Abstract
Monocyte chemoattractant protein-1 (MCP-1), a member of the C-C subfamily of chemokines, is important for the local recruitment of leukocytes to sites of inflammatory challenge. Here, we investigated endothelial signaling pathways involving members of the mitogen-activated protein (MAP) kinase superfamily and studied their role for MCP-1 expression in endothelium. We show that tumor necrosis factor- (TNF-), a potent inflammatory activator of endothelium, leads to activation of MAP kinases ERK, p38, and JNK in human umbilical vein endothelial cells (HUVEC). Contribution of MAP kinase pathways to TNF-–induced synthesis of endothelial MCP-1 was then studied by pharmacologic inhibition and transient expression of dominant negative or constitutively active kinase mutants using flow cytometry, Northern blot, and luciferase reporter gene assays. Inhibition of Raf/MEK/ERK or SEK/JNK pathways had no significant effect on MCP-1 levels, whereas blocking the MKK6/p38 pathway by p38 inhibitors SB203580 or SB202190 or by a dominant negative mutant of MKK6, the upstream activator of p38, strongly inhibited TNF-–induced expression of MCP-1. Consistent with that finding, expression of wild-type or constitutively active MKK6 significantly enhanced the effect of limiting TNF- concentrations on MCP-1 synthesis. These data suggest a crucial role for the MKK6/p38 stress kinase cascade in TNF-–mediated endothelial MCP-1 expression.
MONOCYTE CHEMOATTRACTANT protein-1 (MCP-1), a member of the C-C chemokine family, attracts blood monocytes both in vitro and in vivo.1,2 It is produced by a variety of cells including vessel wall elements such as endothelial cells and is thought to play a major role in the recruitment of leukocytes to sites of prospective inflammation. Abundant amounts of MCP-1 have been detected in diseases such as atherosclerosis, thus strongly suggesting a role for the recruitment of monocytes to the evolving atheromatous plaque.3,4 Besides subendothelial macrophages and smooth muscle cells, endothelial cells have been considered to be the major source of MCP-1 in atheromatous lesions.5 MCP-1 appears to be important for activation of leukocyte subsets and triggers their adhesiveness and transmigration through the endothelial layer. Inhibition of induced MCP-1 expression is associated with reduced transmigration of monocytes,6 as well as with diminished recruitment of T lymphocytes.7 Accordingly, neutralizing antibodies against MCP-1 have been shown to inhibit T-cell recruitment and cutaneous delayed-type hypersensitivity,8 as well as pulmonary granuloma formation9 in rat models of experimental inflammation, thus illustrating the importance of MCP-1 at the onset of inflammatory processes.
Endothelial cells have been found to produce MCP-1 in response to cytokines such as tumor necrosis factor-α (TNF-α), interleukins (IL)-1, 4, and 13, interferon-γ (IFN-γ), as well as by agents such as thrombin (see Mantovani et al2 10 for review). However, the intracellular signaling pathways that couple endothelial activation from receptor levels to the level of MCP-1 expression are so far only poorly understood.
A major mechanism through which signals from environmental stimuli are transmitted to the nucleus involves activation of kinases related to the mitogen-activated protein (MAP) kinase superfamily. To date, at least three subgroups of MAP kinase family members have been identified to be involved in a wide range of cellular responses to extracellular signals.11 The first subgroup includes two isoforms of the extracellular signal-regulated kinases, ERK1 and ERK2. ERKs are strongly activated on stimulation of cells with mitogens such as epidermal growth factor, platelet-derived growth factor, serum, and phorbol-myristate acetate (PMA) through sequential activation of the upstream kinases Raf and MEK. This classical mitogenic kinase cascade plays an important role in mediating signals that control cell proliferation, differentiation, or survival.12-14 In contrast to the ERKs, the two other subgroups of the MAP kinase family, namely the p38 and the c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK) subgroups, are only weakly activated by mitogens, but are highly stimulated on exposure to inflammatory cytokines such as TNF-α and IL-1 and a wide variety of environmental stress inducers such as lipopolysaccharides (LPS), arsenite, anisomycin, heat shock, or ultraviolet (UV) light.15 The upstream activator of p38 is the dual specificity kinase MKK6, whereas JNK/SAPK is activated by MKK4/SEK. Among the downstream targets of JNK and p38 are the transcription factor c-jun and the MAPKAP-kinases 2 and 3, respectively. While the physiologic role of the Raf/MEK/ERK cascade is well established, the function of the stress-activated kinase cascades is far less understood. p38 has originally been identified through the action of a specific p38 inhibitor, SB203580, which partially blocks LPS-induced TNF-α and IL-1 biosynthesis in monocytes.16,17 Using the same inhibitor, p38 has also been shown to be involved in regulation of IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and human immunodeficiency virus (HIV)-long terminal repeat (LTR)-dependent gene expression.18-20
So far, little is known about the role of the different MAP kinase cascades in endothelial activation. Especially, the regulation of endothelial chemokines such as MCP-1 by different MAP kinase pathways is still an open issue.
Thus, we have analyzed the functional role of the different MAP kinase cascades in MCP-1 transcription and synthesis in endothelium. Besides the use of specific kinase inhibitors, we used a transient transfection approach for human umbilical vein endothelial cells (HUVEC) to study expression of endothelial MCP-1 in the presence of dominant negative or constitutively active components of different signaling cascades. Here, we present data to show that activation of the MKK6/p38 pathway is important for expression of MCP-1, while SEK/JNK and Raf/MEK/ERK cascades do not appear to be of major relevance.
MATERIALS AND METHODS
Reagents.
Human recombinant (r)TNF-α was obtained from R & D Systems (Wiesbaden, Germany); phorbol 12-myristate 13-acetate (PMA) from Serva (Heidelberg, Germany). Inhibitor SB203580 was kindly provided by Dr J.C. Lee (Smith Kline Beecham Pharmaceuticals, King of Prussia, PA) or purchased from Calbiochem (Bad Soden, Germany). Inhibitors SB202190 and PD98059 were obtained from Calbiochem. All other reagents were purchased from Sigma (Deisenhofen, Germany) unless otherwise specified.
Cells and cell culture.
HUVEC were obtained from Clonetics (via Cell Systems, Remagen, Germany) and cultured with endothelial basal medium (EBM; Clonetics) supplemented with 2% fetal bovine serum, 1.0 μg/mL hydrocortisone, 10 ng/mL human epidermal growth factor, 12 μg/mL bovine brain extract, 50 μg/mL gentamicin, and 50 ng/mL amphotericin B (ie, EGM). HUVEC were used between passages 3 and 5.
Immunoprecipitation and kinase assays.
Cells were lysed with 20 mmol/L Tris, pH 7.4, 137 mmol/L NaCl, 10% (vol/vol) glycerol, 1% (vol/vol) Triton X-100, 2 mmol/L EDTA, 50 mmol/L sodium-β–glycerophosphate, 20 mmol/L sodium pyrophosphate, 1 mmol/L Pefabloc (Merck, Darmstadt, Germany), 5 μg/mL aprotinin, 5 μg/mL leupeptin, and 5 mmol/L benzamidine (TLB buffer) at 4°C for 30 minutes. Cell debris was removed by centrifugation at 13,000g for 20 minutes. After quantification of the protein content in the supernatants (Biorad Protein Assay; Biorad, Munich, Germany), equal amounts were incubated with 25 μL protein A agarose (Boehringer Mannheim, Mannheim, Germany) and 1 μg/mL rabbit antisera against ERK1/ERK2, JNK (Santa Cruz, Heidelberg, Germany), p38 (kindly provided by J. Han, The Scripps Research Institute, La Jolla, CA) or an antiserum specific for both, MAPKAP kinases 2 and 321 for 2 hours at 4°C. After washing in TLB buffer supplemented with 500 mmol/L NaCl and, thereafter, kinase buffer (25 mmol/L HEPES pH 7.5, 10 mmol/L MgCl2, 25 mmol/L sodium-β–glycerophosphate supplemented with 5 mmol/L benzamidine, 1 mmol/L sodium orthovanadate, and 0.5 mmol/L dithiothreitol), each sample was incubated with 3pK K>M, myelin basic protein (MBP), glutathione-S-transferase (GST)-c-Jun(1-135) or Hsp27 as substrates for p38, ERK, JNK, or MAPKAP kinases 2 and 3 in the presence of 100 μmol/L unlabeled adenosine triphosphate (ATP), 5 μCi [γ32P]-ATP, and kinase buffer in a volume of 20 μL for 15 minutes at 30°C. Thereafter, reactions were terminated by boiling in 5× Lämmli sodium dodecyl sulfate (SDS) sample buffer for 4 minutes. Samples were subsequently subjected to SDS-polyacrylamide gel electrophoresis (PAGE), blotted onto nitrocellulose, and visualized by autoradiography. Western blot analysis was performed to confirm equal loading of ERK1/ERK2, JNK, p38, or MAPKAP kinases 2/3 proteins.
Plasmids and transient transfection procedures.
Plasmids used for transient transfections included KRSPA eukaryotic expression vector,22 pGreen Lantern for expression of green fluorescent protein (GFPS65T; Life Technologies, Eggenstein, Germany), and KRSPA expression plasmids for wild-type MKK6, dominant negative MKK6(Ala) or constitutively active MKK6(Glu) (kindly provided by Dr R.J. Davis, Howard Hughes Medical School, University of Massachusetts, Worchester, MA23). Dominant negative versions of Raf (KRSPA Raf-C4B), ERK2 (KRSPA ERK2C3), SEK (KRSPA SEK K>R), and SAPKβ (KRSPA SAPK KK>RR) were obtained from various sources and have been described in detail in Ludwig et al.21 The dominant negative p38 mutants [pCDNA3 p38(TY>AF), pCDNA3 p38(K>M)]24 were kindly provided by J. Han (La Jolla, CA).
HUVEC were cultured to 50% to 60% confluence using Falcon 10-cm tissue culture plates or 6-well plates (Becton Dickinson, Heidelberg, Germany) before transient transfection, which basically followed a diethyl aminoethyl (DEAE)-dextran protocol described by Karmann et al.25 After washing with 1 mmol/L HEPES/phosphate-buffered saline (PBS), cells were incubated with 20 μg (10-cm plates) or 5 μg DNA (6-well plates), 250 μg/mL DEAE-dextran (Pharmacia, Freiburg, Germany) in 4 or 1 mL 1 mmol/L HEPES/PBS for 30 minutes at 37°C. Thereafter, 6 or 1.5 mL EGM containing 0.15 mmol/L chloroquine was added to each plate and cells incubated for another 2.5 hours. Medium was then removed and cells treated with 10% (vol/vol) dimethyl sulfoxide (DMSO) in EGM for 2.5 minutes. HUVEC were subsequently cultured for 36 hours in EGM and finally stimulated with cytokines or other reagents as indicated. Expression and function of the transfected kinase mutants was confirmed in Western blots and immune complex kinase assays.
Flow cytometry.
Cell surface expression of intracellular adhesion molecule (ICAM)-1 was performed as described earlier.26 27 Briefly, HUVEC were incubated with 1% (wt/vol) bovine serum albumin (BSA)/PBS for 30 minutes and then exposed to mouse IgG1 monoclonal antibody (MoAb) against ICAM-1 (clone 84H10; Immunotech, via Dianova, Hamburg, Germany) or mouse IgG1 of nonrelevant specificity (R & D Systems). After several washes, cells were stained with F(ab′)2fragment of fluorescein isothiocyanate (FITC)-labeled rabbit antimouse IgG (Dako, Hamburg, Germany). Thereafter, propidium iodide was added to allow exclusion of nonviable cells. Fluorescence was measured on 5,000 or 10,000 cells per sample using a FACScan (Becton Dickinson).
To determine expression of the chemokine MCP-1, an intracellular staining procedure was applied. HUVEC were stimulated with cytokines and other reagents as indicated in the presence of 2 μmol/L monensin. Cells were washed, fixed with 4% (wt/vol) paraformaldehyde in PBS at 4°C for 20 minutes, and then incubated with phycoerythrin-labeled mouse MoAb against MCP-1 (mouse IgG1; clone 5D3-F7) or corresponding phycoerythrin-labeled isotype control MoAb (PharMingen, Hamburg, Germany), which had been diluted in permeabilization buffer containing 1% fetal calf serum (FCS), 0.1% (wt/vol) saponin, PBS. Fluorescence was then measured with a FACScan.
MCP-1 expression by HUVEC cotransfected with vectors expressing dominant negative or constitutively active kinases and GFPS65T was detected as follows. Thirty-six hours after transfection, cells were exposed to cytokine and subsequently harvested. Cells were then permeabilized as described above and successively stained with unlabeled MoAb 5D3-F7 against MCP-1, biotin-SP–conjugated goat antimouse IgG F(ab′)2 and streptavidin-Cy-Chrome (PharMingen), respectively. Only those cells, which expressed GFPS65T (as detected in the FL-1 channel), were considered for the detection of MCP-1 (as measured in the FL-3 channel). Nonviable cells were excluded using forward scatter (FSC) and side scatter (SSC) parameters.
Northern blot.
HUVEC were stimulated with TNF-α in the absence or presence of pharmacological inhibitors as indicated. For the assessment of an inhibitory effect of SB202190 on mRNA stability, some samples were treated with the transcriptional inhibitor, actinomycin D (Act D), for distinct time intervals as indicated. Total cellular RNA was isolated using a Quiagen RNeasy kit (Hilden, Germany) according to the manufacturer’s instructions. Denaturated total RNA (10 μg) was separated on 1% agarose/formaldehyde gels and transferred to Hybond N+ membranes (Amersham Buchler, Braunschweig, Germany). Filters were UV cross-linked and subsequently hybridized with a human MCP-1 cDNA probe28 labeled with [α32P]-deoxycytidine triphosphate (dCTP) using the random primed DNA labeling kit (Boehringer Mannheim, Mannheim, Germany). For control of RNA loading of lanes, blots were either rehybridized with an actin probe or densitometrically analyzed for 28S rRNA amounts using NIH Image 1.6 Software (NIH, Bethesda, MD). Autoradiography was performed at −80°C using Amersham Hyperfilm.
Luciferase assays.
HUVEC were seeded in 6-well plates and transfected using the DEAE-dextran protocol described above after having reached subconfluence. The luciferase constructs used contained the proximal promoter region and distal enhancer region of the human MCP-1 gene and have recently been described in detail.29 Transfections for luciferase assays were performed with 1 μg of reporter construct with or without 4 μg of expression vectors containing MKK6(Ala), MKK6(Glu), p38(TY>AF), or p38(K>M) cDNAs. In a set of experiments, HUVEC were cotransfected with increasing amounts (1 μg, 4 μg, and 8 μg/per well) of MKK6(Ala) cDNA or corresponding empty expression vector. Thirty-six hours after transfection, cells were incubated with TNF-α in the absence or presence of pharmacological inhibitors for another 12 hours as indicated. HUVEC of each well were then harvested in 100 μL lysis buffer (50 mmol/L sodium 2-(N-morpholino)ethanesulfonic acid (MES) (Sigma), 50 mmol/L Tris-HCl, pH 7.8, 10 mmol/L dithiothreitol, and 2% Triton X-100) for 30 minutes at 4°C. The crude cell lysates were cleared by centrifugation, and 50 μL of precleared cell extracts were added to 50 μL luciferase assay buffer containing 125 mmol/L sodium MES, 125 mmol/L Tris-HCl, pH 7.8, 25 mmol/L magnesium acetate, and 2 mg/mL ATP. Immediately after the addition of 50 μL of 1 mmol/L D-luciferin (AppliChem, Darmstadt, Germany) for each sample, the luminescence was detected using a LB96P luminometer (Berthold, Bad Wildbach, Germany). Cell lysates were normalized on the basis of protein contents; relative luciferase activities of cells transfected with each cDNA expression vector were based on activities of cells transfected with the same amount of empty expression vector (KRSPA or pCDNA3). Data were obtained from four independent experiments and are expressed as fold stimulation of relative luciferase activity.
RESULTS
TNF-α–induced activation of endogenous MAP kinase cascades in endothelial cells.
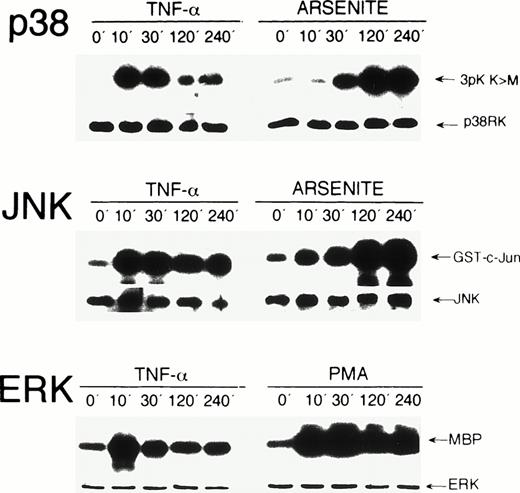
To study activation of endogenous MAP kinases by TNF-α, HUVEC were exposed for 0, 10, 30, 120, and 240 minutes to the cytokine. Kinase activation was determined in immunecomplex kinase assays. Exposure of HUVEC to TNF-α induced a significant activation of stress kinases p38 and JNK, as well as of ERK within 10 minutes. p38 and ERK activities decreased after 30 minutes, whereas JNK activity remained elevated up to 240 minutes (Fig 1). Essentially similar results, albeit weaker, were obtained when HUVEC were exposed to IL-1β, another potent inducer of endothelial activation (data not shown). The effects of stress inducer sodium arsenite on p38 and JNK kinase activity and of phorbol ester PMA on ERK activation were analyzed for control purposes (Fig 1, see legend for detail), which resulted in activation kinetics similar to those found in other cell types.
Activation kinetics of endogenous MAP kinases in HUVEC after stimulation with TNF-, PMA, and arsenite. HUVEC were stimulated with 5 ng/mL TNF-, 20 ng/mL PMA, or 0.25 mmol/L arsenite for the time intervals indicated. Immunecomplex kinase assays were performed as described in Materials and Methods using 3pK K>M, GST-c-Jun(1-135), and myelin basic protein (MBP) as substrates for p38, JNK, and ERK, respectively. Protein loads were controlled in Western blot assays using appropriate antisera. On stimulation with PMA, a strong activation of ERK was detected, which persisted over 240 minutes (lower panel). Arsenite strongly induced p38 and JNK kinases with peak levels after 240 minutes (upper and middle panels).
Activation kinetics of endogenous MAP kinases in HUVEC after stimulation with TNF-, PMA, and arsenite. HUVEC were stimulated with 5 ng/mL TNF-, 20 ng/mL PMA, or 0.25 mmol/L arsenite for the time intervals indicated. Immunecomplex kinase assays were performed as described in Materials and Methods using 3pK K>M, GST-c-Jun(1-135), and myelin basic protein (MBP) as substrates for p38, JNK, and ERK, respectively. Protein loads were controlled in Western blot assays using appropriate antisera. On stimulation with PMA, a strong activation of ERK was detected, which persisted over 240 minutes (lower panel). Arsenite strongly induced p38 and JNK kinases with peak levels after 240 minutes (upper and middle panels).
The observation that all three MAP kinases, p38, JNK and ERK, are activated by TNF-α, one of the most potent physiological activators of endothelium, does not prove their functional involvement in endothelial activation. We therefore evaluated the potential role of all three MAP kinase cascades for endothelial expression of MCP-1.
The p38 inhibitors, SB203580 and SB202190, inhibit MCP-1 synthesis.
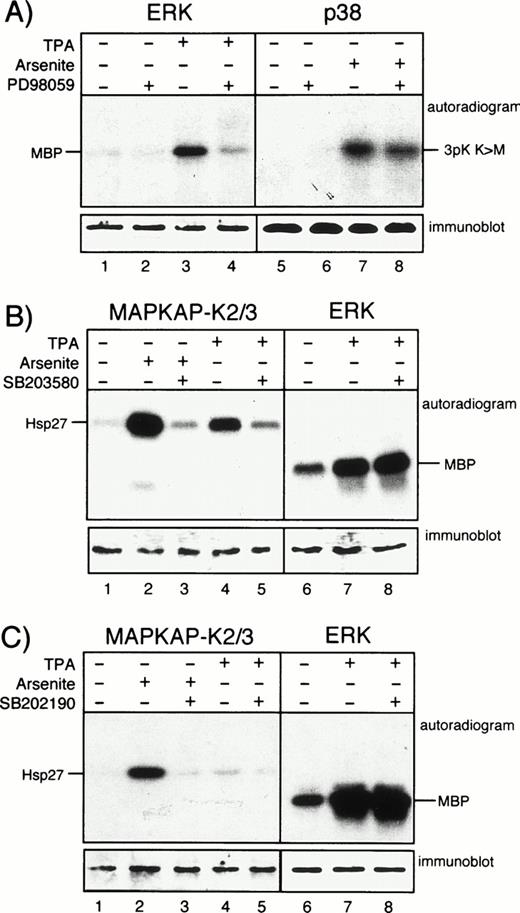
To elucidate a potential contribution of different MAP kinase cascades in TNF-α–induced expression of MCP-1, we studied the effects of pharmacologic kinase inhibitors, namely the selective MAP kinase kinase (MEK) inhibitor PD9805930 and the pyridinyl imidazoles, SB203580 and SB202190,20,31 both inhibitors of p38. PD98059 acts by preventing activation of MEK by Raf, whereas the p38 inhibitors function via blocking the active p38 kinase.20 The inhibitors were used in a concentration that specifically blocked ERK or p38 activity in other cells.21 32 Specific activity of the inhibitors in HUVEC was verified by immunecomplex kinase assays using downstream targets of MEK and p38, ie, ERK or MAPKAP kinases 2 and 3, respectively. A total of 20 μmol/L PD98059 efficiently blocked activation of ERK in endothelial cells stimulated with PMA, but had no effect on arsenite-induced activation of p38 (Fig 2A). A total of 20 μmol/L SB203580 strongly inhibited the activation of the p38 effectors MAPKAP kinases 2 and 3 in HUVEC either incubated with arsenite or PMA, whereas ERK activation was not affected (Fig 2B). Essentially similar results were obtained with SB202190 (Fig 2C).
PD98059, SB203580, and SB202190 function as specific kinase inhibitors in HUVEC. (A) Cells were left unstimulated or were exposed to PMA (for 30 minutes) or arsenite (for 60 minutes) in the presence or absence of 20 μmol/L PD98059 after 30 minutes of pretreatment as indicated. After cell lysis, ERK or p38 were immunoprecipitated and assayed for activity as described. (B) HUVEC were left unstimulated or were stimulated with arsenite or PMA as described above either without or with 30 minutes of preincubation with 20 μmol/L SB203580. After cell lysis, kinases MAPKAP kinases 2 and 3 or ERK were immunoprecipitated and assayed for activity as described in Materials and Methods. (C) Experiments were performed as described in (B), but with the use of 20 μmol/L SB202190 instead of SB203580. Western blots show equal protein loading of the respective kinases.
PD98059, SB203580, and SB202190 function as specific kinase inhibitors in HUVEC. (A) Cells were left unstimulated or were exposed to PMA (for 30 minutes) or arsenite (for 60 minutes) in the presence or absence of 20 μmol/L PD98059 after 30 minutes of pretreatment as indicated. After cell lysis, ERK or p38 were immunoprecipitated and assayed for activity as described. (B) HUVEC were left unstimulated or were stimulated with arsenite or PMA as described above either without or with 30 minutes of preincubation with 20 μmol/L SB203580. After cell lysis, kinases MAPKAP kinases 2 and 3 or ERK were immunoprecipitated and assayed for activity as described in Materials and Methods. (C) Experiments were performed as described in (B), but with the use of 20 μmol/L SB202190 instead of SB203580. Western blots show equal protein loading of the respective kinases.
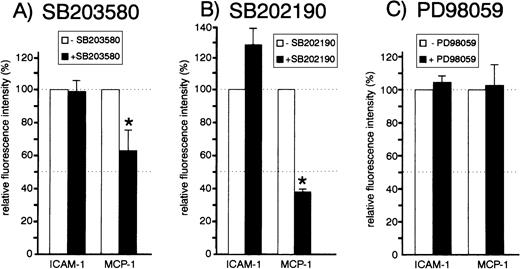
We analyzed the effect of all three inhibitors on the expression of the endothelial chemokine MCP-1, which is upregulated during endothelial activation. HUVEC were exposed to 20 μmol/L SB203580, SB202190, or PD98059 before stimulation with TNF-α. Cells were then processed for flow cytometric analysis of MCP-1 using an intracellular staining procedure. PD98059 did not significantly affect the TNF-α–induced production of MCP-1 or adhesion molecule ICAM-1, which was included for control purposes (Fig 3C). Both SB203580 and SB202190 also did not exert significant inhibitory effects on ICAM-1 expression (Fig 3A and B). However, both p38 inhibitors had a strong effect on TNF-α–induced MCP-1 production (up to 60% inhibition), suggesting an essential role of the MKK6/p38 pathway for synthesis of that chemokine.
Effect of inhibitors SB203580, SB202190, and PD98059 on MCP-1 and ICAM-1 expression. Cells were left untreated or pretreated with 20 μmol/L SB203580 (A), 20 μmol/L SB202190 (B), or 20 μmol/L PD98059 (C) for 30 minutes. Cells were stimulated with 1 ng/mL TNF- for 12 hours in the presence or absence of inhibitors and subsequently processed for flow cytometry analysis as described in Materials and Methods. The mean ± SEM of relative fluorescence intensities of three to six independent experiments is shown. Asterisks denote statistically significant differences as compared with control (P < .05, Wilcoxon test).
Effect of inhibitors SB203580, SB202190, and PD98059 on MCP-1 and ICAM-1 expression. Cells were left untreated or pretreated with 20 μmol/L SB203580 (A), 20 μmol/L SB202190 (B), or 20 μmol/L PD98059 (C) for 30 minutes. Cells were stimulated with 1 ng/mL TNF- for 12 hours in the presence or absence of inhibitors and subsequently processed for flow cytometry analysis as described in Materials and Methods. The mean ± SEM of relative fluorescence intensities of three to six independent experiments is shown. Asterisks denote statistically significant differences as compared with control (P < .05, Wilcoxon test).
TNF-α–induced MCP-1 synthesis in HUVEC is partially blocked by expression of a dominant negative mutant of MKK6.
To confirm the data obtained with pharmacologic inhibitors, we developed a transient transfection approach to study the effect of dominant negative mutants on synthesis of MCP-1. Efficiency of mutants had been verified in various cell systems as described.21,32 33 Cells transfected with the kinase mutants were identified by coexpression of GFPS65T. Thirty-six hours after transfection, cells were exposed to TNF-α for another 12 hours before detection of MCP-1. Expression was determined by flow cytometry using Cy-Chrome–conjugated tertiary stage reagents as described in Materials and Methods. Only those cells expressing GFPS65T detected in the FL-1 channel were considered for analysis of the cotransfected kinase inactive mutants. Additional experiments were performed in the presence of 50 μg/mL polymyxin B sulfate to avoid LPS-induced activation of endothelium. This helped to exclude that LPS potentially contaminating the DNA preparations confounded the results.
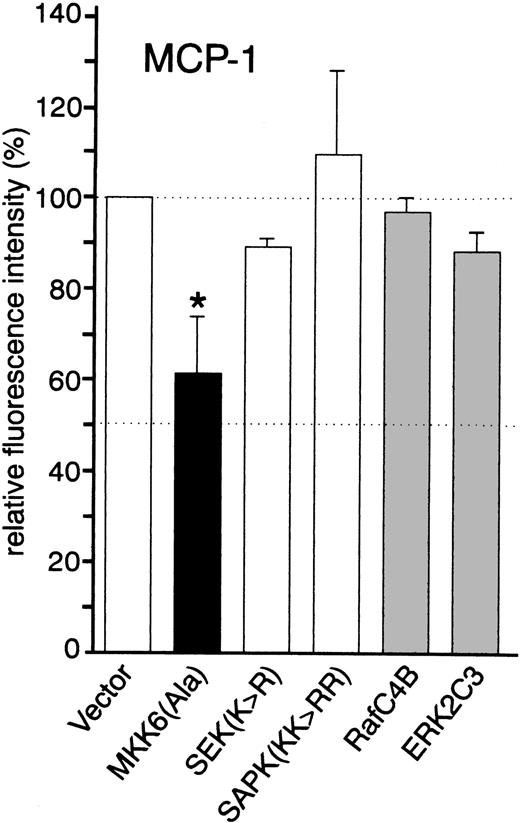
Consistent with our findings obtained with the p38 inhibitors, TNF-α–induced expression of MCP-1 was partially inhibited in cells transfected with kinase inactive MKK6(Ala) (approximately 40% inhibition), suggesting that MKK6 and its downstream kinase p38 are involved in TNF-α–mediated upregulation of MCP-1 (Fig 4; Fig 5A shows a set of representative fluorescence-activated cell sorting [FACS] profiles). Dominant negative JNK (SAPK KK>RR) and a kinase inactive mutant of its upstream activator MKK4/SEK (SEK K>R), as well as dominant negative versions of Raf (Raf-C4B) and ERK (ERK2C3), did not inhibit TNF-α–induced expression of MCP-1 (Fig 4), excluding a critical role of both the SEK/JNK and the Raf/MEK/ERK cascade in MCP-1 synthesis.
Effect of dominant negative kinase mutants on the synthesis of MCP-1 by HUVEC. Cells were transfected in a 1:3 ratio with pGreenLantern expressing GFPS65T and either empty expression vector KRSPA or plasmids expressing dominant negative kinase mutants as indicated and stimulated with 2 ng/mL TNF- for 12 hours. Successfully transfected cells were analyzed for MCP-1 synthesis by flow cytometry as described in Materials and Methods. The data shown represent mean values ± SEM from four independent experiments. The asterisk indicates a statistically significant difference as compared with control (P < .05, Wilcoxon test).
Effect of dominant negative kinase mutants on the synthesis of MCP-1 by HUVEC. Cells were transfected in a 1:3 ratio with pGreenLantern expressing GFPS65T and either empty expression vector KRSPA or plasmids expressing dominant negative kinase mutants as indicated and stimulated with 2 ng/mL TNF- for 12 hours. Successfully transfected cells were analyzed for MCP-1 synthesis by flow cytometry as described in Materials and Methods. The data shown represent mean values ± SEM from four independent experiments. The asterisk indicates a statistically significant difference as compared with control (P < .05, Wilcoxon test).
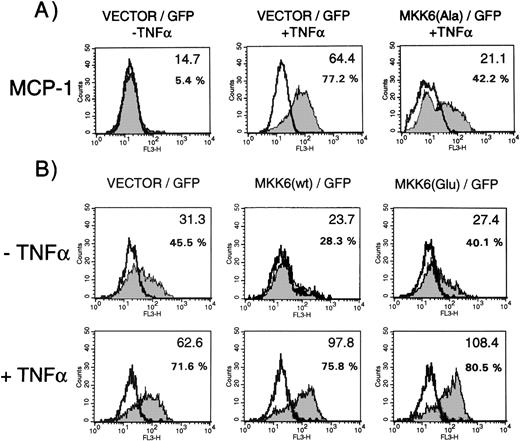
MCP-1 synthesis in cells expressing dominant negative MKK6(Ala), MKK6 wild-type, or constitutively active MKK6(Glu) in the presence or absence of TNF-. Cells were transfected in a 1:3 ratio with pGreenLantern expressing GFPS65T and plasmids expressing either empty expression vector KRSPA, KRSPA MKK6(Ala), KRSPA MKK6 wild-type, or KRSPA MKK6(Glu). (A) Cells transfected with empty expression vector or dominant negative MKK6, ie, MKK6(Ala), were either left unstimulated or stimulated with 2 ng/mL TNF- for 12 hours. Successfully transfected cells were analyzed for MCP-1 synthesis by flow cytometry as described in Materials and Methods. Flow cytometry profiles of one representative experiment are shown. Open profiles represent isotype controls, shaded profiles represent cells labeled for MCP-1 expression. Bold letters indicate the mean fluorescence intensities; additionally, percentages of MCP-1–positive cells are given. (B) HUVEC transfected with empty expression vector, wild-type MKK6, or a constitutively active mutant of MKK6, ie, MKK6(Glu), were exposed to a limited concentration of TNF- (0.2 ng/mL) and evaluated for MCP-1 expression as indicated.
MCP-1 synthesis in cells expressing dominant negative MKK6(Ala), MKK6 wild-type, or constitutively active MKK6(Glu) in the presence or absence of TNF-. Cells were transfected in a 1:3 ratio with pGreenLantern expressing GFPS65T and plasmids expressing either empty expression vector KRSPA, KRSPA MKK6(Ala), KRSPA MKK6 wild-type, or KRSPA MKK6(Glu). (A) Cells transfected with empty expression vector or dominant negative MKK6, ie, MKK6(Ala), were either left unstimulated or stimulated with 2 ng/mL TNF- for 12 hours. Successfully transfected cells were analyzed for MCP-1 synthesis by flow cytometry as described in Materials and Methods. Flow cytometry profiles of one representative experiment are shown. Open profiles represent isotype controls, shaded profiles represent cells labeled for MCP-1 expression. Bold letters indicate the mean fluorescence intensities; additionally, percentages of MCP-1–positive cells are given. (B) HUVEC transfected with empty expression vector, wild-type MKK6, or a constitutively active mutant of MKK6, ie, MKK6(Glu), were exposed to a limited concentration of TNF- (0.2 ng/mL) and evaluated for MCP-1 expression as indicated.
Expression of wild-type or constitutively active MKK6 enhances TNF-α–induced MCP-1 synthesis.
To further support these data, we analyzed whether overexpression of MKK6 exerts positive effects on MCP-1 synthesis. When HUVEC are stimulated with limiting concentrations of TNF-α, expression of MKK6 wild-type enhanced synthesis of MCP-1, as shown by a significant upshift in the mean fluorescence intensity (bold letters), whereas transfection of a control vector had no effect (Fig 5B, lower panel, middle). In addition, we tested a constitutive active mutant of MKK6, which activates p38 in several cell types including HUVEC (data not shown). Although expression of this mutant was not sufficient to induce MCP-1 synthesis in unstimulated cells (Fig 5B, upper panel, left), MCP-1 levels significantly increased in the presence of MKK6(Glu) when cells were stimulated with limiting concentrations of TNF-α.
The observation that specific p38 inhibitors and inactive or active mutants of MKK6 negatively or positively interfere with the expression of MCP-1 shows that the MKK6/p38 cascade is one of the crucial pathways for TNF-α–induced synthesis of MCP-1.
The MKK6/p38 pathway enhances TNF-α–induced MCP-1 expression at the mRNA level.
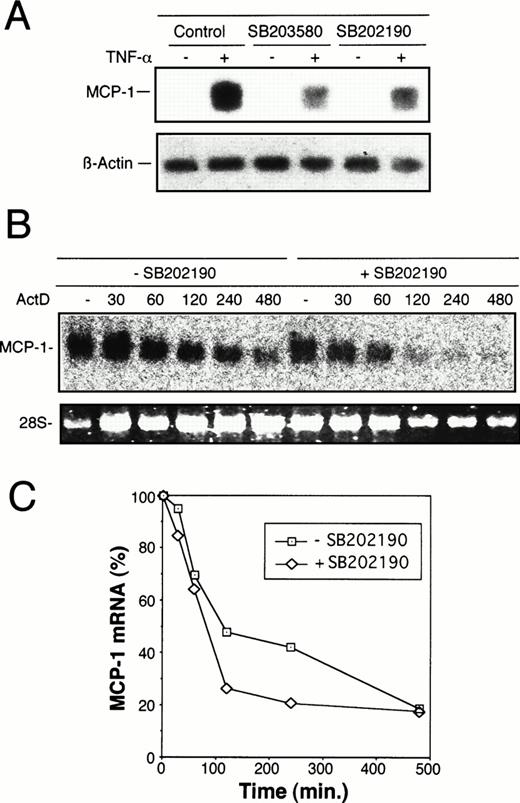
To elucidate whether regulation of MCP-1 synthesis by MKK6/p38 occurs at the transcriptional level, Northern blot analysis was performed. As shown in Fig 6A, stimulation of HUVEC with TNF-α strongly induced MCP-1 mRNA levels, whereas exposure of cells to SB203580 or SB202190 30 minutes before and during an 8-hour stimulation with TNF-α markedly reduced MCP-1 mRNA levels. To analyze whether the reduction of steady-state mRNA levels in the presence of p38 inhibitor is due to an alteration of mRNA stability, the experiments were performed in the presence of a transcriptional inhibitor, Act D. As shown in Fig 6B and C, the degree of decline of MCP-1 mRNA amounts in HUVEC exposed to SB202190 is similar to that of untreated cells. In both treated and untreated cells, approximately 20% of MCP-1 message is still present after 8 hours of transcriptional inhibition, indicating that the p38 pathway is not significantly involved in regulation of MCP-1 mRNA stability.
Inhibition of TNF-–induced MCP-1 mRNA synthesis by p38 inhibitors, SB203580 and SB202190. (A) Total mRNA was extracted from HUVEC exposed to 20 μmol/L SB203580 or SB202190 30 minutes before and during an 8-hour period of stimulation with 2 ng/mL TNF-. Control samples were incubated without p38 inhibitors in the absence or presence of TNF- as indicated. MCP-1 mRNA levels were determined by Northern blot analysis as described in Materials and Methods. Blots were reprobed with an actin cDNA probe to control RNA loading. (B) To assess the effect of p38 inhibition on stability of TNF-–induced MCP-1 mRNA, HUVEC were treated with TNF- in the absence or presence of SB202190 as in (A). After 8 hours (time zero), Act D was added at 2 μg/mL and total RNA isolated at the time intervals indicated (in minutes). Northern blot analysis was performed as described above. (C) Autoradiography bands shown in (B) were analyzed by densitometry and normalized to the RNA content as judged by the levels of 28S rRNA. TNF-–induced MCP-1 mRNA levels at time zero were set as 100%. The decrease of MCP-1 mRNA amounts is shown after exposure to Act D for different time intervals as indicated. Treatment with Act D alone had no effect on the levels of MCP-1 mRNA (data not shown).
Inhibition of TNF-–induced MCP-1 mRNA synthesis by p38 inhibitors, SB203580 and SB202190. (A) Total mRNA was extracted from HUVEC exposed to 20 μmol/L SB203580 or SB202190 30 minutes before and during an 8-hour period of stimulation with 2 ng/mL TNF-. Control samples were incubated without p38 inhibitors in the absence or presence of TNF- as indicated. MCP-1 mRNA levels were determined by Northern blot analysis as described in Materials and Methods. Blots were reprobed with an actin cDNA probe to control RNA loading. (B) To assess the effect of p38 inhibition on stability of TNF-–induced MCP-1 mRNA, HUVEC were treated with TNF- in the absence or presence of SB202190 as in (A). After 8 hours (time zero), Act D was added at 2 μg/mL and total RNA isolated at the time intervals indicated (in minutes). Northern blot analysis was performed as described above. (C) Autoradiography bands shown in (B) were analyzed by densitometry and normalized to the RNA content as judged by the levels of 28S rRNA. TNF-–induced MCP-1 mRNA levels at time zero were set as 100%. The decrease of MCP-1 mRNA amounts is shown after exposure to Act D for different time intervals as indicated. Treatment with Act D alone had no effect on the levels of MCP-1 mRNA (data not shown).
The MKK6/p38 pathway mediates TNF-α–induced induction of the MCP-1 promoter.
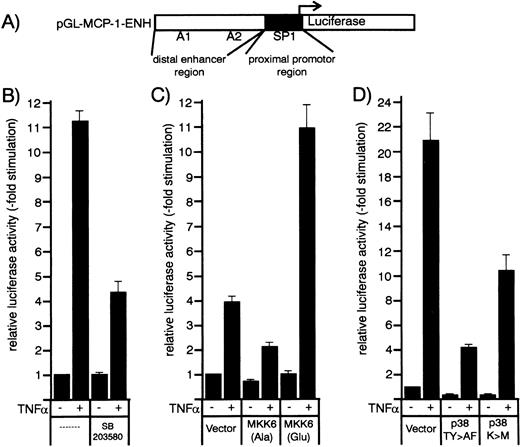
Transcriptional regulation of the MCP-1 promoter by the MKK6/p38 pathway was studied using promoter-luciferase constructs. HUVEC were transfected with a MCP-1 promoter/enhancer luciferase construct containing the enhancer (between −2742 and −2513) and promoter regions (between −107 and +60) of the human MCP-1 promoter (Fig 7A, see Ueda et al29 for details). Thirty-six hours after transfection, HUVEC were stimulated with TNF-α in the absence or presence of p38 inhibitors SB203580 or SB202190 (Fig 7B and8A). Both inhibitors were found to partially block TNF-α–induced transcription of the MCP-1 promoter/enhancer luciferase construct in a concentration-dependent manner (Fig 8A and data not shown).
Effect of p38 inhibitors and kinase mutants of the MKK6/p38 signaling module on TNF-–induced MCP-1 promoter activity in HUVEC. (A) Schematic representation of the human MCP-1 promoter/enhancer construct with the SP1 and NF-κB (A1 and A2) sites marked. The proximal promoter and the distal enhancer region are indicated by closed and open boxes. (B) An MCP-1 promoter/enhancer luciferase construct was transfected into HUVEC according to a DEAE-dextran protocol as described in Materials and Methods. After 36 hours, cells were stimulated with 1 ng/mL TNF- for 12 hours with or without a 30-minute pretreatment with 20 μmol/L SB203580 as indicated. (C and D) HUVEC were cotransfected with MCP-1 promoter/enhancer luciferase constructs and kinase mutants MKK6(Ala), MKK6(Glu), p38(TY>AF), or p38(K>M) as indicated. Thirty-six hours later, cells were stimulated with 2 ng/mL TNF-. Relative luciferase activity is expressed as fold stimulation as described in Materials and Methods.
Effect of p38 inhibitors and kinase mutants of the MKK6/p38 signaling module on TNF-–induced MCP-1 promoter activity in HUVEC. (A) Schematic representation of the human MCP-1 promoter/enhancer construct with the SP1 and NF-κB (A1 and A2) sites marked. The proximal promoter and the distal enhancer region are indicated by closed and open boxes. (B) An MCP-1 promoter/enhancer luciferase construct was transfected into HUVEC according to a DEAE-dextran protocol as described in Materials and Methods. After 36 hours, cells were stimulated with 1 ng/mL TNF- for 12 hours with or without a 30-minute pretreatment with 20 μmol/L SB203580 as indicated. (C and D) HUVEC were cotransfected with MCP-1 promoter/enhancer luciferase constructs and kinase mutants MKK6(Ala), MKK6(Glu), p38(TY>AF), or p38(K>M) as indicated. Thirty-six hours later, cells were stimulated with 2 ng/mL TNF-. Relative luciferase activity is expressed as fold stimulation as described in Materials and Methods.
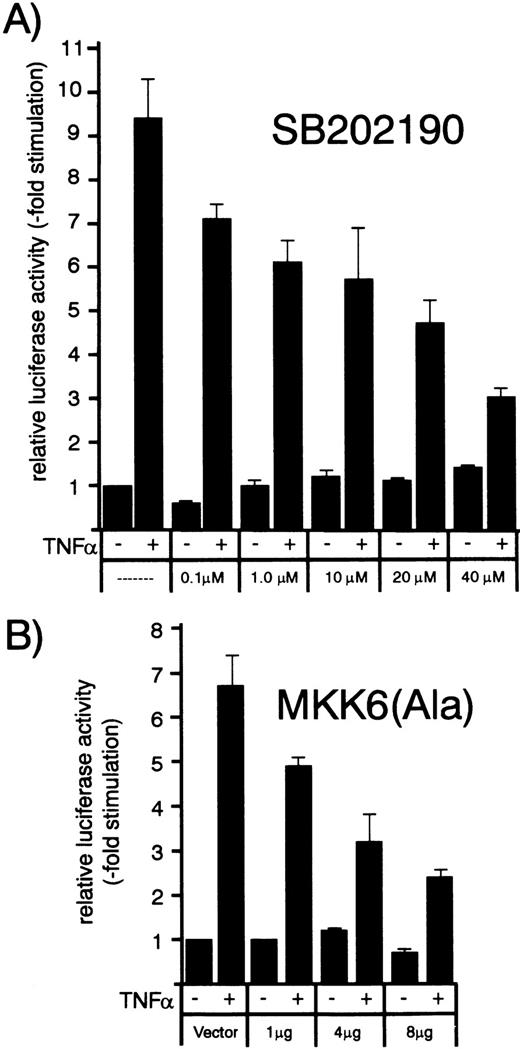
Concentration/response dependence of increasing amounts of p38 inhibitor SB202190 or dominant negative MKK6 kinase mutant MKK6(Ala) on TNF-–induced MCP-1 promoter activity. HUVEC were transfected with a MCP-1 promoter/enhancer luciferase construct as described in Materials and Methods. (A) Thirty-six hours later, cells were exposed to increasing concentrations of p38 inhibitor SB202190 as indicated and stimulated with 1 ng/mL TNF- 30 minutes later for another 12 hours. Luciferase activity was determined as described in the legend to Fig 7. (B) HUVEC were cotransfected with the MCP-1 promoter/enhancer construct and increasing amounts of MKK6(Ala) as indicated and stimulated with 2 ng/mL TNF- 36 hours later for another 12 hours. Relative luciferase activity is expressed as fold stimulation.
Concentration/response dependence of increasing amounts of p38 inhibitor SB202190 or dominant negative MKK6 kinase mutant MKK6(Ala) on TNF-–induced MCP-1 promoter activity. HUVEC were transfected with a MCP-1 promoter/enhancer luciferase construct as described in Materials and Methods. (A) Thirty-six hours later, cells were exposed to increasing concentrations of p38 inhibitor SB202190 as indicated and stimulated with 1 ng/mL TNF- 30 minutes later for another 12 hours. Luciferase activity was determined as described in the legend to Fig 7. (B) HUVEC were cotransfected with the MCP-1 promoter/enhancer construct and increasing amounts of MKK6(Ala) as indicated and stimulated with 2 ng/mL TNF- 36 hours later for another 12 hours. Relative luciferase activity is expressed as fold stimulation.
We then evaluated whether cotransfection of dominant negative or constitutively active mutants of MKK6, ie, MKK6(Ala) or MKK6(Glu), influenced TNF-α–induced MCP-1 promoter-dependent transcription. As shown in Figs 7C and 8B, the dominant negative mutant MKK6(Ala) inhibited TNF-α–induced expression of the reporter gene in a concentration-dependent way, whereas MKK6(Glu) significantly enhanced it. Similarly, dominant negative mutants of p38, ie, p38(TY>AF) and p38(K>M), were able to significantly block MCP-1 promoter-dependent transcription (Fig 7D). Although the levels of stimulation by TNF-α differ somewhat between distinct sets of experiments dependent on vector background or potential variability of primary cells, the degree of transcriptional inhibition or enhancement by the different mutants or inhibitors greatly match the data obtained at the level of protein synthesis. In conclusion, our data provide strong evidence that the MKK6/p38 pathway accounts for at least 50% of the TNF-α–induced signals controlling MCP-1 expression.
DISCUSSION
Mitogen- and stress-activated protein kinases of the MAP kinase family respond to a plethora of extracellular signals integrating different inputs, which may result in the functional reprograming of cellular activities.20 These signals include factors such as TNF-α, which are delivered under conditions of inflammation. Our studies were based on the observation that TNF-α caused p38, JNK, and ERK activation in endothelial cells. However, because cytokine-induced activation of MAP kinases may exert pleiotropic effects on various other cellular activities, a functional relevance for expression of certain endothelial adhesion molecule genes or chemokines cannot necessarily be deduced from these data.
We have now shown that the p38 inhibitors, SB203580 and SB202190, inhibit the TNF-α–induced expression of MCP-1 at a transcriptional level. Besides the use of inhibitors, which may have potential side effects, we followed an independent approach to evaluate the contribution of each MAP kinase pathway to MCP-1 transcription and synthesis. We used transient transfection assays with a panel of dominant negative or constitutively active kinase mutants. Besides promoter-reporter gene-based transfection experiments, our approach allowed us to analyze the expression of endogenous proteins. Although TNF-α was found to be an efficient agonist of three different MAP kinase cascades in HUVEC, only the MKK6/p38 pathway has now been identified to be of major relevance for TNF-α–induced expression of the MCP-1 gene. MCP-1 synthesis and transcription were affected to the same degree by the different inhibitors and mutants of the MKK6/p38 pathway. Further, block of the p38 pathway does not significantly affect MCP-1 mRNA stability, indicating that regulation of MCP-1 expression by the MKK6/p38 module mainly occurs at the transcriptional level. This adds MCP-1 to the list of MKK6/p38-regulated genes such as IL-6 or GM-CSF.18 A still open question is the determination of the MKK6/p38-responsive regions of the MCP-1 promoter. Structural features of the human MCP-1 promoter have recently been described.29,34 While basal promoter activity is dependent on a proximal promoter region containing an SP-1 site, cytokine-inducible promoter activity is mainly mediated by a distal enhancer region (Fig 7A). Two NF-κB binding sites (A1 and A2) have been identified in the distal enhancer region, which are crucial for enhancer activity.29 Because another promoter-luciferase construct containing solely the proximal promoter region was neither responsive to TNF-α or influenced by mutants or inhibitors of the MKK6/p38 pathway (M.G. and S.L., unpublished observation, May 1998), it is most likely that the MKK6/p38-responsive target sites are localized in the MCP-1 distal enhancer. Mutation of the A1 or A2 NF-κB sites in this region resulted in loss of the enhancer activity,29 suggesting that MCP-1 regulation by the cytokine-inducible MKK6/p38 pathway is also mediated by NF-κB sites. Consistent with our observations, the MKK6/p38 cascade has been reported to be involved in NF-κB–dependent gene expression in several other systems.18-20 35
MCP-1 transcription and synthesis were inhibited by roughly 50% to 60% of induced levels, indicating that at least half of the TNF-α signal leading to induction of MCP-1 expression is transmitted by the MKK6/p38 signaling module. Using the p38 inhibitor, SB203580, similar degrees of inhibition were observed for LPS-induced TNF-α and IL-1 expression in monocytes,17 as well as for TNF-α–induced vascular cell adhesion molecule-1 (VCAM-1) expression in HUVEC.36 Involvement of this pathway in TNF-α–induced MCP-1 synthesis may be even higher taking into account that the dominant negative mutants act in competition and do not completely shut off the upstream signal32 and that the p38 inhibitors may not be fully stable during incubation times of 12 hours.21
TNF-α activates a variety of intracellular signaling pathways, which may act in concert with the MKK6/p38 pathway to support MCP-1 expression in HUVEC. These include several other MAP kinase cascades besides the MKK6/p38 pathway (Fig 1). However, according to our data, the additional pathways, which account for full TNF-α–induced expression of the MCP-1 gene, are different from other MAP kinase signaling pathways, namely the Raf/MEK/ERK and the JNK/SAPK pathways.
The characterization of MCP-1 as a novel target gene of the MKK6/p38 cascade identifies the latter as an important mediator of physiologic responses to TNF-α signals in endothelial cells. This might be important with respect to inflammatory dysfunctions. It has been shown previously that inhibition of induced MCP-1 expression is associated with reduced transmigration of monocytes through the vessel wall,6 as well as with diminished recruitment of T lymphocytes,7 indicating that the level of MCP-1 synthesis is critical for the course of inflammatory reactions.
The MKK6/p38 pathway might be further important in the control of inflammatory responses in general, as this cascade appears to act on several levels of leukocyte recruitment by endothelium. Besides the effects on MCP-1 reported here, this pathway was discussed to be of relevance for TNF-α–induced VCAM-1-36 and E-selectin expression in HUVEC,37 as well as for expression of the proinflammatory cytokines TNF-α and IL-1β in monocytes.17 Thus, in analogy to the mitogenic Raf/MEK/ERK cascade, the MKK6/p38 module might be considered to act as an inflammatory pathway.
Finally, the identification of MCP-1 as a novel physiologic target for the MKK6/p38 pathway might be important with respect to our understanding of the mechanisms leading to the recruitment of monocytes to the site of prospective plaque formation during atherosclerosis.
ACKNOWLEDGMENT
The authors thank Angelika Hoffmeyer and Christoph K. Weber for critically reading the manuscript and Martina Gropengieβer, Heide Häfner, Sybille Schmid, and Atiye Toksoy for excellent technical assistance.
Supported by Grants No. GO 811/1-1 and LU 477/2-3 from the Deutsche Forschungsgemeinschaft (DFG) (to M.G. and S.L.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Stephan Ludwig, PhD, Institut f. Med. Strahlenkunde u. Zellforschung (MSZ), University of Würzburg, Versbacher Str. 5, D-97078 Würzburg, Germany; or Matthias Goebeler, MD, Klinik für Haut und Geschlechtskrankheiten, University of Würzburg, Josef-Schneider-Str. 2, 97080 Würzburg, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal