. This T-cell clone was designated HO-1.
Alloantigen-specific CD8+ CTLs were also generated as the control CTLs, which exert granule exocytosis-mediated and apoptosis-inducing cytotoxicity, as follows: PBMCs from an individual whose HLA class I and class II were nonidentical to those of a donor for HO-1 and a patient with Fas deficiency were cocultured with MMC-treated B-LCL established from HO or that from a patient with Fas deficiency at a responder to stimulator ratio of 5:1. After 5 days, CD8+ T cells were isolated using anti-CD8 monoclonal antibody (MoAb)-coated magnetizable polystyrene beads (DYNAL, Oslo, Norway). CD8+ T cells were then cultured in IL-2–containing culture medium and restimulated with MMC-treated B-LCL cells. Following these procedures, CD8+ CTL lines directed against allogeneic B-LCL were established.
Establishment of a Fas-deficient B-LCL.
A Fas-deficient B-LCL, designated YK-B1, was established from the peripheral blood B lymphocytes of a patient with a homozygousFas gene mutation by in vitro transformation using Epstein-Barr virus.21 A homozygous point mutation was present in the splice acceptor of intron 3 of the Fas gene of this patient. This resulted in the skipping of exon 4 and complete loss of Fas expression.19 The HLA type of the patient was as follows: HLA-A24/24, B48/48, Cw8/-, DRB1*0401/0401, DRB4*0103/0103, DQB1*0301/0301, DPB1*0201/0201. A B-LCL, designated YK-B2, was also established from the peripheral blood B lymphocytes of the patient’s father, who is a heterozygote for the Fas gene mutation. The B-LCLs were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and were shown not to be infected with mycoplasma.
Flow cytometric analysis.
The expression of various membrane-bound molecules including Fas, TNF-α, and LT was determined by direct or indirect immunofluorescence using the following antibodies (Abs): fluorescence isothiocyanate (FITC)-conjugated anti-Fas MoAb (MBL, Nagoya, Japan), polyclonal rabbit anti–TNF-α Ab (Genzyme, Cambridge, MA), and polyclonal rabbit anti–LT-α and β Abs, which were prepared by immunizing rabbits with human recombinant LT-α and β (Kanegafuchi Chemical Co, Tokyo, Japan) as described previously.14 Stained cells were analyzed with a flow cytometer (FACSCalibur; Becton Dickinson, San Jose, CA).
Proliferative response to synthetic peptide.
Proliferative response assays were performed as described previously with a slight modification.22 Briefly, 2 × 104 clone cells and 2 × 105 MMC-treated autologous PBMCs or 3 × 104 MMC-treatedHLA-DR gene-transfected murine L cells as a source of antigen presenting cells (APCs) in 0.2 mL culture medium were seeded into flat-bottomed microtiter wells, to which synthetic peptide was added. In preliminary experiments to determine the optimal concentration of peptide, a DEK-CAN fusion peptide-specific T-cell clone proliferated maximally in the presence of more than 10 μg/mL peptide. Therefore, we used DEK-CAN fusion peptide at a concentration of 10 μg/mL in the present series of experiments. The culture was incubated at 37°C in an atmosphere containing 5% CO2 for 72 hours. For the final 16 hours of incubation, 1 μCi [3H]thymidine (3H-TdR) (New England Nuclear, Boston, MA) was added to each well, and the incorporation of 3H-TdR was determined by liquid scintillation counting. To determine the restriction element governing the interaction between the T-cell clones and APCs, MoAb L243 (anti–HLA-DR) (ATCC, Rockville, MD), MoAb TÜ169 (anti-HLA-DQ) (Pharmingen, San Diego, CA), or MoAb HI43 (anti-HLA-DP) (Pharmingen) was added to the wells at an optimal concentration, and the inhibitory effect of each MoAb on the proliferative response of the T-cell clones was examined as described previously.20
Cytotoxicity assays.
51Cr-release assays were performed as described previously.22 Briefly, 1 × 10451Cr (Na251CrO4) (New England Nuclear)-labeled target cells suspended in 0.1 mL RPMI 1640 medium supplemented with 10% FCS (referred to hereafter as the assay medium) were seeded into round-bottomed microtiter wells and incubated with or without synthetic peptide for 2 hours. Various numbers of effector cells suspended in 0.1 mL assay medium were then added to each well. After various incubation periods, 0.1 mL supernatant was collected from each well. The percentage of specific 51Cr release was calculated as follows: (cpm experimental release − cpm spontaneous release)/(cpm maximal release − cpm spontaneous release) × 100. To examine the Ca2+ dependency of the cytotoxicity, cytotoxicity assays were performed in the presence of EGTA (Sigma, St Louis, MO) at various concentrations. To evaluate the role of perforin in CTL-mediated cytotoxicity, effector T cells were pretreated with concanamycin A (CMA) (Wako Pure Chemical Industries, Osaka, Japan), an inhibitor of vacuolar type H+-adenosine triphosphate (ATPase), at various concentrations for 2 hours, then incubated with the target cells in the presence of CMA.23Moreover, to clarify the contribution of the granule exocytosis pathway to CTL-mediated cytotoxicity, strontium chloride (SrCl2) (Sigma), which induces degranulation of CTL,24,25 was added to effector cells at various concentrations. After incubation at 37°C for 18 hours, the effector cells were washed twice and then51Cr release assays were performed as described above. To examine the effect of an anti–TNF-α Ab on cytotoxicity, effector cells were incubated with polyclonal rabbit anti–TNF-α Ab (Genzyme) at a final concentration of 1:10, which neutralizes 1,000 U TNF-α bioactivity for 30 minutes, after which 51Cr release assay was performed as described above. This procedure inhibited TNF-α–dependent cytotoxicity.26 27 Each cytotoxicity assay was performed at least twice, and identical data were obtained.
DNA fragmentation assays.
DNA fragmentation in the target cells was determined by 5-[125I]Iodo-2′-deoxyuridine (125IUdR) release assays as described previously.28 Briefly, target cells were incubated with 125IUdR (Amersham, Arlington Heights, IL) for 2 hours at 37°C. Various numbers of effector cells and 1 × 104125IUdR-labeled target cells, which had been incubated with or without peptide for 2 hours, were incubated together in 0.2 mL assay medium in round-bottomed microtiter wells. After various incubation periods, 0.1 mL supernatant was removed, and 0.1 mL 0.2% Triton X-100 was added to each well and mixed by pipetting. The solubilized samples were centrifuged, 0.1 mL supernatant was collected from each well, and the radioactivity was counted. The total radioactivity of the target cells was estimated by directly counting the radioactivity of the cell suspension without solubilization. The specific 125IUdR release was estimated as described for the 51Cr release assays.
Cytokine production.
For the assays of cytokine production, 5 × 105 clone cells and 2 × 106 MMC-treated autologous PBMCs were suspended in 2 mL assay medium and cultured in 16-mm wells in the presence or absence of peptide. After 72 hours, the supernatants were collected from each well and assayed for the production of various cytokines by enzyme-linked immunosorbent assay (ELISA) (PerSeptive Diagnostics, Inc, Cambridge, MA).
Detection of cytolytic mediator mRNA expression.
Expression of the mRNAs for various cytolytic mediators in HO-1 was investigated by reverse transcriptase-polymerase chain reaction (RT-PCR). Total RNA was extracted from HO-1, which had been stimulated with peptide 5 days previously (activated phase) and 14 days previously (resting phase), and cDNA was synthesized by reverse transcription with Moloney murine leukemia virus reverse transcriptase, as described previously.29 Amplification of the cDNAs by PCR was performed using the following primers: perforin, 5′-ACCAGCAATGTGCATGTGTCTGTG-3′ and 5′-GAAGGAGGCCGTCATCTTGTGCTT-3′; granzyme B, 5′-TGCAGGAAGATCGAAAGTGCG-3′ and 5′-GAGGCATGCCATTGTTTCGTC-3′; Fas ligand, 5′-ATAGGATCCATGTTTCTGCTCTTCCACCTACAGAAGGA-3′ and 5′-ATAGAATTCTGACCAAGAGAGAGCTCAGATACGTTGAC-3′; TNF-α, 5′-TGAGCACTGAAAGCATGATC-3′ and 5′-TTATCTCTCAGCTCCACGCC-3′; and LT-β, 5′-ATAAGCTTGATCAGGGAGGACTGGTAACGG-3′ and 5′-TAGGTACCTCGCACCACGCACTCATATTC-3′. The expected lengths of the amplified cDNA sequences for each cytolytic mediator were as follows: perforin, 459 bp; granzyme B, 180 bp; Fas ligand, 506 bp; TNF-α, 375 bp; and LT-β, 635 bp.
Analysis of DNA fragmentation by agarose gel electrophoresis.
The cloned T cells and B-LCL cells were cocultured at an effector to target (E:T) ratio of 3:1 for 4 hours in the presence or absence of DEK-CAN fusion peptide, then harvested by centrifugation. The pellet was lysed with hypotonic lysing buffer, and the DNA was extracted from each lysate using a DNA extraction kit (Sepa Gene; Sanko Junyaku Co, Tokyo, Japan). The samples were electrophoresed in 2% agarose gel. The gel was stained with ethidium bromide and visualized by transillumination with ultraviolet light.
Transmission electron microscopy.
Morphologic changes in the target cells after incubation with the effector T cells were examined by transmission electron microscopy. After cocultivation of the cloned T cells and peptide-loaded LCL at an E:T ratio of 3:1 for 4 hours, the cells were fixed with 2.0% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.4), postfixed with 1% osmium tetroxide, and gradually dehydrated. The samples were embedded in Epon 812, sectioned, stained with uranyl acetate and lead citrate, and examined with an H-800 microscope (Hitachi, Ibaragi, Japan).
RESULTS
Generation of a T-cell clone directed against the DEK-CAN fusion peptide and analysis of HLA restriction.
PBMCs from a healthy individual, whose HLA-DR type was DRB1*0405/1502, DRB4*0103/−, were repeatedly stimulated with DEK-CAN fusion peptide in microtiter wells as detailed in Materials and Methods. From a total of 192 wells seeded, a T-cell line, which proliferated in response to stimulation with DEK-CAN peptide in the presence of autologous APCs was generated. After cloning by a limiting dilution method, a T-cell clone, designated HO-1, was established. The phenotype of HO-1 was CD2+ CD3+ CD4+CD8− CD56−. HO-1 proliferated on stimulation with the 16-mer DEK-CAN fusion peptide, but not in response to stimulation with the physiological 16-mer counterpart peptides DEK or CAN (data not shown). The proliferative response of HO-1 to stimulation with DEK-CAN peptide was inhibited by adding anti-DR MoAb to the culture medium, but not by adding anti-DQ or anti-DP MoAbs, suggesting that the proliferative response of HO-1 was restricted by HLA-DR (Table 1, experiment no. 1). HO-1 appeared to proliferate in response to stimulation with the DEK-CAN peptide in the presence of allogeneic APCs bearing HLA-DR4, -DR7, and -DR9, but showed no response in the presence of APCs bearing only HLA-DR2 or -DR6 (data not shown). Because expression of HLA-DRB4 (DR53) is associated with HLA-DR4, -DR7 and -DR9, we speculated that the restriction element for HO-1 might be HLA-DRB4. To verify this possibility, we used HLA-DRA and HLA-DRB4*0103gene-transfected murine L cells (L-DR53) as APC. As shown in Table 1, experiment no. 2, HO-1 proliferated in response to stimulation with the DEK-CAN peptide in the presence of L-DR53, but not in the presence ofHLA-DRA and DRB1*0401 gene-transfected L cells (L-DR4) or control L cells transfected with the selection markerNeor gene alone (L-Neo). These data show that the proliferative response of HO-1 is restricted by HLA-DRB4*0103. According to a recent study of peptide motifs for the HLA-DR53 molecule,30 the seventh amino acid (K) in the DEK portion and the tenth amino acid (I) in the CAN portion might be binding motifs for HLA-DR53 positions 1 and 4, respectively.
HLA-DR53 Restriction of the Proliferative Response of HO-1 to the DEK-CAN Fusion Peptide
| Clone . | APC . | MoAb Added . | Peptide Stimulation . | Proliferative Response (cpm) (mean ± SD) . |
|---|---|---|---|---|
| Experiment No. 1* | ||||
| HO-1 | Auto PBMCs | None | + | 23,084 ± 1,395 |
| − | 129 ± 24 | |||
| Anti–HLA-DR | + | 209 ± 15 | ||
| Anti–HLA-DQ | + | 29,599 ± 1,461 | ||
| Anti–HLA-DP | + | 18,138 ± 151 | ||
| Experiment No. 2† | ||||
| HO-1 | None | + | 604 ± 48 | |
| − | 89 ± 8 | |||
| L-Neo | + | 271 ± 25 | ||
| − | 213 ± 20 | |||
| L-DR4 | + | 640 ± 139 | ||
| − | 764 ± 150 | |||
| L-DR53 | + | 24,475 ± 1,227 | ||
| − | 242 ± 46 | |||
| None | L-DR53 | + | 367 ± 188 | |
| − | 227 ± 25 | |||
| Clone . | APC . | MoAb Added . | Peptide Stimulation . | Proliferative Response (cpm) (mean ± SD) . |
|---|---|---|---|---|
| Experiment No. 1* | ||||
| HO-1 | Auto PBMCs | None | + | 23,084 ± 1,395 |
| − | 129 ± 24 | |||
| Anti–HLA-DR | + | 209 ± 15 | ||
| Anti–HLA-DQ | + | 29,599 ± 1,461 | ||
| Anti–HLA-DP | + | 18,138 ± 151 | ||
| Experiment No. 2† | ||||
| HO-1 | None | + | 604 ± 48 | |
| − | 89 ± 8 | |||
| L-Neo | + | 271 ± 25 | ||
| − | 213 ± 20 | |||
| L-DR4 | + | 640 ± 139 | ||
| − | 764 ± 150 | |||
| L-DR53 | + | 24,475 ± 1,227 | ||
| − | 242 ± 46 | |||
| None | L-DR53 | + | 367 ± 188 | |
| − | 227 ± 25 | |||
Incorporation of 3H-TdR into HO-1 cells was determined in the presence of autologous APCs with or without DEK-CAN peptide and with or without anti-HLA MoAb.
Incorporation of 3H-TdR into HO-1 cells was determined in the presence or absence of various types of APC with or without DEK-CAN peptide.
Cytotoxic activity against various allogeneic target cells and L-cell transfectants.
We next examined the ability of HO-1 to lyse DEK-CAN peptide-loaded target cells. Table 2 shows the cytotoxicity of HO-1 against autologous and various allogeneic B-LCLs in the presence or absence of the peptide. HO-1 exerted strong cytotoxicity (70.0%, 68.4%, and 57.7% specific cytotoxicity at E:T ratios of 10:1, 5:1, and 2.5:1, respectively) against peptide-loaded autologous B-LCL. As with the proliferative response, the DEK-CAN fusion peptide-specific cytotoxicity of HO-1 was restricted by HLA-DR53, as allogeneic targets bearing HLA-DR53 and L-DR53, but not HLA-DR53-negative allogeneic or L-Neo cells, were lysed by HO-1.
Cytotoxicity of HO-1 Against Various Target Cells
| Target . | HLA-DRB1* . | HLA-DR53 . | % Cytotoxicity . |
|---|---|---|---|
| Autologous LCL | 0405/1502 | + | 68.4 |
| Allogeneic LCL #1 | 0901/1406 | + | 67.2 |
| Allogeneic LCL #2 | 0405/0901 | + | 64.6 |
| Allogeneic LCL #3 | 0401/0401 | + | 63.4 |
| Allogeneic LCL #4 | 0101/0405 | + | 50.7 |
| Allogeneic LCL #5 | 0405/0410 | + | 57.0 |
| Allogeneic LCL #6 | 0406/0406 | + | 65.3 |
| Allogeneic LCL #7 | 0701/0701 | + | 64.2 |
| Allogeneic LCL #8 | 1306/1501 | − | −0.3 |
| Allogeneic LCL #9 | 1405/1501 | − | 1.7 |
| Allogeneic LCL #10 | 1501/1601 | − | 0.3 |
| Allogeneic LCL #11 | 1401/1502 | − | −0.3 |
| L-DR53 | — | + | 43.5 |
| L-Neo | — | − | 1.0 |
| Target . | HLA-DRB1* . | HLA-DR53 . | % Cytotoxicity . |
|---|---|---|---|
| Autologous LCL | 0405/1502 | + | 68.4 |
| Allogeneic LCL #1 | 0901/1406 | + | 67.2 |
| Allogeneic LCL #2 | 0405/0901 | + | 64.6 |
| Allogeneic LCL #3 | 0401/0401 | + | 63.4 |
| Allogeneic LCL #4 | 0101/0405 | + | 50.7 |
| Allogeneic LCL #5 | 0405/0410 | + | 57.0 |
| Allogeneic LCL #6 | 0406/0406 | + | 65.3 |
| Allogeneic LCL #7 | 0701/0701 | + | 64.2 |
| Allogeneic LCL #8 | 1306/1501 | − | −0.3 |
| Allogeneic LCL #9 | 1405/1501 | − | 1.7 |
| Allogeneic LCL #10 | 1501/1601 | − | 0.3 |
| Allogeneic LCL #11 | 1401/1502 | − | −0.3 |
| L-DR53 | — | + | 43.5 |
| L-Neo | — | − | 1.0 |
The cytotoxicity of HO-1 against various target cells loaded with the DEK-CAN peptide was determined by 4-hour 51Cr release assays at an E:T ratio of 5:1.
Direct lysis of target cells.
We addressed the question of whether HO-1 exerts cytotoxicity via the release of an antigen-nonspecific soluble cytolytic factor into the culture medium or via direct contact with the target cells. To clarify this issue, we performed “innocent bystander” experiments, in which the effector cells were incubated with 51Cr-labeled HLA-DR53− allogeneic LCL in the presence of unlabeled peptide-loaded autologous LCL. As shown in Table 3, HO-1 mediated no apparent51Cr release from HLA-DR53− allogeneic cells. These data suggest that HO-1 directly lyses target cells in a peptide-specific and HLA-restricted manner.
Direct Killing of Target Cells by HO-1
| %Specific 51Cr Release From Target Cells . | |||
|---|---|---|---|
| 51Cr-Labeled Auto LCL . | 51Cr-Labeled DR53 (−) LCL . | 51Cr-Labeled Auto LCL + Unlabeled DR53 (−) LCL3-150 . | 51Cr-Labeled DR53 (−) LCL + Unlabeled Auto LCL3-151 . |
| 61.3 | −3.6 | 61.6 | 1.1 |
| %Specific 51Cr Release From Target Cells . | |||
|---|---|---|---|
| 51Cr-Labeled Auto LCL . | 51Cr-Labeled DR53 (−) LCL . | 51Cr-Labeled Auto LCL + Unlabeled DR53 (−) LCL3-150 . | 51Cr-Labeled DR53 (−) LCL + Unlabeled Auto LCL3-151 . |
| 61.3 | −3.6 | 61.6 | 1.1 |
The 51Cr release assay was performed in the presence of 51Cr-labeled DEK-CAN peptide-loaded autologous LCL and unlabeled peptide-loaded HLA-DR53− allogeneic LCL.
The 51Cr release assay was performed in the presence of51Cr-labeled DEK-CAN peptide-loaded HLA-DR53−allogeneic LCL and unlabeled peptide-loaded autologous LCL.
Cytokine production.
HO-1 cells were cultured with autologous MMC-treated PBMCs as APC in the presence or absence of the peptide, and the supernatants were analyzed for the production of IL-4, IL-10, TNF-α, and interferon-γ (IFN-γ). As shown in Table 4, HO-1 secreted all of these cytokines after stimulation with the DEK-CAN fusion peptide. Therefore, HO-1 can be classified as a Th0 type CD4+ T-cell clone.
Cytokine Production by HO-1 in Response to Peptide Stimulation
| Cells Cultured . | Peptide Stimulation . | Cytokine Production (pg/mL) . | |||
|---|---|---|---|---|---|
| IL-4 . | IL-10 . | TNF-α . | IFN-γ . | ||
| HO-1 + Auto APC | + | 762 | 1,558 | 1,524 | 3,088 |
| − | 0 | 43 | 73 | 166 | |
| Auto APC | + | 0 | 26 | 64 | 0 |
| Cells Cultured . | Peptide Stimulation . | Cytokine Production (pg/mL) . | |||
|---|---|---|---|---|---|
| IL-4 . | IL-10 . | TNF-α . | IFN-γ . | ||
| HO-1 + Auto APC | + | 762 | 1,558 | 1,524 | 3,088 |
| − | 0 | 43 | 73 | 166 | |
| Auto APC | + | 0 | 26 | 64 | 0 |
HO-1 cells were cultured in the presence of autologous APCs with or without DEK-CAN peptide for 72 hours, and the concentration of each cytokine in the culture supernatant was determined by ELISA.
Expression of cytolytic mediators.
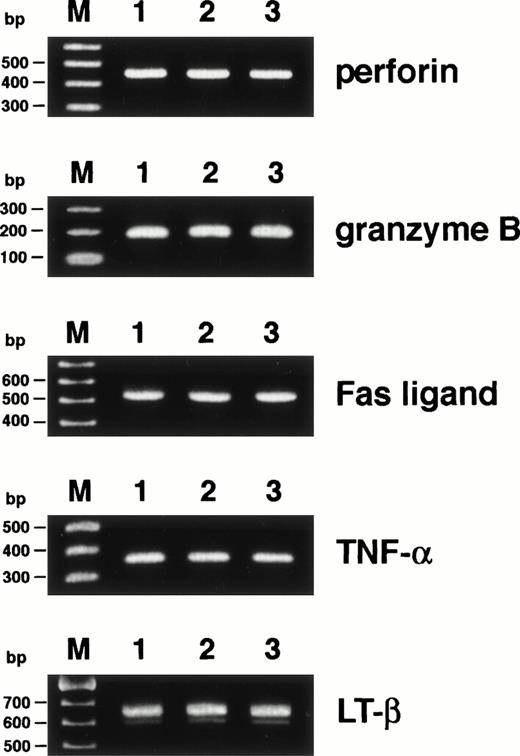
Because the mechanism underlying CD4+ CTL-mediated cytotoxicity remains obscure, we attempted to study the pathway by which HO-1 cytotoxicity is mediated. First, we examined the expression of cytolytic mediators, which have been reported to be important in CD8+ and CD4+ CTL- and natural killer (NK) cell-mediated cytotoxicity, including perforin, granzyme B, Fas ligand, TNF-α, and LT, by RT-PCR. As shown in Fig1, mRNAs for all of the cytolytic mediators examined were expressed by HO-1 in both the activated and resting phases.
Expression of cytolytic mediators in HO-1. Expression of mRNAs for perforin, granzyme B, Fas ligand, TNF-, and LT-β was investigated by RT-PCR as detailed in Materials and Methods. mRNAs were extracted from HO-1, which had been stimulated with the DEK-CAN peptide and autologous APCs for 5 days (lane 1) and for 14 days (lane 2) and from PBMCs, which had been stimulated with phytohemagglutinin for 3 days (lane 3). Lane M shows 100-bp ladder markers.
Expression of cytolytic mediators in HO-1. Expression of mRNAs for perforin, granzyme B, Fas ligand, TNF-, and LT-β was investigated by RT-PCR as detailed in Materials and Methods. mRNAs were extracted from HO-1, which had been stimulated with the DEK-CAN peptide and autologous APCs for 5 days (lane 1) and for 14 days (lane 2) and from PBMCs, which had been stimulated with phytohemagglutinin for 3 days (lane 3). Lane M shows 100-bp ladder markers.
Ca2+-dependent cytotoxicity.
The perforin/granzyme pathway is known to be Ca2+-dependent, and recent studies have shown that extracellular Ca2+ is also necessary for the Fas/Fas ligand system.4 31 With these findings in mind, the cytotoxic activity of HO-1 was examined in the absence of extracellular Ca2+. As shown in Fig 2, no HO-1 cytotoxicity was observed in the presence of the Ca2+-chelating agent EGTA. Thus, HO-1–mediated cytotoxicity appears to be completely Ca2+-dependent.
Ca2+-dependent cytotoxicity of HO-1. HO-1–induced lysis of DEK-CAN peptide-loaded autologous LCL was examined by 4-hour 51Cr release assay at an E:T ratio of 5:1 in the absence or presence of various concentrations of EGTA.
Ca2+-dependent cytotoxicity of HO-1. HO-1–induced lysis of DEK-CAN peptide-loaded autologous LCL was examined by 4-hour 51Cr release assay at an E:T ratio of 5:1 in the absence or presence of various concentrations of EGTA.
Cytotoxicity against a Fas-deficient cell line.
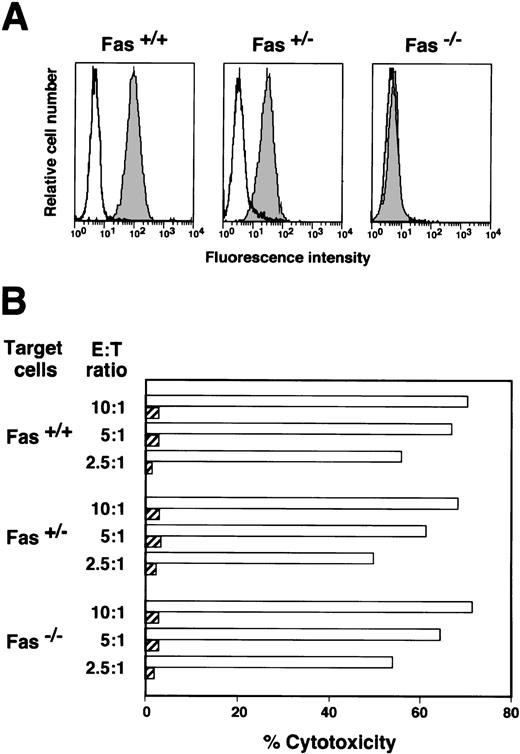
The significance of the Fas/Fas ligand pathway in HO-1–mediated cytotoxicity was examined by measuring its cytotoxic activity against Fas-deficient target cells. The results of a flow cytometric analysis of Fas expression on the B-LCLs used in the present study are shown in Fig 3A. A complete lack of Fas expression was detected in the YK-B1 cell line, which was established from a patient with a homozygous Fas gene mutation. The expression of Fas on YK-B2, which was established from the patient’s father who carried a heterozygous Fas gene mutation, was decreased as compared with B-LCL established from a donor for HO-1. Because the Fas-deficient B-LCL, YK-B1, was positive for HLA-DR53, the degree of cytotoxicity against peptide-loaded YK-B1 was expected to be reduced if the Fas/Fas ligand system was involved in the cytolytic pathway. However, the percentage cytotoxicities against autologous, heterozygousFas mutant and homozygous Fas mutant target cells were almost the same (Fig 3B). These data indicate that the HO-1 line has a Fas-independent cytotoxic pathway.
Cytotoxicity of HO-1 against Fas-positive and -negative target cells. (A) B-LCLs were established from the donor for HO-1 (Fas+/+), the patient’s father (Fas+/−), and the patient with Fas deficiency (Fas−/−). Cells were stained with FITC-conjugated mouse anti-Fas MoAb (shaded histograms) or FITC-conjugated mouse IgG (open histograms). The fluorescence profiles were analyzed with a flow cytometer. (B) HO-1–induced lysis of DEK-CAN peptide-loaded (open columns) and unloaded (shaded columns) target cells was determined by51Cr release assays over 4 hours at E:T ratios of 10:1, 5:1, and 2.5:1.
Cytotoxicity of HO-1 against Fas-positive and -negative target cells. (A) B-LCLs were established from the donor for HO-1 (Fas+/+), the patient’s father (Fas+/−), and the patient with Fas deficiency (Fas−/−). Cells were stained with FITC-conjugated mouse anti-Fas MoAb (shaded histograms) or FITC-conjugated mouse IgG (open histograms). The fluorescence profiles were analyzed with a flow cytometer. (B) HO-1–induced lysis of DEK-CAN peptide-loaded (open columns) and unloaded (shaded columns) target cells was determined by51Cr release assays over 4 hours at E:T ratios of 10:1, 5:1, and 2.5:1.
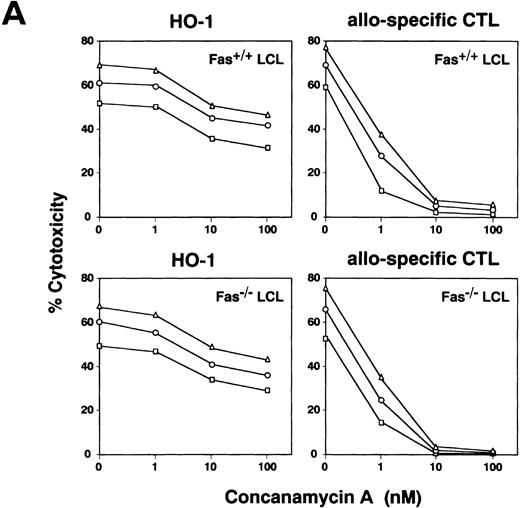
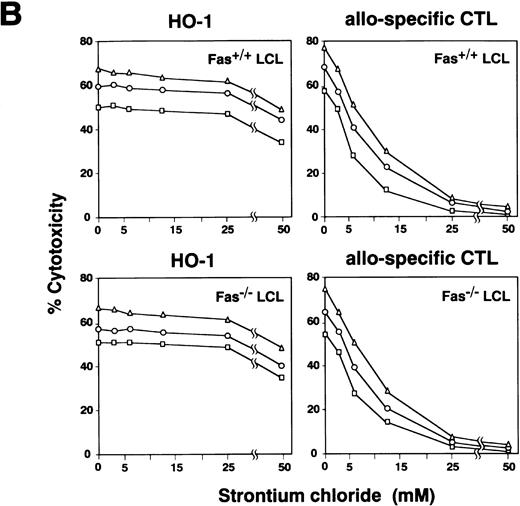
Inhibition of cytotoxicity by CMA and Sr2+.
The significance of the granule exocytosis pathway was examined using an inhibitor of vacuolar type H+-ATPase, CMA. Recent studies have shown that CMA is a selective inhibitor blocking only perforin-based cytotoxicity, but not affecting Fas-dependent cytotoxicity, due mostly to accelerated degradation of perforin by an increase in the pH of lytic granules.23 It has been reported that treatment of CTLs with CMA at a concentration of more than 10 nmol/L almost completely inhibits perforin-based cytotoxic activity.23,32 As treatment of HO-1 cells with CMA at concentrations of more than 100 nmol/L produced toxic effects, CMA was used at concentrations of 1 to 100 nmol/L. The results shown in Fig 4A demonstrate that while pretreatment of HO-1 with CMA produced some inhibition of peptide-specific cytotoxicity against both autologous B-LCL (Fas+/+) and Fas-deficient B-LCL (Fas−/−), this inhibition was only partial. On the other hand, cytotoxicity of alloantigen-specific CD8+ CTLs directed against Fas-deficient target cells, which was considered to be mediated via granule exocytosis pathway, was completely inhibited by treatment with CMA at a concentration of more than 10 nmol/L, as reported previously.23 32
Effect of CMA and Sr2+ on cytotoxicity mediated by HO-1. (A) HO-1 cells were preincubated with various concentrations of CMA for 2 hours. The CMA-treated or untreated HO-1 cells were then cocultured with 51Cr-labeled autologous LCL (Fas+/+ LCL) and Fas-deficient LCL (Fas−/− LCL), which had been loaded with the DEK-CAN peptide in the presence or absence of CMA at E:T ratios of 10:1 (▵), 5:1 (○), and 2.5:1 (□) for 4 hours. Effect of CMA on cytotoxicity mediated by alloantigen-specific CD8+ CTLs directed against Fas+/+ LCL and Fas−/− LCL was also examined. (B) HO-1 cells were preincubated with various concentrations of SrCl2 for 18 hours. The Sr2+-treated or untreated HO-1 cells were then cocultured with 51Cr-labeled autologous LCL (Fas+/+LCL) and Fas-deficient LCL (Fas−/− LCL), which had been loaded with the DEK-CAN peptide at E:T ratios of 10:1 (▵), 5:1 (○), and 2.5:1 (□) for 4 hours. The effect of Sr2+ on cytotoxicity mediated by alloantigen-specific CD8+ CTLs directed against Fas+/+ LCL and Fas−/−LCL was also examined.
Effect of CMA and Sr2+ on cytotoxicity mediated by HO-1. (A) HO-1 cells were preincubated with various concentrations of CMA for 2 hours. The CMA-treated or untreated HO-1 cells were then cocultured with 51Cr-labeled autologous LCL (Fas+/+ LCL) and Fas-deficient LCL (Fas−/− LCL), which had been loaded with the DEK-CAN peptide in the presence or absence of CMA at E:T ratios of 10:1 (▵), 5:1 (○), and 2.5:1 (□) for 4 hours. Effect of CMA on cytotoxicity mediated by alloantigen-specific CD8+ CTLs directed against Fas+/+ LCL and Fas−/− LCL was also examined. (B) HO-1 cells were preincubated with various concentrations of SrCl2 for 18 hours. The Sr2+-treated or untreated HO-1 cells were then cocultured with 51Cr-labeled autologous LCL (Fas+/+LCL) and Fas-deficient LCL (Fas−/− LCL), which had been loaded with the DEK-CAN peptide at E:T ratios of 10:1 (▵), 5:1 (○), and 2.5:1 (□) for 4 hours. The effect of Sr2+ on cytotoxicity mediated by alloantigen-specific CD8+ CTLs directed against Fas+/+ LCL and Fas−/−LCL was also examined.
It has been reported that Sr2+ causes degranulation and release of granule contents from mast cells, basophils, and large granular lymphocytes and induces inhibition of NK cell activity.24 Recent studies have also demonstrated that pretreatment of human CTL clones with Sr2+ selectively inhibits the cytotoxicity mediated by the granule exocytosis pathway.25 In view of these findings, we examined the effect of Sr2+ on cytotoxicity mediated by HO-1. As shown in Fig 4B, the treatment of HO-1 resulted in only partial inhibition of cytotoxicity against peptide-loaded target cells, while cytotoxicity mediated by alloantigen-specific CD8+ CTLs was completely abrogated by treatment with Sr2+ at concentrations of more than 25 mmol/L. Taken together with the results of the RT-PCR, which showed the expression of perforin and granzyme B mRNAs in HO-1, these results suggest that the granule exocytosis pathway is involved to some extent, but that other mechanisms may also exist and may be more important in HO-1–mediated cytotoxicity.
Expression of membrane-bound forms of TNF-α.
TNF-α and LT have been reported to exist in both membrane-bound and secretory forms. Membrane-bound TNF-α and LT are known to be expressed on some CD8+ CTLs, CD4+ CTLs and lymphokine-activated killer cells, and exert cytotoxicity against appropriate target cells.13-15,33 Flow cytometric analysis showed that TNF-α was expressed; however, membrane-bound LT-α and β were not detected on the surface of HO-1 (Fig 5A). Negative reactivity of HO-1 against anti–LT-α and β Abs did not result from uselessness of these Abs, as lymphokine-activated killer cells14 and other CTL clones33 were stained with these Abs. To evaluate the roles of membrane-bound TNF-α in HO-1–mediated cytotoxicity against peptide-loaded autologous (Fas+/+) and Fas-deficient (Fas−/−) B-LCLs, inhibition assay using anti–TNF-α Ab was performed. As shown in Fig 5B, no inhibition of cytotoxicity was induced by the addition of anti–TNF-α Ab, suggesting that membrane-bound TNF-α is not involved in HO-1–mediated antigen-specific cytotoxicity.
Expression of membrane-bound forms of TNF- and LT on HO-1 cells and the effects of anti–TNF- Ab on cytotoxicity. (A) HO-1 cells were stained by indirect immunofluorescence with rabbit anti–TNF- IgG, rabbit anti–LT- IgG, or rabbit anti–LT-β IgG, then with FITC-conjugated goat anti-rabbit IgG (shaded histograms). Control stainings were performed using normal rabbit IgG and FITC-conjugated goat anti-rabbit IgG (open histograms). (B) HO-1–induced lysis of DEK-CAN peptide-loaded autologous LCL (Fas+/+ LCL) and Fas-deficient (Fas−/−LCL) was examined in 51Cr release assays conducted over 4 hours at E:T ratios of 10:1, 5:1, and 2.5:1 in the presence of normal rabbit IgG or anti–TNF- rabbit IgG. The effect of Ab against TNF- was also examined using TNF-–dependent killer cells, MY-3.11, and L929 as TNF-–sensitive target cells.
Expression of membrane-bound forms of TNF- and LT on HO-1 cells and the effects of anti–TNF- Ab on cytotoxicity. (A) HO-1 cells were stained by indirect immunofluorescence with rabbit anti–TNF- IgG, rabbit anti–LT- IgG, or rabbit anti–LT-β IgG, then with FITC-conjugated goat anti-rabbit IgG (shaded histograms). Control stainings were performed using normal rabbit IgG and FITC-conjugated goat anti-rabbit IgG (open histograms). (B) HO-1–induced lysis of DEK-CAN peptide-loaded autologous LCL (Fas+/+ LCL) and Fas-deficient (Fas−/−LCL) was examined in 51Cr release assays conducted over 4 hours at E:T ratios of 10:1, 5:1, and 2.5:1 in the presence of normal rabbit IgG or anti–TNF- rabbit IgG. The effect of Ab against TNF- was also examined using TNF-–dependent killer cells, MY-3.11, and L929 as TNF-–sensitive target cells.
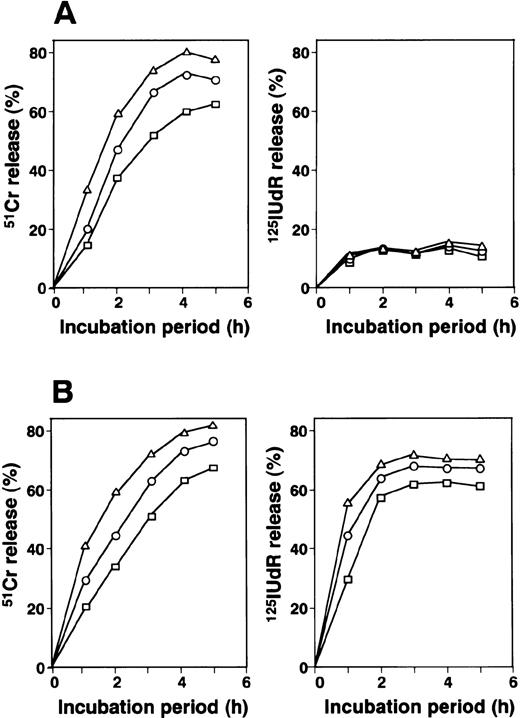
Kinetics of 51Cr and 125IUdR release assays.
The results of kinetic studies of 51Cr release, which reflects membrane damage, and 125IUdR release, which reflects DNA fragmentation in target cells, are shown in Fig 6. 51Cr release from target cells incubated with HO-1 was first detected after 1 hour of incubation, then increased rapidly. The 51Cr release level was almost maximal after 4 hours of incubation. In contrast, a low level of 125IUdR release was detected after 1 hour of incubation and did not increase further during continuous culture. As shown in Fig 6B, rapid DNA fragmentation from target cells is induced by alloantigen-specific CD8+ CTLs via perforin/granzyme system. Taken together with the data shown in Fig 4, we strongly suggest that the granule exocytosis system is not the main pathway governing HO-1–mediated cytotoxicity.
Kinetics of 51Cr and 125IUdR release from target cells mediated by HO-1. (A) HO-1 cells were cocultured with 51Cr- or 125IUdR-labeled autologous LCL (HO-LCL), which had been loaded with the DEK-CAN peptide at E:T ratios of 10:1 (▵), 5:1 (○), and 2.5:1 (□) for various incubation periods. (B) 51Cr and 125IUdR release from HO-LCL mediated by alloantigen-specific CD8+CTLs was also examined. The percentage 51Cr and125IUdR release levels were determined as described in Materials and Methods.
Kinetics of 51Cr and 125IUdR release from target cells mediated by HO-1. (A) HO-1 cells were cocultured with 51Cr- or 125IUdR-labeled autologous LCL (HO-LCL), which had been loaded with the DEK-CAN peptide at E:T ratios of 10:1 (▵), 5:1 (○), and 2.5:1 (□) for various incubation periods. (B) 51Cr and 125IUdR release from HO-LCL mediated by alloantigen-specific CD8+CTLs was also examined. The percentage 51Cr and125IUdR release levels were determined as described in Materials and Methods.
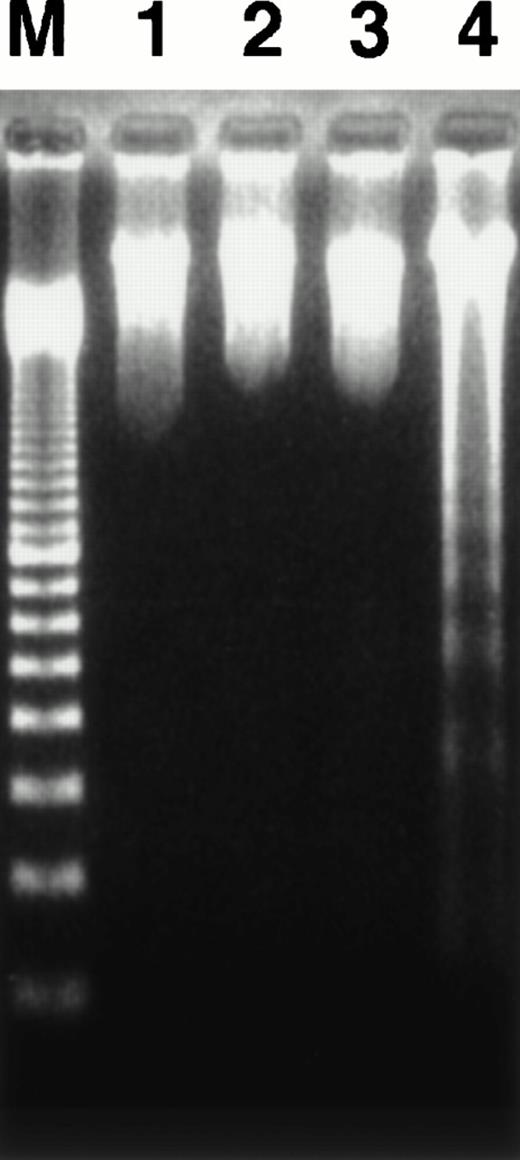
Agarose gel electrophoresis of DNA.
We investigated whether apoptosis is induced in target cells after coculture with HO-1 by agarose gel electrophoresis of their DNA. As shown in Fig 7, a ladder pattern, which reflects DNA fragmentation into oligonucleosome units, was rarely detected in DNA from a mixture of target cells and HO-1 cells, which had been incubated at an E:T ratio of 3:1 for 4 hours. Taken together with the results of the 51Cr and 125IUdR release assays, it was concluded that HO-1 mediates rapid cytotoxicity without inducing apparent DNA fragmentation in target cells.
Agarose gel electrophoresis of cellular DNA. HO-1 cells were cocultured with peptide-free autologous LCL (lane 1), DEK-CAN peptide-loaded autologous LCL (lane 2), or DEK-CAN peptide-loaded HLA-DR53–negative allogeneic LCL (lane 3) at an E:T ratio of 3:1 for 4 hours. The DNA was extracted from each sample and electrophoresed in 2% agarose gel. DNA extracted from B-LCL and alloantigen-specific CD8+ CTLs, which had been cocultured for 4 hours was also electrophoresed as the control for fragmented DNA (lane 4). Lane M shows 100-bp ladder markers.
Agarose gel electrophoresis of cellular DNA. HO-1 cells were cocultured with peptide-free autologous LCL (lane 1), DEK-CAN peptide-loaded autologous LCL (lane 2), or DEK-CAN peptide-loaded HLA-DR53–negative allogeneic LCL (lane 3) at an E:T ratio of 3:1 for 4 hours. The DNA was extracted from each sample and electrophoresed in 2% agarose gel. DNA extracted from B-LCL and alloantigen-specific CD8+ CTLs, which had been cocultured for 4 hours was also electrophoresed as the control for fragmented DNA (lane 4). Lane M shows 100-bp ladder markers.
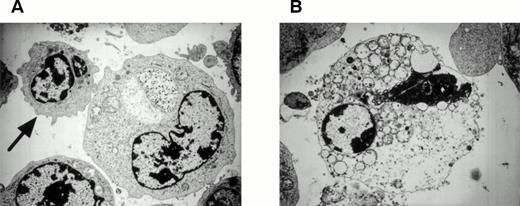
Transmission electron microscopy of target cells.
CTLs are well known to mediate apoptosis in target cells. This is characterized morphologically by condensation of the nuclear chromatin, shrinkage and blebbing of the cytoplasm, and the formation of apoptotic bodies. Therefore, we investigated morphologic changes in target cells cocultured with HO-1 by transmission electron microscopy. As shown in Fig 8, severe degeneration of the mitochondria occurred in the target cells after incubation with HO-1 cells; however, none of the aforementioned characteristics of apoptosis were detected, suggesting that the cytotoxicity of HO-1 against peptide-loaded target cells was mediated by nonapoptotic mechanisms.
Transmission electron micrographs of DEK-CAN peptide-loaded autologous LCL cocultured with HO-1. (A) A target cell, which is in contact with a CTL (arrow). Note the degeneration of the mitochondria in the target cell. (B) A target cell, which was lysed by HO-1 cells. Note the degeneration of the mitochondria and rough endoplasmic reticula in the target cell. Original magnification: (A), ×6,300; (B), ×7,200.
Transmission electron micrographs of DEK-CAN peptide-loaded autologous LCL cocultured with HO-1. (A) A target cell, which is in contact with a CTL (arrow). Note the degeneration of the mitochondria in the target cell. (B) A target cell, which was lysed by HO-1 cells. Note the degeneration of the mitochondria and rough endoplasmic reticula in the target cell. Original magnification: (A), ×6,300; (B), ×7,200.
DISCUSSION
In the present study, we established a human CD4+ CTL clone directed against an AML-associated DEK-CAN fusion peptide, designated HO-1. The characteristics of the peptide-specific and HLA-DR–restricted cytotoxicity mediated by HO-1 were as follows: first, HO-1 produced direct, but not “innocent bystander,” cytotoxicity over a short culture period, suggesting that its cytotoxicity was not mediated by soluble cytolytic factor(s), but by direct contact with the target cells. Second, the cytotoxicity of HO-1 was Ca2+-dependent. Third, HO-1 lysed peptide-loaded Fas-deficient cells, which were established from a patient with a homozygous Fas gene mutation, indicating that the HO-1 line has a Fas-independent cytotoxic pathway. Fourth, treatment of HO-1 with CMA and Sr2+, which are inhibitors of the granule exocytosis pathway, resulted in only partial inhibition of its cytotoxicity. Fifth, although HO-1 expressed the membrane-bound form of TNF-α, a neutralizing Ab against TNF-α had no effect on HO-1–mediated cytotoxicity. Sixth, no apparent DNA fragmentation was detected in target cells by agarose gel electrophoresis, and transmission electron microscopy of the target cells showed a necrotic appearance rather than apoptosis, suggesting that the main pathway involved in HO-1–mediated cytotoxicity was not apoptosis. These characteristics of CTL-mediated cytotoxicity have not been shown previously.
The mechanisms underlying cell-mediated cytotoxicity have mainly been studied in CD8+ CTLs and NK cells, and two main pathways, granule exocytosis mediated by perforin and granzymes and the Fas/Fas ligand system, have been identified.3-10 Antigen-specific and major histocompatibility complex (MHC)-restricted cytotoxicity was formerly thought to be mediated only by CD8+ CTLs, but it is now well known that some CD4+ T cells can also exert cytotoxic activity34-39; thus, the mechanisms underlying CD4+ CTL-mediated cytotoxicity are of considerable interest. These mechanisms have mainly been studied in murine systems using various mutant and knockout mice. Recent studies have shown that CD4+ CTLs derived from gld/gld mice, which lack the expression of a functional Fas ligand, do not exert Fas-mediated cytotoxicity.17 In addition, it has been reported that CD4+ Th1 cells, which lyse target cells derived from wild-type mice, do not show cytotoxicity against targets derived from Fas-deficient lpr/lpr mice.18 These reports strongly suggest that the Fas/Fas ligand system acts as the main pathway for CD4+ CTL-mediated cytotoxicity. In contrast to the extensive studies in murine systems, the mechanisms involved in human CD4+ CTL-mediated cytotoxicity have not been studied precisely due to the lack of a suitable experimental system. In the present study, we first examined the Fas-dependency of human CTL-mediated cytotoxicity using Fas-deficient target cells derived from a patient with a homozygous Fas gene mutation, reflecting the situation in lpr/lpr mice.19,40 41 The results obtained using this experimental system unexpectedly showed that the HLA-DR–restricted cytotoxicity mediated by a DEK-CAN peptide-specific human CD4+ CTL clone was Fas-independent.
The granule exocytosis model mediated by perforin and granzymes, the other important mechanism involved in CTL-mediated cytotoxicity, is thought to be the main pathway in CD8+ CTLs and NK cells. It has recently been reported that perforin expression is detectable in some CD4+ T cells, and that the granule exocytosis pathway also plays an important role in CD4+ CTL-mediated cytotoxicity.33,42-45 As addition of the Ca2+-chelating agent EGTA to the assay medium resulted in complete inhibition of cytotoxicity, HO-1–mediated cytotoxicity appeared to be completely Ca2+-dependent. Because perforin-mediated cytotoxicity depends on extracellular Ca2+, we addressed the question of whether the perforin/granzyme system is involved in HO-1–mediated cytotoxicity. To clarify this issue, we performed an experiment using CMA and Sr2+, which inhibit the granule exocytosis pathway, and found that treatment of HO-1 with optimal concentrations of CMA and Sr2+ resulted in only partial inhibition of cytotoxicity. In addition, a 125IUdR release assay showed that HO-1 induced only partial DNA fragmentation in the target cells within 1 hour, and no further 125IUdR release was detected during continuous culture. Taking into consideration the evidence that51Cr release, which reflects membrane damage, increased continuously over 4 hours of incubation, we suggest that the granule exocytosis pathway certainly plays a role in HO-1–mediated cytotoxicity, but that the main pathway is mediated by cytolytic mediator(s) other than the perforin/granzyme system. It has been reported that mice deficient in both granzyme B and Fas ligand can produce residual cytotoxic activity, which seems to be mediated by membrane damage induced by perforin alone.46-48 Although this finding suggests that HO-1–mediated cytotoxicity may be due to a perforin-dependent, but granzyme-independent mechanism, this possibility is unlikely, as RT-PCR and Western blotting showed that granzyme B mRNA and protein were expressed in HO-1.
The residual cytotoxic activity seen in CTLs deficient in both a functional Fas ligand and perforin suggests the presence of a third pathway of CTL-mediated cytotoxicity.11,12 Recent studies have shown that TNF-α and LT are major candidates as mediators of such Fas ligand- and perforin-independent cytotoxicity. Because it has recently been reported that both TNF-α and LT are expressed on some CTLs in their membrane-bound forms and that they induce cytotoxicity through contact with target cells,13-15,27 the expression of these cytokines in HO-1 was examined. mRNAs for both TNF-α and LT were found to be expressed in HO-1 and membrane-bound form of TNF-α was detectable by flow cytometry. However, it is well known that cytotoxicity mediated by TNF-α and LT needs a longer culture period to become apparent than that mediated by the perforin/granzyme and Fas/Fas ligand systems.12 In the present study, HO-1 exerted strong cytotoxicity within 2 hours, whereas it usually takes more than 10 hours to detect TNF-α– and LT-mediated cytotoxicity. In addition, the addition of a neutralizing Ab against TNF-α did not have any effect on the cytotoxic activity of HO-1, and the B-LCL appeared to be relatively resistant to cytotoxicity mediated by soluble form of TNF-α (data not shown). These findings strongly suggest that TNF-α and LT are not involved in HO-1–mediated antigen-specific cytotoxicity. Similar results were reported for a study of TNF-α by Liu et al,49 who found that human immunodeficiency virus-specific CD4+ CTL clones produced membrane-bound and secreted forms of TNF-α, although these molecules were not required for cytolysis of the target cells.
On the basis of the findings described above, we conclude that the mechanism underlying HO-1–mediated cytotoxicity is independent of the previously reported main pathways of CTL-mediated cytotoxicity, ie, granule exocytosis mediated by perforin and granzymes, the Fas/Fas ligand system, and membrane-bound TNF-α and LT. It is noteworthy that although HO-1 expressed mRNAs for all major cytolytic mediators, including perforin, granzyme B, Fas ligand, TNF-α, and LT, its cytotoxic activity was not mediated by these mediators, except for weak perforin activity. The main cytolytic mechanism(s) of HO-1 may thus act predominantly against these conventional cytotoxic pathways.
Whether leukemia-associated fusion peptide-specific CD4+CTL clones are able to inhibit the growth of leukemia cells is a point of particular interest. However, because the frequency of AML with t(6;9) is low (<1% of all cases of AML), we were unable to obtain t(6;9) AML cells bearing HLA-DRB4*0103, and thus could not investigate the cytotoxic and growth inhibitory effects of HO-1 on such leukemia cells. It has been shown that cancer-associated intracellular proteins, such as ras p21, can be recognized by CD4+ T cells in the context of MHC class II antigens.50,51 In addition, recent studies have shown that chronic myelogenous leukemia-associated BCR-ABL fusion peptide-specific CD4+ T cells can specifically recognize leukemia cells in an HLA class II–restricted manner.52 These findings strongly suggest that DEK-CAN fusion peptide-specific CD4+ T cells should be able to react against leukemia cells, as the leukemic cells in most cases of t(6;9) AML are positive for HLA-DR.53 Although the Fas antigen is frequently expressed on AML cells, these cells have been reported to be relatively resistant to anti-Fas antibody-induced apoptosis.54 This finding suggests that CTL-mediated cytotoxicity acting via the Fas/Fas ligand system may be relatively ineffective against leukemia cells, thus a Fas-independent pathway of cytotoxicity mediated by CTLs could be anticipated to inhibit leukemia growth effectively. Considering these possibilities, we strongly suggest that adoptive transfer of CTL clones possessing the characteristics of cytotoxic activity described in this study may be a hopeful prospect in immunotherapy for leukemia.
ACKNOWLEDGMENT
We thank Drs Yasuharu Nishimura (Kumamoto University, Kumamoto, Japan) and Takahiko Horiuchi (Kyushu University, Fukuoka, Japan) for providing the cell lines and for their helpful suggestions. We also thank Dr Seiji Matsuda (Ehime University, Ehime, Japan) for his helpful comments on the electron micrographs.
Supported by grants from the Ministry of Education, Science, Sports and Culture of Japan, the Ministry of Health and Welfare of Japan, the Mochida Foundation for Medical and Pharmaceutical Research, the Inamori Foundation, and the Suzuken Memorial Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Masaki Yasukawa, MD, PhD, The First Department of Internal Medicine, Ehime University School of Medicine, Shigenobu, Ehime 791-0295, Japan; e-mail: yasukawa@m.ehime-u.ac.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal