Abstract
The possible involvement of Fas and Fas ligand (FasL) in the regulation of erythropoiesis was evaluated. Immunohistochemistry of normal bone marrow specimens revealed that several immature erythroblasts undergo apoptosis in vivo. Analysis of bone marrow erythroblasts and purified progenitors undergoing unilineage erythroid differentiation showed that Fas is rapidly upregulated in early erythroblasts and expressed at high levels through terminal maturation. However, Fas crosslinking was effective only in less mature erythroblasts, particularly at basophilic level, where it induced apoptosis antagonized by high levels of erythropoietin (Epo). In contrast, FasL was selectively induced in late differentiating Fas-insensitive erythroblasts, mostly at the orthochromatic stage. FasL is functional in mature erythroblasts, as it was able to kill Fas-sensitive lymphoblast targets in a Fas-dependent manner. Importantly, FasL-bearing mature erythroblasts displayed a Fas-based cytotoxicity against immature erythroblasts, which was abrogated by high levels of Epo. These findings suggest the existence of a negative regulatory feedback between mature and immature erythroid cells, whereby the former cell population might exert a cytotoxic effect on the latter one in the erythroblastic island. Hypothetically, this negative feedback operates at low Epo levels to moderate the erythropoietic rate; however, it is gradually inhibited at increasing Epo concentrations coupled with enhanced erythrocyte production. Thus, the interaction of Fas and FasL may represent an apoptotic control mechanism for erythropoiesis, contributing to the regulation of red blood cell homeostasis.
ERYTHROPOIESIS IS A multistep process involving the differentiation of pluripotent hematopoietic stem cells through the lineage-committed burst-forming unit–erythroid (BFU-E) and colony-forming unit–erythroid (CFU-E) progenitor cells, which give rise to a series of early and late erythroblasts, eventually leading to the formation of reticulocytes and mature erythrocytes.1
During this process, the sequential formation of proerythroblasts, basophilic, polychromatophilic, and orthochromatic erythroblasts is positively regulated by erythropoietin (Epo), a glycoprotein hormone produced by the kidney in response to tissue hypoxia.2 Epo displays multiple positive effects on early erythroblasts, including increased proliferation, progression through maturation, and protection from programmed cell death.3,4 Because of the low expression of antiapoptotic genes,5 immature erythroblasts are particularly vulnerable in the absence of Epo, which has been shown to repress apoptosis through the induction of Bcl-XL, a member of the Bcl-2 family involved in protection from cell death in a number of systems.6 Bcl-XL expression is very low during early erythroid differentiation and gradually increases in intermediate and late erythroblasts, along with the loss of Epo dependence.5Thus, it has been proposed that the levels of Epo determine the fate of erythroid differentiation by promoting the survival of early erythroblasts.4 5
Fas (CD95/APO-1) is a major member of the newly characterized family of “Death Receptors.”7 Several tissues express Fas, including spleen, lymph nodes, bone marrow, heart, lung, kidney, and ovary.8 Molecular crosslinking of Fas by its natural ligand or by agonistic antibodies results in the sequential triggering of caspases,9 a family of aspartate-specific cysteine proteases whose activation is required for the propagation of the biochemical events responsible for induction of apoptotic cell death.10 The expression and function of Fas in hematopoietic cells directly correlate with the rate of proliferation, suggesting a potential role for Fas and its ligand in the regulation of hematopoietic homeostasis.11
The ligand for Fas (FasL) is a type II membrane protein predominantly expressed by activated lymphocytes and monocytes, neutrophils, thyrocytes, and stroma cells of the retina.12-16 Moreover, a number of other cell types can express FasL under different pathological conditions, including hepatocytes exposed to ethanol, macrophages infected with human immunodeficiency virus (HIV), leukemia cells exposed to chemotherapy drugs, and several cell types following tumor transformation.17 The interaction of Fas with FasL promotes the physiological deletion of potentially harmful or unnecessary cells.8 Impaired Fas-induced apoptosis results in cell accumulation, whereas inappropriate expression or excessive Fas activity causes tissue damage.18
Primitive hematopoietic progenitor cells express low amounts of Fas.19-21 However, different cytokines have been shown to upregulate Fas expression on CD34+ cells, including tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ).19-21 Fas crosslinking enhances TNF-α– and IFN-γ–mediated suppression of colony formation from bone marrow CD34+ cells.19,20 Moreover, IFN-γ has been shown to prime erythroid colony-forming cells for Fas-induced apoptosis, suggesting Fas-FasL interaction as a possible pathogenetic mechanism contributing to immune destruction of erythroid cells.22
In the bone marrow, erythropoiesis occurs in discrete anatomic units, the erythroblastic islands, consisting of one or two macrophages surrounded by one or more rings of erythroblasts at different maturation stages. The inner erythroblastic layers contain immature cells, whereas the more mature cells are at the periphery of the island.23 This spatial association of mature and immature erythroblasts may play an important role in erythropoiesis as homocellular cell-cell interaction seems to be required for erythroid cell growth and maturation.24 However, we speculated that maturating erythroblasts might deliver negative signals to neighboring cells, as a consequence of a decreased requirement for erythroid cell production. We therefore studied the possible involvement of Fas and FasL in the regulation of erythropoiesis.
MATERIALS AND METHODS
In situ apoptosis detection.
Normal human bone marrow specimens were obtained from hematologically normal patients after their informed consent and the approval by the Committee for Human Studies. Bone marrow biopsies were fixed in 4% paraformaldehyde and paraffin embedded at 50°C. Serial bone marrow sections (6 mm) were mounted onto polylysine-coated microscope slides and allowed to equilibrate to room temperature. The sections were exposed to xylene for 6 minutes for deparaffinization and then rehydrated using scalar dilution of ethanol (100% to 50%), followed by Tris-buffer saline (TBS) incubation for 5 minutes before starting the labeled streptavidin-biotin (Dako LSAB Kit, Dako Corporation, Santa Barbara, CA) staining technique. Sections stained with anti-CD68 (Dako) or anti–glycophorin-A (JC159, Dako) were treated with biotinylated antimouse immunoglobulins prediluted in TBS, following by a 30-minute incubation at room temperature with peroxidase-conjugated streptavidin. The binding was revealed by aminoethylcarbazole (AEC) colorimetric substrate. For in situ TUNEL staining, CD68- or glycophorin-A–labeled bone marrow sections were permeabilized with 0.1% Triton X-100 and 0.1% sodium citrate for 2 minutes on ice and washed twice with phosphate-buffered saline (PBS). The labeling of 3′-OH fragmented DNA ends (TdT-mediated dUTP nick end labeling) was performed by an in situ apoptosis detection kit (In Situ Cell Death Detection, AP; Boehringer Mannheim, Indianapolis, IN). Detection of labeled ends was performed with a Fab2 antifluorescein antibody conjugated with alkaline phosphatase (AP). 5-bromo-4-chloro-3-indolyl-phosphate (BCIP; Dako) was used as colorimetric substrate. Control tissue sections were prelabeled with irrelevant isotype-matched monoclonal antibodies (MoAbs) and subjected to identical treatment for TUNEL staining without terminal deoxynucleotidyl transferase (TdT).
Bone marrow erythroblast purification.
Bone marrow cells from healthy donors were obtained by needle aspiration and purified over a Ficoll-Hypaque density gradient (Pharmacia, Piscataway, NJ). A greater than 97% pure erythroid population was then obtained by flow cytometry sorting on the basis of glycophorin-A expression. Briefly, 106 cells/mL were incubated with saturating amounts of fluorescein isothiocyanate (FITC)-conjugated anti–glycophorin-A MoAb (Dako) or an isotype-matched control antibody for 30 minutes on ice. Cells were washed twice with cold PBS and run on a fluorescence-activated cell sorter (FACS) VANTAGE cell sorter (Becton Dickinson, San Jose, CA). Glycophorin-A positive cells were then sorted on the basis of fluorescence emission at 525 nm.
Adult peripheral blood (PB) human progenitor cell purification and erythropoietic unilineage cultures.
Adult PB was obtained from male donors after their informed consent and the approval by the Committee for Human Studies.25Hematopoietic progenitor cells (HPCs) were purified as reported26 and modified as described.27Briefly, (IA) PB samples were separated over a Ficoll-Hypaque density gradient and (IB) PB mononuclear cells resuspended in Iscove’s modified Dulbecco’s medium (IMDM) containing 20% heat-inactivated fetal calf serum (FCS) (GIBCO, Grand Island, NY) for three cycles of plastic adherence. Thereafter, (II) cells were separated by centrifugation on a discontinuous Percoll gradient (Biochrom KG, Berlin, Germany). (III) Step III purification was potentiated (Step IIIP) as described.26 For unilineage cultures, Step IIIP HPCs were seeded at 5 × 104/mL and grown in liquid suspension cultures supplemented with low doses of interleukin-3 (IL-3; 0.01 U/mL) and granulocyte-macrophage colony-stimulating factor (GM-CSF; 0.001 ng/mL), and high levels of Epo (3 U/mL).26HPCs grown under these conditions undergo selective erythroid differentiatiation. Recombinant human IL-3 (rhIL-3; 2 × 106 U/mg) and rhGM-CSF (2 × 108 U/mg) were supplied by the Genetics Institute (Cambridge, MA); rhEpo (1.2 × 105 U/mg) was obtained from Amgen (Thousand Oaks, CA).
Fas and FasL staining.
For immunofluorescence staining and flow cytometry analysis, cells were incubated for 30 minutes on ice with phycoerythrin (PE)-conjugated anti-Fas MoAb (DX2, IgG1; Pharmigen, San Diego, CA) or control IgG1, and washed twice with cold PBS. Alternatively, cells were incubated for 30 minutes at 4°C with 5 μg/mL of purified rabbit polyclonal anti-FasL (Ab-1; Calbiochem-Novachem Corp, La Jolla, CA) or control rabbit IgG, washed, and treated with PE-conjugated donkey anti-rabbit IgG (Chemicon, Temecula, CA). After an additional washing, cells were analyzed on a FACSCAN cytofluorimeter (Becton Dickinson).
Cytospins of sorted bone marrow erythroid cells were allowed to equilibrate to room temperature and fixed in 4% paraformaldheyde for 15 minutes, followed by air-drying. Bound rabbit polyclonal anti-FasL Ab (Ab-1) or control rabbit IgG were detected by immunoenzymatic alkaline phosphatase antialkaline phosphatase (APAAP) complex procedure (Dako).
Reverse-transcription polymerase chain reaction (RT-PCR) analysis.
Total RNA from erythroblasts at different maturation stages was extracted by the standard guanidinium thyocianate-CsCl technique and quantitated by dot hybridization with a human rRNA probe. After densitometric analysis, the same amount of RNA was reverse-transcribed with oligo (dT) as a primer, and the products were normalized by amplification of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene together with HuT78 cDNA.
cDNA amplification was obtained by 35 PCR cycles, which generated a 346-bp fragment of human FasL (forward primer, from 641to 661 bp; reverse primer, from 966 to 986 bp).15 PCR products were verified by sequencing and analyzed by Southern blotting with an internal oligomer as a probe. The probe used for GAPDH gene was from 880 to 914 bp.
Evaluation of caspase activity and apoptosis in mature and immature erythroblasts.
Day-7 and day-14 erythroblasts were incubated with 200 ng/mL of agonistic anti-Fas MoAb (CH-11, IgM; UBI, Lake Placid, NY) or control IgM. The percentage of erythroblasts undergoing apoptosis was measured by DNA staining and flow cytometry analysis, as previously described.12 Briefly, the cell pellet was gently resuspended in hypotonic fluorochrome solution (propidium iodide 50 μg/mL [Sigma, St Louis, MO] in 0.1% sodium citrate plus 0.1% Triton X-100) and kept overnight at 4°C in the dark until flow cytometry analysis. The percentage of apoptotic cells was determined by evaluating the number of hypodiploid nuclei.
Evaluation of caspase activation in erythroblasts at different maturation stages was performed by the ApoAlert assay kit (Clontech, Palo Alto, CA), according to the manufacturer’s instructions. Lysates from 106 cells were incubated with the fluorogenic substrate (DEVD-AFC) for 60 minutes at 37°C in a buffer containing 5 mmol/L dithiotreitol (DTT). Samples were then analyzed on a plate fluorimeter (Flow Laboratories, McLean, VA) at 538 nm.
Cytotoxicity studies.
Fas-sensitive and Fas-resistant Epstein-Barr virus (EBV)-transformed lymphoblastoid target cells were exposed overnight to 1 mmol/L 3,3′-dioctadecyloxacarbocyanine (DioC18), washed twice with RPMI containing 5% FCS, transferred to round bottom tubes containing different numbers of day-7 or day-14 erythroblasts, and centrifuged at 1,000 rpm for 10 minutes. Cells were analyzed after 8 hours of culture.
Day-7 erythroblasts, loaded with DioC18 as described above, were used as target cells and incubated for 36 hours with day-14 effector cells in the presence of either 30 mU/mL or 3 U/mL recombinant Epo. In some cases, 10 μg/mL of an anti-Fas antagonist MoAb ZB4 (UBI) was added to the target cells 15 minutes before the assay. At the end of the incubation, cells were treated with 5 μg/mL propidium iodide and immediately analyzed by flow cytometry. The percentage of specific lysis was determined by comparing the number of DioC18-labeled propidium iodide–positive (dead) and –negative (living) cells among the different samples.15
RESULTS
Immature erythroblasts are the major apoptotic population in normal bone marrow.
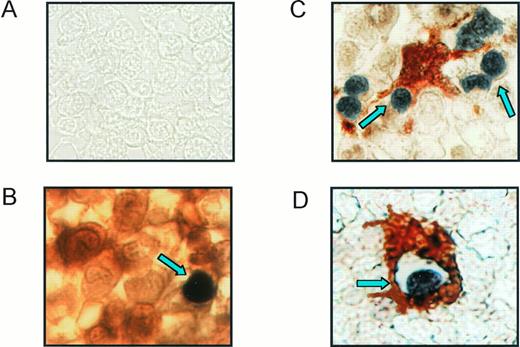
To determine whether erythroblast apoptosis is a process occurring in vivo under physiological conditions, bone marrow specimens from healthy donors were analyzed for in situ apoptosis. Immunohistochemistry of formalin-fixed sections double labeled for glycophorin staining and TUNEL reaction revealed the presence of several apoptotic erythroblasts (about 2% to 3% of the immature erythroid population), showing phenotypical characteristics of immature cells, such as large volume, thin cytoplasm, and the cell nucleus occupying at least three fourths of the whole cell area (Fig 1A and B). A minority of cells from other lineages seem to undergo apoptosis in normal bone marrow, as glycophorin-negative/TUNEL-positive cells were only occasionally observed (not shown). Moreover, analysis of sections double stained for the macrophage marker CD68 and TUNEL showed bone marrow macrophages surrounding (Fig 1C) and phagocytosing (Fig 1D) apoptotic cells, suggesting that macrophages in erythroblastic islands are responsible for the clearance of apoptotic erythroblasts.
Erythroblast apoptosis in normal bone marrow. Sections from bone marrow biopsies were fixed in paraformaldehyde and paraffin and double-labeled for TUNEL reaction with BCIP (black) and anti–glycophorin-A or anti-CD68 with AEC (red). (A) Control section treated with irrelevant IgG Abs and TUNEL reaction without TdT. (B) Section stained with anti–glycophorin-A and TUNEL reaction showing an immature apoptotic erythroblast (arrowhead). (C and D) Sections treated with anti-CD68 and TUNEL reaction representing macrophages surrounding and phagocytosing apoptotic cells are indicated by arrows. Representative sections from one donor out of four are shown.
Erythroblast apoptosis in normal bone marrow. Sections from bone marrow biopsies were fixed in paraformaldehyde and paraffin and double-labeled for TUNEL reaction with BCIP (black) and anti–glycophorin-A or anti-CD68 with AEC (red). (A) Control section treated with irrelevant IgG Abs and TUNEL reaction without TdT. (B) Section stained with anti–glycophorin-A and TUNEL reaction showing an immature apoptotic erythroblast (arrowhead). (C and D) Sections treated with anti-CD68 and TUNEL reaction representing macrophages surrounding and phagocytosing apoptotic cells are indicated by arrows. Representative sections from one donor out of four are shown.
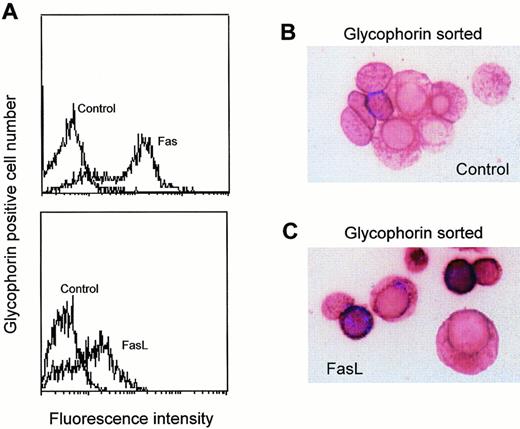
Fas and FasL expression in bone marrow erythroblasts. Bone marrow erythroid cells obtained by needle aspiration were purified by Ficoll and flow cytometry sorting based on glycophorin-A positivity. Erythroblasts were subsequently analyzed by flow cytometry for Fas and FasL expression (A), and by immunohistochemistry on cytospins stained with control (B) or anti-FasL polyclonal Abs (C) and revealed with alkaline phosphatase. A representative experiment out of five performed with cells from three different donors is shown.
Fas and FasL expression in bone marrow erythroblasts. Bone marrow erythroid cells obtained by needle aspiration were purified by Ficoll and flow cytometry sorting based on glycophorin-A positivity. Erythroblasts were subsequently analyzed by flow cytometry for Fas and FasL expression (A), and by immunohistochemistry on cytospins stained with control (B) or anti-FasL polyclonal Abs (C) and revealed with alkaline phosphatase. A representative experiment out of five performed with cells from three different donors is shown.
Erythroblasts express Fas and FasL.
The expression of Fas and FasL was analyzed on freshly isolated bone marrow erythroid cells sorted for glycophorin-A expression. Figure 2A shows that most glycophorin-positive erythroblasts (>90%) constitutively express Fas, presumably because they have a high proliferative rate, which is associated with Fas upregulation in hematopoietic cells.8 In contrast, the expression of FasL in these cells is heterogeneous, FasL being present on about 60% to 70% of bone marrow erythroblasts. To determine whether the differential expression of FasL is related to different maturation stages, we analyzed the morphology of purified bone marrow erythroid cells stained with control or anti-FasL Abs. As shown in Fig 2B and C, FasL was expressed only on small mature erythroblasts, suggesting that FasL expression is acquired during the late phases of erythroid differentiation (ie, at the stage of late polychromatophilic and orthochromatic erythroblasts).
Expression of Fas and FasL during unilineage erythroid differentiation.
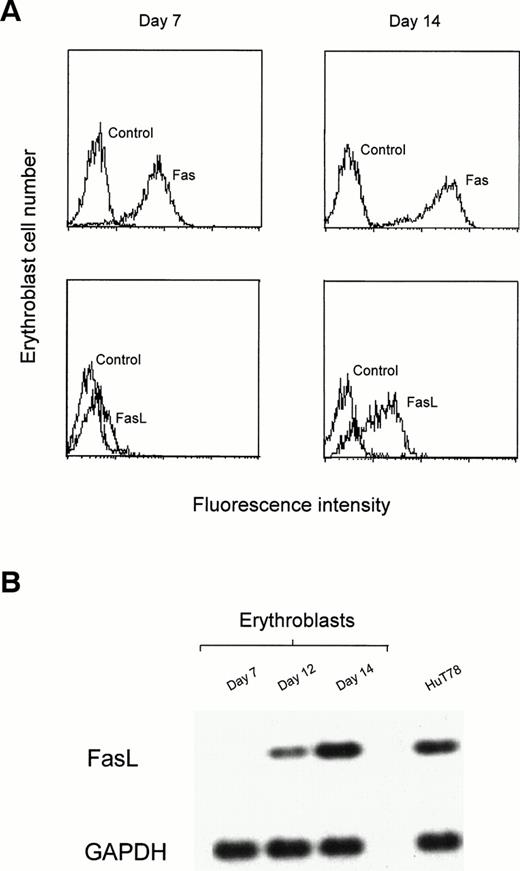
The unilineage erythroid culture system allows the study of discrete steps of cell maturation, from undifferentiated CD34+ cells to the terminal stages of erythroid differentiation.28Purified CD34+ progenitor cells, grown in the presence of very low doses of IL-3 and GM-CSF and high amounts of Epo, differentiate to basophilic erythroblasts after 7 days and orthochromatic erythroblasts after 14 days of culture. The comparative analysis of early (day-7) and late (day-14) erythroblasts showed that Fas is expressed in immature cells and further upregulated in mature erythroblasts, whereas FasL is absent or barely detectable in immature erythroblasts and highly expressed in late erythroblasts (Fig 3A). Membrane-bound FasL is rapidly cleaved to a soluble form by metalloproteases. We therefore investigated FasL mRNA expression by semiquantitative RT-PCR to facilitate accurate quantification of FasL production.29 As shown in Fig 3B, FasL mRNA is absent in immature erythroblasts but gradually increases in more mature erythroblasts starting from day 12. At day 14, FasL mRNA levels are comparable or higher than those found in the positive control HuT78 T lymphoma cell line, known to produce considerable amounts of FasL.29
Expression of Fas and FasL in unilineage erythroid differentiation. Purified peripheral blood CD34+ cells were cultivated with high concentration of Epo and very low amounts of IL-3 and GM-CSF for up to 14 days. (A) After 7 and 14 days cells were stained with control, anti-Fas (Fas), and anti-FasL (FasL) Abs, and analyzed by flow cytometry. (B) Semiquantitative RT-PCR analysis from erythroblasts at different days of culture and from HuT78 cells. Top lane, human FasL cDNA amplification; bottom lane, GAPDH cDNA amplification used to normalize RT-RNAs. A representative experiment out of three performed with cells from different donors is shown.
Expression of Fas and FasL in unilineage erythroid differentiation. Purified peripheral blood CD34+ cells were cultivated with high concentration of Epo and very low amounts of IL-3 and GM-CSF for up to 14 days. (A) After 7 and 14 days cells were stained with control, anti-Fas (Fas), and anti-FasL (FasL) Abs, and analyzed by flow cytometry. (B) Semiquantitative RT-PCR analysis from erythroblasts at different days of culture and from HuT78 cells. Top lane, human FasL cDNA amplification; bottom lane, GAPDH cDNA amplification used to normalize RT-RNAs. A representative experiment out of three performed with cells from different donors is shown.
Immature erythroid cells undergo Fas-induced apoptosis in the absence of high levels of Epo.
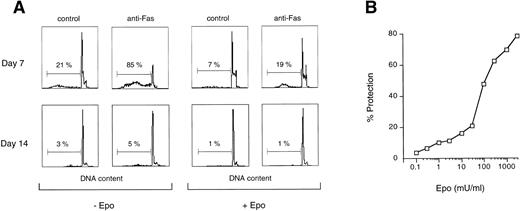
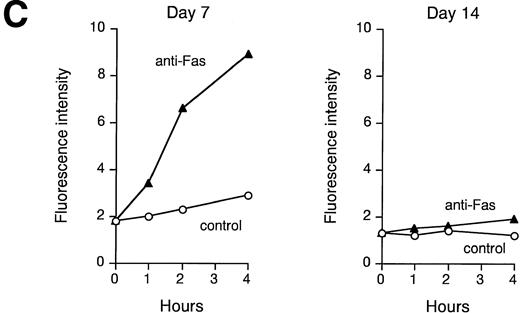
Fas expression does not always correlate with its ability to transduce an apoptotic signal. We therefore analyzed Fas sensitivity of early and late erythroblasts and the possible interference of Epo in the death signal generated by Fas crosslinking. Figure 4A and B show that Fas triggering induces massive apoptosis of immature erythroblasts in the absence of Epo, which is extremely effective in protection from Fas-induced apoptosis. In contrast, Fas crosslinking in mature erythroblasts is not able to transduce a death signal, even in the absence of Epo (Fig 4A), suggesting that Fas in mature erythroblasts is not coupled to the apoptotic machinery. Accordingly, Fas crosslinking in immature erythroblasts resulted in a strong and persistent caspase activation, as evaluated by the ability of cell extracts to cleave the fluorogenic substrate DEVD, whereas no increase in caspase activity was detected in Fas-stimulated mature erythroblasts (Fig 4C), even in the presence of high Fas expression.
Fas is functional in immature erythroblasts in the absence of high levels of Epo. (A) Peripheral CD34-derived day-7 and day-14 erythroblasts were incubated for 24 hours with or without 200 ng/mL of agonistic anti-Fas MoAb in the absence (−Epo) or in the presence (+Epo) of 3 U/mL recombinant Epo. Apoptosis was quantitated by DNA staining and flow cytometry analysis. (B) Day-7 cells were treated as described above with different concentrations of Epo. Percentage of protection was calculated by comparison with cells stimulated in the absence of Epo. A representative experiment out of five performed with cells from different donors is shown. (C) Lysates from day-7 and day-14 cells untreated (control) or stimulated for different times with agonistic anti-Fas MoAb (anti-Fas) were analyzed for their ability to cleave the fluorogenic caspase substrate DEVD-AFC. Data are expressed in arbitrary fluorescence units and show a representative experiment out of four performed with cells from different donors.
Fas is functional in immature erythroblasts in the absence of high levels of Epo. (A) Peripheral CD34-derived day-7 and day-14 erythroblasts were incubated for 24 hours with or without 200 ng/mL of agonistic anti-Fas MoAb in the absence (−Epo) or in the presence (+Epo) of 3 U/mL recombinant Epo. Apoptosis was quantitated by DNA staining and flow cytometry analysis. (B) Day-7 cells were treated as described above with different concentrations of Epo. Percentage of protection was calculated by comparison with cells stimulated in the absence of Epo. A representative experiment out of five performed with cells from different donors is shown. (C) Lysates from day-7 and day-14 cells untreated (control) or stimulated for different times with agonistic anti-Fas MoAb (anti-Fas) were analyzed for their ability to cleave the fluorogenic caspase substrate DEVD-AFC. Data are expressed in arbitrary fluorescence units and show a representative experiment out of four performed with cells from different donors.
FasL is functional in mature erythroblasts and able to kill immature erythroblasts in the absence of high levels of Epo.
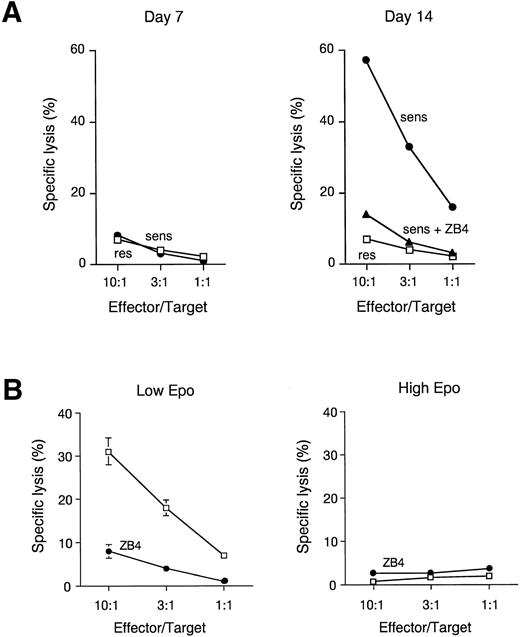
To determine whether FasL present on the membrane of mature erythroblasts is capable of eliciting a cytotoxic response, we exposed Fas-sensitive and Fas-resistant EBV-transformed lymphoblasts to increasing numbers of immature (day-7) or mature (day-14) erythroblasts. As shown in Fig 5A, FasL-positive mature erythroblasts were able to massively kill Fas-sensitive, but not Fas-resistant, lymphoblast targets in a Fas-dependent fashion, as the target lysis was completely prevented by the addition of anti-Fas antagonist MoAb. As expected, FasL-negative immature erythroblasts were unable to kill either lymphoblast target (Fig 5A). To investigate whether Fas-FasL interaction among erythroblasts results in apoptotic cell death of immature erythroblasts, we evaluated the ability of FasL-expressing mature erythroblasts to kill immature Fas-sensitive erythroblasts. Figure 5B shows that in the presence of Epo levels comparable to those routinely found in normal sera, mature erythroblasts are able to efficiently kill immature erythroblasts through the induction of Fas-mediated apoptosis, as pretreatment of immature erythroblasts with anti-Fas antagonist MoAb essentially abolished the cell cytotoxicity. In contrast, no cytotoxicity against immature erythroblast targets was observed in the presence of high levels of Epo, indicating that elevated Epo concentrations are required for protection of immature erythroblasts against the abundant FasL production of mature erythroblasts.
FasL is functional in mature erythroblasts and able to kill immature erythroblasts in the absence of high levels of Epo. (A) Cytotoxic activity of day-7 and day-14 erythroblasts toward Fas-sensitive (sens) and Fas-resistant (res) EBV-transformed lymphoblastoid cell lines. Effector and target cells were spun down together and incubated for 8 hours. In some experiments anti-Fas antagonist MoAb ZB4 (10 μg/mL) was added to Fas-sensitive cells (sens + ZB4). Data show a representative experiment out of three performed with cells from different donors. (B) Cytotoxic activity of day 14 erythroblasts toward day-7 erythroblasts pretreated or not with 10 μg/mL ZB4, in the presence of low (30 mU/mL) or high (3 U/mL) amounts of Epo. Effector and target cells were spun down together and incubated for 36 hours. Data show mean ± SD of three experiments performed with cells from different donors.
FasL is functional in mature erythroblasts and able to kill immature erythroblasts in the absence of high levels of Epo. (A) Cytotoxic activity of day-7 and day-14 erythroblasts toward Fas-sensitive (sens) and Fas-resistant (res) EBV-transformed lymphoblastoid cell lines. Effector and target cells were spun down together and incubated for 8 hours. In some experiments anti-Fas antagonist MoAb ZB4 (10 μg/mL) was added to Fas-sensitive cells (sens + ZB4). Data show a representative experiment out of three performed with cells from different donors. (B) Cytotoxic activity of day 14 erythroblasts toward day-7 erythroblasts pretreated or not with 10 μg/mL ZB4, in the presence of low (30 mU/mL) or high (3 U/mL) amounts of Epo. Effector and target cells were spun down together and incubated for 36 hours. Data show mean ± SD of three experiments performed with cells from different donors.
DISCUSSION
The erythroblastic island is a relatively autonomous unit.23 It is therefore of interest to identify potential regulators of erythroid cell homeostasis among the cell population forming the island.
Several studies have shown that the interaction of Fas with FasL plays a major role in the maintenance of hematopoietic cell homeostasis, because physiological deletion of T and B lymphocytes, granulocytes, and natural killer cells seems primarily due to the engagement of Fas following homocellular or heterocellular FasL production.8,14 30 Due to the adjacent location of mature and immature erythroblasts in the bone marrow, the possible interaction between these cells may be critical for their fate. Here we have examined the possible apoptotic role of Fas and FasL in the regulation of erythropoiesis.
In situ TUNEL analysis of normal bone marrow specimens has shown here that several immature erythroblasts are apoptotic. Although an accurate quantification of this phenomenon is not feasible, due to the unknown clearance rate of these apoptotic cells in vivo, it is likely that apoptosis of immature erythroblasts significantly contributes to the ineffective quote of erythropoietic process described in normal subjects.31 32
The level of Epo dictates the fate of immature erythroblasts, which display a high proliferative potential and therefore represent a key target for the regulation of erythropoiesis in physiological and pathological conditions.4 We found that immature erythroblasts, mostly at the basophilic stage, are Epo-dependent and more susceptible to apoptosis upon Epo deprivation. These cells express a functional Fas molecule, and in the presence of FasL-producing mature erythroblasts undergo apoptosis, unless exposed to high levels of Epo. In contrast, because mature erythroblasts are completely resistant to Fas-induced apoptosis, they are not susceptible to the autotoxic lysis mediated by high FasL production. Interestingly, the Epo levels necessary for protection from Fas-induced apoptosis in immature erythroblasts are comparable to those found in sera of subjects with low hemoglobin concentrations or decreased red blood cell precursor mass.33 We hence postulate that the inhibitory effect of mature erythroblasts on erythropoiesis may act as a negative regulatory feedback by inducing cell death of immature erythroblasts when there is a lower metabolic requirement for new erythrocytes, and consequently low levels of Epo are present in the bone marrow. On the other hand, this inhibitory effect is gradually blocked at increasing Epo concentrations coupled with enhanced erythrocyte production. Thus, the Fas/FasL system may contribute, together with low Epo levels, to the negative regulation of physiological erythropoiesis.
Several other regulatory systems, including the interaction of other death receptors with their ligands, may be also involved in the physiological inhibition of erythropoiesis. This would result in redundancy and may explain why mice with targeted mutation of the Fas gene do not show major abnormalities in the erythroid compartment.34
The presence of high levels of functional FasL in mature erythroblasts may play an important pathogenetic role in a number of diseases resulting in dyserythropoiesis and anemia. Cytokines or inflammatory factors able to increase Fas-sensitivity in immature erythroblasts are likely to alter the balance between Epo and Fas/FasL, with deleterious effects on erythropoiesis. This may account for the potent erythroid suppression induced by IFN-γ, which has been shown to upregulate both Fas and its apoptotic machinery.35 This suppressor mechanism might be operating in patients suffering from aplastic anemia, who often present overproduction of IFN-γ in the bone marrow.36,37 Similarly, mice with targeted mutations of the Fanconi anemia group C gene, a murine model for Fanconi anemia, show hypersensitivity to IFN-γ, which, at doses ineffective in normal mice, primes progenitor cells for Fas-mediated destruction and impaired clonal growth of erythroid and granulocyte-macrophage lineages.38
Alternatively, it is possible that FasL-producing erythroblasts may increase their Fas-sensitivity following exposure to priming agents such as IFN-γ, resulting in erythroblast suicide as recently proposed.22 However, here we identify prebasophilic/basophilic erythroblasts as the most sensitive maturation stage for Fas-induced apoptosis during erythroid differentiation. These cells do not express FasL and are more sensitive to the inhibitory effect of IFN-γ than mature erythroblasts, which gradually lose the susceptibility to apoptosis while increasing FasL expression. Thus, immature erythroblasts seem to be a major target for both physiological and pathological inhibition of erythropoiesis.
In conclusion, we provide evidence for an apoptotic role of Fas and FasL in the regulation of erythropoiesis. Massive FasL production following mature erythroblast accumulation may act as a negative regulator of erythroblast development and is likely to contribute to homeostasis of the erythroblastic island. In this context, basophilic erythroblasts are the most sensitive target of FasL-induced antierythropoietic effects and therefore represent a preferential target for potential Fas/FasL-based anemia.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to C. Peschle, MD, Kimmel Cancer Center, Room #902, Thomas Jefferson University, Philadelphia, PA 19107-5541; e-mail:cesare.peschle@mail.tju.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal