Abstract
The median survival in primary systemic (AL) amyloidosis is less than 18 months. No published series of patients with AL amyloidosis have reported survival of more than 10 years. The records of all Mayo Clinic patients with a diagnosis of AL amyloidosis between January 1, 1966 and March 1, 1987 were reviewed. Patients with secondary amyloidosis, familial amyloidosis, senile systemic amyloidosis, and localized amyloidosis were excluded. During the 21 years of the study, 841 patients with AL amyloidosis were seen. Of these, 29 were excluded because the diagnosis was made at autopsy, and 2 others were excluded because no follow-up data were available. Actuarial survival for the 810 patients was 51% at 1 year, 16% at 5 years, and 4.7% at 10 years. Thirty patients survived for 10 years or more after the histologic diagnosis of AL amyloidosis; all received alkylating-agent therapy. In 14 patients, the monoclonal protein disappeared from the serum or urine. Of 10 patients with nephrotic syndrome, 4 had an objective response. Congestive heart failure, older age, creatinine value of 2 mg/dL or more, bone marrow plasma cell value of 20% or more, platelet count of 500 × 109/L or less, and the presence of peripheral neuropathy were underrepresented in the 10-year survivors and are unfavorable prognostic features. Five percent of patients with AL amyloidosis survived for 10 years or more.
THE MEDIAN SURVIVAL in 474 patients with primary systemic (AL) amyloidosis was 13 months.1 Despite the use of melphalan and prednisone, the median survival is still only 17 to 18 months.2 No published series of patients with AL amyloidosis have reported survival of more than 10 years, and there have been only infrequent case reports of patients surviving more than a decade.3 4 This review was undertaken to report our experience with patients with AL amyloidosis who have survived for 10 or more years and to identify any clinical or laboratory features that predicted long-term survival.
MATERIALS AND METHODS
The records of all Mayo Clinic patients with a diagnosis of AL amyloidosis who were seen between January 1, 1966 and March 1, 1987 were reviewed. Patients with secondary amyloidosis, familial amyloidosis, senile systemic amyloidosis, and localized amyloidosis were excluded.
Amyloidosis was confirmed histologically in all patients. Sections stained with Congo red showed green birefringence when reviewed under polarized light.5
The diagnosis of multiple myeloma was made on the basis of a bone marrow containing more than 10% plasma cells, a monoclonal protein in the serum, a monoclonal protein in the urine, or lytic bone lesions. The clinical features of multiple myeloma were also present.
Serum protein electrophoresis was done on agarose gel with Ponceau S staining. Immunoelectrophoresis or immunofixation was performed with monospecific antisera to IgG, IgA, IgM, and κ and λ light chains. All serum samples were screened by Ouchterlony immunodiffusion for excess of IgD and IgE. Immunoglobulin levels were measured with nephelometry. Electrophoresis and immunoelectrophoresis or immunofixation were performed on urine concentrated by ulrafiltration.6
Statistical analysis.
Many of the patients in the study were first referred to Mayo Clinic some months after their initial diagnosis. A naive computation of the survival curve for time from diagnosis will be biased upward in this case. The method of Turnbull7 was used to compute the survival curve, corrected for the delayed entry.
Predictions of long-term survival were assessed with logistic regression. Because a patient’s characteristics measured at a delayed entry date might differ from the patient’s (unknown) values at diagnosis, these comparisons used only subjects who were referred to Mayo Clinic within 6 months of the initial diagnosis of AL amyloidosis. All analyses were done with the SAS and S-Plus statistical packages. All tests were two-tailed.
RESULTS
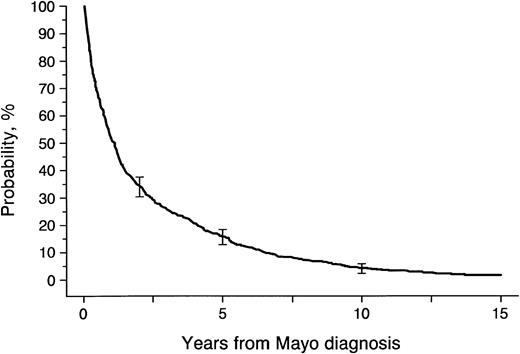
During the 21 years of the study, 841 patients with AL amyloidosis were seen at Mayo Clinic. Of these, 29 were excluded because the diagnosis was made at autopsy, and 2 others were excluded because no follow-up data were available. Of the remaining 810 patients, 685 were seen at Mayo Clinic within 6 months of the initial diagnosis of AL amyloidosis, 93 between 6 months and 2 years, and 32 more than 2 years after initial diagnosis. Sixty-seven patients seen within 6 months are still alive and under observation (Table 1). Overall survival for the 810 patients was 51% at 1 year, 16% at 5 years, and 4.7% at 10 years (Fig 1).
Primary Amyloidosis, Mayo Clinic, 1966 to 1987 (n = 841)
| . | Seen <6 Mo . | Seen ≥6 Mo . |
|---|---|---|
| Diagnosis at autopsy | 29 | 0 |
| No follow-up | 2 | 0 |
| Died ≤10+ yr | 599 | 73 |
| Alive ≤10+ yr | 67 | 41 |
| Alive 10+ yr | 8 | 4 |
| Died 10+ yr | 11 | 7 |
| Total | 716 | 125 |
| . | Seen <6 Mo . | Seen ≥6 Mo . |
|---|---|---|
| Diagnosis at autopsy | 29 | 0 |
| No follow-up | 2 | 0 |
| Died ≤10+ yr | 599 | 73 |
| Alive ≤10+ yr | 67 | 41 |
| Alive 10+ yr | 8 | 4 |
| Died 10+ yr | 11 | 7 |
| Total | 716 | 125 |
Survival for 810 patients with systemic amyloidosis seen at Mayo Clinic between January 1, 1966 and March 1, 1987.
Survival for 810 patients with systemic amyloidosis seen at Mayo Clinic between January 1, 1966 and March 1, 1987.
Long-term survivors.
Thirty patients survived for 10 years or more after the histologic diagnosis of AL amyloidosis; 19 of these were first seen at Mayo Clinic within 6 months of diagnosis (Table 1). Comparison of these two groups showed little difference between them (Table 2). All but 1 of the 30 patients had a monoclonal protein in the serum or urine, monoclonal plasma cells in the bone marrow, or immunohistochemical proof of monoclonal light-chain staining of the amyloid tissue. In the one exception, the diagnosis was made in 1970, immunoelectrophoresis and bone marrow biopsy were not done, and the positive rectal biopsy specimen contained no additional tissue for immunohistochemical studies. The ages of the survivors ranged from 27 to 68 years (median, 54 years) at diagnosis of AL. Fifty-three percent were male. Weight loss was present in 31%; 3 patients reported a loss of 40 pounds or more. The liver was palpable in 31%. Only 3 patients had a liver palpable more than 5 cm below the right costal margin. The spleen was not palpable in any survivor, and lymphadenopathy was noted in only 2 patients. Macroglossia was present in 20%. At the first Mayo Clinic visit, the median hemoglobin value was 13.5 g/dL, and only 2 patients had a value less than 10 g/dL. The median platelet value was 286 × 109/L; 24% of patients had a platelet value more than 500 × 109/L. Fourteen percent had an initial serum creatinine value of 2.0 mg/dL or more. The median serum alkaline phosphatase value was 162 U/L (normal, < 309 U/L); 2 patients had a value twice normal. The serum albumin value was less than 3.0 g/dL in 37%. Only 1 patient had a vitamin B12 value less than 150 ng/L, and none had a serum carotene value less than 50 μg/dL.
Comparison of Patients With Primary Systemic Amyloidosis, by Duration of Survival
| Factor . | Nonsurvivors (<10 yr) Seen ≤6 Mo of Diagnosis (n = 599) . | Survivors ≥10 Yr . | P Value . | |
|---|---|---|---|---|
| Total Group (n = 30) . | Seen ≤6 Mo of Diagnosis (n = 19) . | |||
| Age, median (yr) | 63 | 54 | 54 | .001 |
| Sex, male (%) | 65 | 53 | 47 | .12 |
| Weight loss (%) | 49 | 31 | 33 | .18 |
| Liver palpable (%) | 34 | 31 | 33 | .94 |
| Macroglossia (%) | 19 | 21 | 12 | .75 |
| Hemoglobin <10 g/dL (%) | 12 | 7 | 6 | .71 |
| Platelets >500 × 109/L (%) | 10 | 24 | 33 | .01 |
| Alkaline phosphatase ≥500 U/L (%) | 8 | 7 | 0 | .38 |
| Creatinine ≥2.0 mg/dL (%) | 22 | 14 | 12 | .55 |
| Bone marrow plasma cells ≥20% (%) | 21 | 4 | 6 | .21 |
| Serum albumin <3 g/dL (%) | 59 | 37 | 37 | .14 |
| Serum monoclonal protein (%) | 70 | 64 | 59 | .33 |
| Urine positive (%) | 74 | 62 | 69 | .57 |
| Serum or urine monoclonal protein present (%) | 89 | 86 | 88 | 1.00 |
| Interventricular septal thickness ≥15 mm* (%) | 43 | 36 | 33 | .51 |
| Carpal tunnel syndrome (%) | 25 | 20 | 21 | 1.00 |
| Nephrotic range proteinuria (%) | 34 | 33 | 32 | .74 |
| CHF (%) | 24 | 7 | 11 | .08 |
| Peripheral neuropathy (%) | 18 | 7 | 5 | .22 |
| Orthostatic hypotension (%) | 16 | 13 | 16 | 1.00 |
| Bone marrow biopsy positive for amyloid deposits (%) | 46 | 64 | 61 | .37 |
| Rectal biopsy positive for amyloid deposits (%) | 75 | 65 | 83 | .75 |
| Therapy | ||||
| ≤30 d of diagnosis (%) | 64 | 33 | 44 | .09 |
| ≤180 d of diagnosis (%) | 94 | 52 | 72 | .01 |
| Factor . | Nonsurvivors (<10 yr) Seen ≤6 Mo of Diagnosis (n = 599) . | Survivors ≥10 Yr . | P Value . | |
|---|---|---|---|---|
| Total Group (n = 30) . | Seen ≤6 Mo of Diagnosis (n = 19) . | |||
| Age, median (yr) | 63 | 54 | 54 | .001 |
| Sex, male (%) | 65 | 53 | 47 | .12 |
| Weight loss (%) | 49 | 31 | 33 | .18 |
| Liver palpable (%) | 34 | 31 | 33 | .94 |
| Macroglossia (%) | 19 | 21 | 12 | .75 |
| Hemoglobin <10 g/dL (%) | 12 | 7 | 6 | .71 |
| Platelets >500 × 109/L (%) | 10 | 24 | 33 | .01 |
| Alkaline phosphatase ≥500 U/L (%) | 8 | 7 | 0 | .38 |
| Creatinine ≥2.0 mg/dL (%) | 22 | 14 | 12 | .55 |
| Bone marrow plasma cells ≥20% (%) | 21 | 4 | 6 | .21 |
| Serum albumin <3 g/dL (%) | 59 | 37 | 37 | .14 |
| Serum monoclonal protein (%) | 70 | 64 | 59 | .33 |
| Urine positive (%) | 74 | 62 | 69 | .57 |
| Serum or urine monoclonal protein present (%) | 89 | 86 | 88 | 1.00 |
| Interventricular septal thickness ≥15 mm* (%) | 43 | 36 | 33 | .51 |
| Carpal tunnel syndrome (%) | 25 | 20 | 21 | 1.00 |
| Nephrotic range proteinuria (%) | 34 | 33 | 32 | .74 |
| CHF (%) | 24 | 7 | 11 | .08 |
| Peripheral neuropathy (%) | 18 | 7 | 5 | .22 |
| Orthostatic hypotension (%) | 16 | 13 | 16 | 1.00 |
| Bone marrow biopsy positive for amyloid deposits (%) | 46 | 64 | 61 | .37 |
| Rectal biopsy positive for amyloid deposits (%) | 75 | 65 | 83 | .75 |
| Therapy | ||||
| ≤30 d of diagnosis (%) | 64 | 33 | 44 | .09 |
| ≤180 d of diagnosis (%) | 94 | 52 | 72 | .01 |
Abbreviation: CHF, congestive heart failure.
Determined with echocardiography.
A monoclonal protein was found in the serum in 18 (64%) of 28 patients tested; the monoclonal protein consisted of IgG in 7 patients, IgA in 3, IgM in 1, IgD in 1, κ in only 1, and λ in only 5. λ light chains were found in 15 (83%) of 18 patients. Fifteen of the 28 patients who had serum protein electrophoresis had a γ spike (range, 0.4 to 2.7 g/dL; median, 0.8 g/dL). Only 2 patients had a monoclonal protein spike more than 2.0 g/dL. The urine contained a monoclonal light chain in 16 (62%) of 26 patients tested; in 13 it was λ. The size of the urine monoclonal spike ranged from 0.01 to 0.9 g/24 h in the 16 patients. The 24-hour urine protein content ranged from 0.1 to 11.8 g/24 h (median, 0.5 g/24 h) in the 28 patients in whom it was measured; 11 had a protein loss of more than 3.0 g/24 h.
A monoclonal protein was detected in the serum or urine in 24 (86%) of the 28 patients in whom immunoelectrophoresis or immunofixation was performed. Of the 6 patients without a monoclonal protein in the serum or urine at diagnosis, a bone marrow biopsy specimen contained a monoclonal population of plasma cells in 2, immunohistochemical stains of the amyloid tissue were positive for a monoclonal light chain in 1, a monoclonal protein developed in the serum and urine in 2 patients during the course of their illness, and no studies were done in 1 patient.
The interventricular septal thickness was 15 mm or more in 8 (36%) of the 22 patients in whom it was measured. The ejection fraction was less than 50% in 2 patients (44% and 45%).
Overt congestive heart failure was present in 2 patients at the time of diagnosis. The first patient was a 62-year-old white man who had had progressive dyspnea and paroxysmal nocturnal dyspnea for 1 year. He also had periorbital purpura during this time and had lost 10 pounds. The liver was palpable 3 cm below the right costal margin, and the Tinel sign was positive. He had a small monoclonal λ light chain in the serum and urine (300 mg/24 h). The bone marrow contained 20% plasma cells. A rectal biopsy was positive for amyloid. He was treated with melphalan and prednisone, and the λ light chain disappeared 13 months later. An endomyocardial biopsy was performed 6 years later because the patient was doing so well. All four specimens showed moderate amyloid deposition that was pericellular and nodular. Acute leukemia subsequently developed, and the patient died 12.5 years after diagnosis. Results of cytogenetic studies were normal on two occasions before the diagnosis of acute leukemia. In the second patient, a 64-year-old white man, exertional dyspnea and paroxysmal nocturnal dyspnea developed from congestive heart failure. Biopsies of the tongue, rectum, and pleura were positive for amyloid. The interventricular septal thickness was 13 mm. The urine contained a small monoclonal λ light chain. He was treated with melphalan and prednisone, and the λ light chains disappeared 7 months later. This therapy was discontinued at 20 months (total melphalan dosage, 766 mg). He subsequently required a pacemaker but is alive with compensated congestive heart failure 13 years after diagnosis.
Nephrotic syndrome was present in 10 patients (33%) when first evaluated and developed in 1 other patient during the course of the disease. Six patients had carpal tunnel syndrome at diagnosis, and only 2 had a sensorimotor peripheral neuropathy. Orthostatic hypotension was present in 4 patients before diagnosis.
Bone marrow biopsy was positive for amyloid in 18 (64%) of the 28 patients in whom a bone marrow was done. Four of five liver biopsies were positive for amyloid, and all four endomyocardial biopsies were positive for amyloid. Subcutaneous fat aspiration was performed in only 3 patients, and all had positive results. All seven renal biopsies were positive. Eleven (65%) of 17 rectal biopsies were positive for amyloid.
Chemotherapy.
All 30 patients received alkylating-agent therapy. One patient with an IgM monoclonal protein received chlorambucil, and another patient was given cyclophosphamide. The remaining 28 patients received melphalan and prednisone. The duration of treatment ranged from 5 to 84 months (median, 34.5 months). The total dose of melphalan ranged from 212 to 4,760 mg (median, 1,708 mg).
The monoclonal light chain disappeared from the urine in 13 patients and from the serum in 5 patients. The monoclonal protein disappeared from the serum or urine in a total of 14 patients. Of the 10 patients with nephrotic syndrome, 4 had a reduction of more than 50% in proteinuria and a normal serum creatinine value (1.1 mg/dL or less). The serum albumin value increased 1.0 g/dL or more in 2 of the 3 patients who had an initial serum albumin value less than 3.0 g/dL.
The liver in the patient with marked hepatomegaly (14 cm below the costal margin) became nonpalpable with therapy. The alkaline phosphatase value decreased from 379 to 113 U/L. One patient had a ruptured spleen at diagnosis. Two of the 30 patients had an associated multiple myeloma that required chemotherapy. In 1 patient, autopsy results showed that the amyloidosis had disappeared. This patient had presented with a nephrotic syndrome (24-hour urine protein value of 6.6 g/24 h and a serum albumin level of 1.2 g/dL), an IgG λ monoclonal protein in the serum, and renal and bone marrow biopsies positive for amyloid. At autopsy, there was no evidence of amyloid in any organ.
An acute nonlymphocytic leukemia developed in 3 patients, and 2 others had a myelodysplastic syndrome. Cytogenetic studies revealed a monosomy 7 in 3 of the 4 patients in whom studies were done.
Of 18 deaths, the causes were cardiac in 9, infection in 3, renal insufficiency in 2 (both patients voluntarily stopped dialysis), and 1 each from gastrointestinal bleeding, multiple myeloma and renal failure, ventricular fibrillation (no evidence of amyloid at autopsy), and an unrelated ruptured subclavian artery. Myelodysplasia or leukemia contributed to death in 3 patients. Twelve patients are still alive and under observation at 10 to 15.5 years. One patient had renal transplantation and was doing well 7 years later (serum creatinine value was 1.3 mg/dL and 24-hour urine protein value was 83 mg).
Comparison of long-term survivors with nonsurvivors.
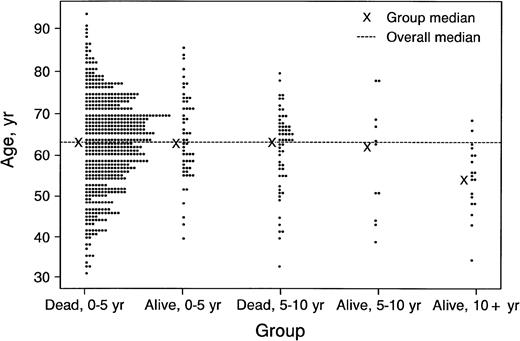
Of the 685 patients seen at Mayo Clinic within 6 months of diagnosis, 599 died before 10 years; these patients were compared with the overall group of 30 patients who survived 10 years or more after diagnosis and with the 19 who were seen within 6 months of diagnosis and survived 10 years or more (Table 1). By logistic regression, the most significant predictors of long-term survival were young age (Fig 2) and initial platelet count. Comparison of the 19 patients seen within 6 months of diagnosis who survived 10 years or more and the overall group of 30 patients who survived 10 years or more revealed no significant differences. Congestive heart failure, older age, creatinine value of 2 mg/dL or more, bone marrow plasma cell value of 20% or more, platelet value of 500 × 109/L or less, and the presence of peripheral neuropathy were underrepresented in the 10-year survivors and are unfavorable prognostic features.
Status of 685 patients with primary systemic amyloidosis seen at Mayo Clinic within 6 months of diagnosis, by age.
Status of 685 patients with primary systemic amyloidosis seen at Mayo Clinic within 6 months of diagnosis, by age.
Response to therapy.
The 19 long-term survivors were compared with 148 patients who received melphalan and prednisone in a prospective study2(Table 3). An objective protein response was noted in 10 (53%) of the 19 patients who survived 10 years or more and in 42 (28%) of the 148 patients in the prospective study (P = .51). Although the percentages appear to differ, the comparison must be adjusted for lead-time bias. The median time of response was 258 days (8.5 months). Responders to chemotherapy at 8.5 months were not more likely to survive 10+ years than nonresponders still alive at that time. In fact, responders are more likely to survive 10+ years only by virtue of still being in the living cohort at 8.5 months. Thus, any patient still alive at 8.5 months is as likely to survive 10+ years as a responder.
Response to Therapy in Patients With Amyloidosis: A Prospective Study and Survivors for 10 Years or More
| Factor . | Melphalan + Prednisone2 (n = 148) . | 10-Yr Survivors (n = 19) . | ||
|---|---|---|---|---|
| No.3-150 . | % . | No.3-150 . | % . | |
| Serum albumin | 13/84 | 15 | 2/7 | 29 |
| Serum monoclonal protein | 21/104 | 20 | 3/10 | 30 |
| Urinary monoclonal protein | 19/99 | 19 | 9/11 | 82 |
| Urinary total protein | 12/68 | 18 | 3/7 | 43 |
| Total no. of patients with protein responses | 42/148 | 28 | 10/19 | 53 |
| Factor . | Melphalan + Prednisone2 (n = 148) . | 10-Yr Survivors (n = 19) . | ||
|---|---|---|---|---|
| No.3-150 . | % . | No.3-150 . | % . | |
| Serum albumin | 13/84 | 15 | 2/7 | 29 |
| Serum monoclonal protein | 21/104 | 20 | 3/10 | 30 |
| Urinary monoclonal protein | 19/99 | 19 | 9/11 | 82 |
| Urinary total protein | 12/68 | 18 | 3/7 | 43 |
| Total no. of patients with protein responses | 42/148 | 28 | 10/19 | 53 |
Number of patients with response to therapy/number with abnormal value. A response was defined as one or more of the following: (1) an increase of 1 g or more in the serum albumin value, given an initial value of less than 3 g/dL and stable renal function; (2) disappearance of or a reduction of at least 50% in the serum monoclonal protein concentration, given an initial value of at least 1 g/dL; (3) disappearance of or a reduction of at least 50% in the urinary monoclonal protein concentration, given an initial value of at least 0.5 g/24 h; and (4) a reduction of at least 50% in the urinary protein concentration, given an initial value of at least 3 g/24 h in the absence of progressive renal failure.
DISCUSSION
Long-term survival among patients with AL amyloidosis is uncommon. Only two case reports of long-term survival have been published. The first was a 72-year-old woman with an 18-year history of periorbital purpura. Similar lesions appeared on the chest 10 years before the diagnosis of AL amyloidosis. She had an IgG κ monoclonal protein in the serum and a bone marrow containing 8% plasma cells. A skin biopsy was positive for amyloid, and a rectal biopsy was negative. She had no evidence of heart, kidney, skeletal, or neurologic system involvement.4The second patient was a 43-year-old woman who presented in October 1981 with a 1-month history of paresthesias of her extremities. Results of neurologic examination were consistent with a polyneuropathy. She had an IgA λ monoclonal protein (1,300 mg/dL) and a bone marrow containing more than 10% plasma cells. Biopsy of the sural nerve revealed amyloid. She was treated with melphalan and prednisone for several years. The IgA level subsequently increased to 2,900 mg/dL, and the bone marrow was consistent with multiple myeloma. Another sural nerve biopsy confirmed the presence of amyloid. She died of cardiorespiratory failure 11 years after diagnosis.3
Of 841 patients with AL amyloidosis seen at Mayo Clinic from 1966 to 1987, 30 survived 10 years or more. In a comparison of the 19 patients who survived 10 years or more and 599 who did not survive for 10 years (both groups seen at Mayo Clinic within 6 months of diagnosis), we found that younger patients and those with an initial platelet count of 500 × 109/L or more were more likely to survive 10 years. Congestive heart failure, older age, creatinine value of 2 mg/dL or more, bone marrow plasma cell value of more than 20%, platelet value of 500 × 109/L or less, and presence of peripheral neuropathy were underrepresented in the 10-year survivors and appear to be unfavorable prognostic features.
The finding of a better prognosis in younger patients with AL is similar to our experience in multiple myeloma. In a series of 72 patients with multiple myeloma who were younger than 40 years, the median survival was 54 months.8 However, it is difficult to explain the possible benefit of thrombocytosis. In fact, one might expect the opposite in that thrombocytosis would be associated with a shorter survival because the increased platelet count may be due to amyloid replacement of the spleen, resulting in hyposplenism producing thrombocytosis. This finding is statistically significant but clinically insignificant. The influence of multiple myeloma cannot be ascertained because only 1 of the 30 patients had multiple myeloma at the time of diagnosis of AL. In the second patient, multiple myeloma developed 12 years after the diagnosis of AL and the patient died of myeloma and renal insufficiency 3 years later.
This experience with 30 patients does not permit one to determine whether high-dose therapy followed by stem-cell rescue is superior to conventional chemotherapy with melphalan and prednisone. The selection of patients for high-dose therapy is most critical. Unfortunately, the decision to proceed with high-dose therapy must be made before the patient has had an adequate trial of melphalan and prednisone because alkylating therapy will damage the hematopoietic stem cells. One cannot reliably identify patients who will respond to high-dose therapy.
Long-term survival in AL amyloidosis is rare. The factors affecting long-term survival are generally unknown. The response rate to conventional chemotherapy is low, and we hope that stem-cell transplantation9 or therapy with 4′-iodo-4′-deoxydoxorubicin10 will increase the number of long-term survivors. Patients with AL amyloidosis should be treated at institutions with ongoing amyloidosis trials in order to make progress in this disease.
Supported in part by Research Grant CA 62242 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Robert A. Kyle, MD, Mayo Clinic, 200 First St SW, Rochester, MN 55905.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal