Abstract
We report on a series of 26 patients diagnosed with primary (de novo) plasma cell (PC) leukemia (PCL) in whom we analyzed the clinicobiologic characteristics of the disease together with the immunophenotype, DNA cell content, proliferative index, and numeric chromosomal aberrations of the neoplastic PC, and compared them with 664 multiple myeloma (MM) patients at diagnosis. The median age, sex ratio, and bone lesion extension were similar, but PCL cases displayed a higher prevalence of clinical stage III, extramedullary involvement, and Bence Jones cases, with fewer IgA cases than for MM patients. In addition, according to several prognostic indicators (β2-microglobulin serum level, proportion of S-phase PCs, proteinuria, calcium serum level, lactate dehydrogenase [LDH] and renal function), the incidence of adverse prognostic factors was significantly higher in PCL versus MM. Immunophenotypic expression was similar for CD38, CD138, CD2, CD3, CD16, CD10, CD13, and CD15, but PCL differed from MM in the expression of CD56, CD9 HLA-DR, CD117, and CD20 antigens. Twenty-two PCL cases were diploid and one was hypodiploid, while most MM cases (57%) showed DNA hyperdiploidy. With the fluorescent in situ hydridization (FISH) technique, 12 of 13 PCL cases displayed the numeric aberrations, −13 (86%), ±1 (57%), +18 (43%), and −X in women (25%), but they lacked several numeric aberrations usually found in MM such as +3, +6, +9, +11, and +15. PCL cases had a lower overall response to therapy than MM cases (38% v 63%, P = .01332). Among PCL patients, a trend for a worse response was observed in cases treated with melphalan and prednisone (MP) versus polychemotherapy. Overall survival was significantly worse in PCL versus MM patients (8 v 36 months,P < .0001), but it was significantly better in PCL patients treated with polychemotherapy versus MP (18 v 3 months,P = .0137). By contrast, MM patients did not show significant differences in overall survival according to the treatment used, MP or polychemotherapy. Ten variables seemed to predict survival in PCL patients, but only the β2-microglobulin level and S-phase PCs retained an independent value in multivariate analysis. In summary, our study illustrates that PCs from PCL display singular phenotypic, DNA cell content, and cytogenetic characteristics that lead to a different disease evolution versus MM.
MONOCLONAL GAMMOPATHIES comprise a wide range of entities characterized by the proliferation of a clonal population of terminally differentiated B cells, plasma cells (PCs).1 When the number of circulating PCs is significant, the term plasma cell leukemia (PCL) is usually used. The French-American-British group2 has suggested that this term should be restricted to a de novo presentation in leukemic phase, but others have used it more generally.3,4 To obtain uniform criteria for the diagnosis of PCL, Kyle et al3 proposed an absolute PC count greater than 2 × 109/L, with PCs also comprising greater than 20% of peripheral blood cells, although according to others, these criteria are arbitrary.5 Due to the low frequency of this entity, most publications on PCL are based on case reports, and only two series with more than 20 patients can be found in the literature.4 6 Moreover, information about the intrinsic biology (immunophenotype, proliferative rate, and cytogenetic aberrance) of tumor cells present in primary PCL and possible differences versus myelomatous PCs is still scanty.
We now report on a series of 26 patients diagnosed with primary (de novo) PCL in whom we have analyzed the clinicobiologic characteristics of the disease together with the immunophenotype, DNA cell content, proliferative index, and numeric chromosomal aberrations of the neoplastic PCs, comparing them with 664 multiple myeloma (MM) patients at diagnosis previously reported in part.7 8 Our results show that primary PCL is associated with several biologic features different from MM and follows an aggressive course with a poor response to standard MM therapy.
SUBJECTS AND METHODS
Patients.
Between January 1982 and December 1996, we studied 26 patients with primary PCL who were registered in our laboratory among 690 consecutive untreated patients with MM (3.8%). The criteria for a diagnosis of PCL required greater than 2 × 109/L blood PCs. The diagnosis of MM was based on criteria from the Chronic Leukemia-Myeloma Task Force.9 Patients were treated according to the protocols of the Spanish cooperative group Programa Español de Tratamiento de Hemopatı́as Malignas (PETHEMA), which include melphalan and prednisone (MP) or alternating cycles of vincristine, cyclophosphamide, melphalan, and prednisone/vincristine, bleomycin, adriamycin, and prednisone (VCMP/VABP) at standard or high doses.10 Patients with primary PCL received the same treatment as contemporary MM patients. Twelve received the standard MP regimen, and the remaining 14 patients received polychemotherapy (VCMP/VBAP at standard dose, n = 5; VCMP/VBAP at high dose, n = 9).
In each patient, the most relevant clinical and laboratory disease characteristics documented at diagnosis were evaluated for biologic and prognostic significance. These included clinical features (age, sex, performance status, bone pain and lesions, hepatosplenomegaly, and plasmocytomas), hematologic parameters (hemoglobin level, white blood cell count, platelet count, and erythrocyte sedimentation rate), serum biochemical data (creatinine, urea, calcium, lactate dehydrogenase [LDH], and β2-microglobulin levels), electrophoretic characteristics (total protein, albumin, type of monoclonal [M] component, and presence of urine Ig light chains), the percentage of bone marrow (BM) PCs, and the presence or absence of bone lesions. The performance status and bone lesions were scored according to previously described criteria.10 In addition, patients were grouped into clinical stages according to the Durie and Salmon criteria.11
The response was considered to be complete, objective (OR), partial (PR), or a failure (FR) according to the standard criteria of the PETHEMA group.8 10 Patients who died before completion of the therapy were considered as early deaths. Overall survival was considered from the moment of diagnosis to the moment of death, and response duration from the moment at which the response was obtained until relapse.
Immunophenotypic studies.
Immunophenotypic characterization of BM PCs was performed as previously described.12-14 The following panel of monoclonal antibodies (MoAbs)—whose specificity has been described elsewhere12,15—were used: Leu 17 (CD38), Leu M7 (CD13), anti-CALLA (CD10), anti-HLA-DR (Ia), Leu 16 (CD20), Leu M1 (CD15), FMC56 (CD9), Leu 19 (CD56), Leu 4 (CD3), Leu 5b (CD2), Leu 11c (CD16), c-kit (CD117), and B-B4 (CD138). These MoAbs were used in triple staining, with CD38 included in all combinations for specific identification of PCs.16 Irrelevant isotype-matched mouse Igs were used as negative controls.
Analysis of cell reactivity with the different combinations of MoAbs was performed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Results were analyzed for at least 10,000 cells per test using the PAINT-A-GATE-PRO software program (Becton Dickinson). An antigen was considered positive when at least 15% of the PCs displayed reactivity for this marker. A complete immunophenotype of the PCs was available in 21 PCL and 290 MM cases.
DNA measurements.
DNA measurements were performed with previously described methods.15,17 The DNA index was calculated as the ratio of the modal channel obtained for PCs (CD38+++) and the remaining normal cells (CD38− or CD38+) present in the sample; in addition, the proportion of cells in the different cell-cycle phases for both subsets (PCs and residual normal cells) was calculated according to previously described criteria8 17 using the MODFIT software (Verity Software House, Topsham, ME) after excluding cell doublets and separately gating PCs and residual normal cells. Information about cell DNA content from PCs and normal residual hematopoietic cells was available for 22 PCL and 404 MM cases.
Analysis of numeric chromosomal aberrations.
The analysis of numeric chromosomal aberrations was performed using interphase fluorescent in situ hybridization (FISH) with probes for 15 different human chromosomes according to previously described methods.18 The following panel of probes were used for the analysis of numeric aberrations: chromosomes 1 (pUC1.77; Boehringer Mannheim, Mannheim, Germany), 3 (pAE0.68; Boehringer), 6 (D6Z1; Oncor, Gaithersburg, MD), 7 (pZ7.6B; Boehringer), 8 (pZ8.4; Boehringer), 9 (D9Z1; Oncor), 10 (CEP10; Vysis, Framingham, MA), 11 (CEP11; Vysis), 12 (D12Z3; Oncor), 15 (pMC15; Boehringer), 17 (pZ17-1.6A; Boehringer), 18 (pZXba; Boehringer), X (pDMX1; Boehringer), and Y (pHY2.1; Boehringer). In addition, a locus-specific DNA probe for the Rb gene sequence in chromosome 13 was used (LSI13; Vysis). Hybridization spots were evaluated by fluorescence microscopy, counting the hybridization spots per cell in at least 200 cells per sample. In all slides analyzed, the number of unhybridized cells in the assessed areas was less than 1%, and only spots with a similar size, intensity, and shape were counted. The mean percentage of trisomic/monosomic cells in control samples (BM cells from 20 healthy individuals) was 0% to 2% for trisomies and 0% to 5% for monosomies. A patient was considered to be carrying a numeric chromosomal abnormality when the percentage of cells displaying a proportion of events with an abnormal number of spots was higher than the mean ± 2 SD for the percentage obtained for that specific chromosome in normal controls. FISH analysis for numeric aberrations was available in 13 PCL and 56 MM patients.
Statistical methods.
To estimate the statistical significance of differences observed between mean values for PCL and MM patients for continuous variables, the Mann-Whitney U and Kruskal-Wallis tests were used with SPSS statistical software (SPSS Inc, Chicago, IL).19 The chi-square test (crosstabs; SPSS) was used for comparison of dichotomous variables between groups.19 Survival curves were plotted according to the method of Kaplan and Meier and compared using the log-rank test (survival; SPSS). The variables considered for possible inclusion in a regression analysis (Coxreg; SPSS) were those displaying a significant association with survival in the univariate analysis (P < .05) or for which prior studies suggested a possible prognostic value. The stepwise regression method was discontinued when the P value for entering an additional factor was greater than .05. The model was tested by including the variables in a continuous manner.
RESULTS
Clinical features.
Twenty-six (3.8%) of 690 patients with PC malignancies referred to our institution between 1983 and 1996 were identified as having primary PCL. The most relevant clinical features of all 26 primary PCL patients and the remaining MM patients are presented in Table 1. Upon comparing the tumor burden according to the Durie and Salmon criteria, a higher incidence of clinical stage III was found (P = .00093) in primary PCL versus MM. In the PCL group, there was a higher prevalence of Bence Jones protein cases and fewer IgA cases than in the MM group (Table 1). Although the prevalence of Bence Jones protein cases was higher in PCL, the degree of proteinuria was similar in PCL and MM (3.7 ± 4.0 v 4.3 ± 5.2 g/d, P > .05). The median age, sex ratio, and bone lesion extension were similar in both groups of patients. Extramedullary involvement was noted in 4% of MM cases and 23% (n = 6) of primary PCL cases (P < .05). The six cases of extramedullary involvement were subcutaneous nodes (n = 3), peritoneal plasmacytoma, meningeal infiltration, and parapleural mass. In addition, according to several prognostic indicators such as the β2-microglobulin serum level, proportion of S-phase PCs, proteinuria, calcium serum level, LDH serum level, and renal function, the incidence of adverse prognostic features was significantly higher in PCL versus MM (P < .01). Residual BM function was poorer in PCL cases, as assessed by both the hemoglobin level and platelet count (Table1), as well as the percentage of normal residual BM cells in S phase (see Table 3).
Clinical and Biologic Data for the MM and Primary PCL Cases
| Parameter . | MM (n = 664) . | PCL (n = 26) . | P . |
|---|---|---|---|
| Age ≥65 years (median) | 65.0% (68 ± 10) | 50.0% (66 ± 10) | .12216 |
| Male | 53% | 46% | .49355 |
| ECOG ≥2 | 33% | 58% | .01993 |
| Extramedullary involvement | 4% | 23% | .01407 |
| Bone scale ≥2 | 67% | 48% | .17767 |
| Hemoglobin <8.5 g/dL | 31% (10.1 ± 2.7) | 54% (8.2 ± 2.3) | .01451 |
| Platelets <100 × 109/L | 9% (209 ± 83) | 48% (123 ± 88) | <.00001 |
| Stage III | 59% | 92% | .00093 |
| BM PCs ≥40% | 43% | 92% | <.00001 |
| LDH ≥460 U/L | 9% | 48% | <.00001 |
| Albumin <3.5 g/dL | 46% | 52% | .58077 |
| Type of monoclonal component | |||
| IgG | 55% | 54% | .00027 |
| IgA | 30% | 4% | |
| IgD | 1% | 8% | |
| Bence Jones | 13% | 31% | |
| Nonsecretory | 1% | 4% | |
| Monoclonal proteinuria | 40% | 68% | .00068 |
| Creatinine ≥2 mg/dL | 21% | 44% | .00634 |
| Calcium ≥11.0 mg/dL | 20% | 48% | .00071 |
| β2-Microglobulin ≥6 mg/mL | 27% | 65% | .00012 |
| C-reactive protein ≤6 mg/dL | 18% | 61% | .00015 |
| S-phase PCs ≥3% | 32% | 71% | <.00001 |
| S-phase residual BM cells <4.5% | 26% | 83% | <.00001 |
| Global response | 63% | 38% | .01330 |
| OR and complete response | 40% | 29% | .27427 |
| Parameter . | MM (n = 664) . | PCL (n = 26) . | P . |
|---|---|---|---|
| Age ≥65 years (median) | 65.0% (68 ± 10) | 50.0% (66 ± 10) | .12216 |
| Male | 53% | 46% | .49355 |
| ECOG ≥2 | 33% | 58% | .01993 |
| Extramedullary involvement | 4% | 23% | .01407 |
| Bone scale ≥2 | 67% | 48% | .17767 |
| Hemoglobin <8.5 g/dL | 31% (10.1 ± 2.7) | 54% (8.2 ± 2.3) | .01451 |
| Platelets <100 × 109/L | 9% (209 ± 83) | 48% (123 ± 88) | <.00001 |
| Stage III | 59% | 92% | .00093 |
| BM PCs ≥40% | 43% | 92% | <.00001 |
| LDH ≥460 U/L | 9% | 48% | <.00001 |
| Albumin <3.5 g/dL | 46% | 52% | .58077 |
| Type of monoclonal component | |||
| IgG | 55% | 54% | .00027 |
| IgA | 30% | 4% | |
| IgD | 1% | 8% | |
| Bence Jones | 13% | 31% | |
| Nonsecretory | 1% | 4% | |
| Monoclonal proteinuria | 40% | 68% | .00068 |
| Creatinine ≥2 mg/dL | 21% | 44% | .00634 |
| Calcium ≥11.0 mg/dL | 20% | 48% | .00071 |
| β2-Microglobulin ≥6 mg/mL | 27% | 65% | .00012 |
| C-reactive protein ≤6 mg/dL | 18% | 61% | .00015 |
| S-phase PCs ≥3% | 32% | 71% | <.00001 |
| S-phase residual BM cells <4.5% | 26% | 83% | <.00001 |
| Global response | 63% | 38% | .01330 |
| OR and complete response | 40% | 29% | .27427 |
Immunophenotypic characteristics and DNA cell content.
The immunophenotypic characteristics of PCs from both PCL and MM cases are listed in Table 2. CD38 and CD138 antigens were excellent PC markers in both groups of patients, while CD2, CD3, and CD16 were consistently negative in all cases. In addition, the frequency of CD10+, CD13+, and CD15+ cases was similar in both groups. By contrast, statistically significant differences were observed between PCL and MM for the expression of CD20, CD56, CD9, CD117, and HLA-DR antigens: the CD20 antigen displayed higher reactivity in PCL, whereas the other four antigens were more frequently present in MM. These findings indicate that although PCL has a characteristic immunophenotype that differs from the pattern for MM, there is some overlap in antigenic expression.
Immunophenotypical Results in MM and Primary PCL
| Immunophenotype . | MM . | PCL . | P . |
|---|---|---|---|
| BB4+ | 99% | 100% | .90482 |
| CD10+ | 6% | 6% | .72808 |
| CD13+ | 31% | 23% | .40456 |
| CD15+ | 7% | 8% | .60321 |
| CD20+ | 17% | 50% | .00139 |
| CD38+ | 100% | 100% | — |
| CD56+ | 70% | 45% | .02217 |
| CD9+ | 78% | 46% | .01984 |
| CD117+ | 43% | 0% | .03646 |
| DR+ | 56% | 21% | .01549 |
| Immunophenotype . | MM . | PCL . | P . |
|---|---|---|---|
| BB4+ | 99% | 100% | .90482 |
| CD10+ | 6% | 6% | .72808 |
| CD13+ | 31% | 23% | .40456 |
| CD15+ | 7% | 8% | .60321 |
| CD20+ | 17% | 50% | .00139 |
| CD38+ | 100% | 100% | — |
| CD56+ | 70% | 45% | .02217 |
| CD9+ | 78% | 46% | .01984 |
| CD117+ | 43% | 0% | .03646 |
| DR+ | 56% | 21% | .01549 |
All except one PCL cases analyzed were diploid (DNA index, 1), with the remaining case displaying a DNA index less than 1. In contrast, most MM cases (57%) showed a DNA index greater than 1 (Table 3). It should be noted that in one PCL case, two PC subpopulations were found, one diploid (DNA index, 1) and the other tetraploid (DNA index, 2). The distribution of cells along the cell cycle was also different between PCL and MM, with the former showing a higher percentage of S-phase PCs and a lower percentage of S-phase residual normal cells (Table3).
S-Phase Cells and DNA Ploidy by Flow Cytometry in MM and Primary PCL
| Parameter . | MM (n = 404) . | PCL (n = 22) . | P . |
|---|---|---|---|
| S-phase CD38+++ (PCs) | 2.9% | 4.5% | .0038 |
| S-phase CD38−/+ (residual normal cells) | 7.4% | 2.7% | <.0001 |
| DNA index | |||
| <1 | 1.5% | 4.8% | |
| =1 | 42.5% | 95.2% | <.0001 |
| >1 | 56.0% | 0% |
| Parameter . | MM (n = 404) . | PCL (n = 22) . | P . |
|---|---|---|---|
| S-phase CD38+++ (PCs) | 2.9% | 4.5% | .0038 |
| S-phase CD38−/+ (residual normal cells) | 7.4% | 2.7% | <.0001 |
| DNA index | |||
| <1 | 1.5% | 4.8% | |
| =1 | 42.5% | 95.2% | <.0001 |
| >1 | 56.0% | 0% |
Numeric chromosomal aberrations.
Although only one of 13 PCL cases in which FISH studies were available showed an abnormal DNA cell content by flow cytometry (DNA index, 0.88), FISH analysis revealed that 12 cases displayed numeric aberrations (Table 4). In these cases with a DNA index of 1, the abnormalities were not detected by flow cytometry, due to the low sensitivity of the technique for detection of balanced chromosomal gains and losses (eg, coexistence of one trisomy and one monosomy) or single numeric chromosomal abnormalities (monosomy 13 and trisomy 18). The specific chromosomal abnormalities detected were monosomy 13 (85% of cases), chromosome 1 changes (57%), trisomy 18 (43%), and monosomy X in women (25%). In MM cases, a higher frequency of numeric abnormalities were detected, most corresponding to trisomies 1, 6, 9, 11, and 15. Statistically significant differences between PCL and MM were observed for the following chromosomal aberrations: −13 (26% in MM and 84% in PCL, P = .00038), +9 (0% in PCL and 52% in MM, P = .00835), and +6 (0% in PCL and 32% in MM, P = .04231). Conventional cytogenetic analysis was available in only three PCL patients, and no discrepancies were observed versus the FISH analysis.
FISH Results Using Centromeric Probes in 13 Primary PCL Cases and 56 MM Cases
| Chromosome . | PCL Patients (n = 13) . | MM Patients (n = 56) . | ||
|---|---|---|---|---|
| Monosomy . | Trisomy . | Monosomy . | Trisomy . | |
| 1 | 14% | 43% | 3% | 37% |
| 3 | 0% | 0% | 0% | 31% |
| 64-150 | 0% | 0% | 0% | 32% |
| 7 | 0% | 0% | 0% | 28% |
| 8 | 0% | 0% | 5% | 8% |
| 94-150 | 0% | 0% | 0% | 52% |
| 10 | 0% | 0% | 0% | 8% |
| 11 | 0% | 0% | 3% | 33% |
| 12 | 0% | 0% | 0% | 3% |
| 134-150 | 86% | 0% | 26% | 0% |
| 15 | 0% | 0% | 0% | 48% |
| 17 | 0% | 0% | 0% | 22% |
| 18 | 0% | 43% | 6% | 24% |
| X | 25%4-151 | 0% | 32%4-151 | 6%‡ |
| Y | 0% | 0% | 0% | 0% |
| Chromosome . | PCL Patients (n = 13) . | MM Patients (n = 56) . | ||
|---|---|---|---|---|
| Monosomy . | Trisomy . | Monosomy . | Trisomy . | |
| 1 | 14% | 43% | 3% | 37% |
| 3 | 0% | 0% | 0% | 31% |
| 64-150 | 0% | 0% | 0% | 32% |
| 7 | 0% | 0% | 0% | 28% |
| 8 | 0% | 0% | 5% | 8% |
| 94-150 | 0% | 0% | 0% | 52% |
| 10 | 0% | 0% | 0% | 8% |
| 11 | 0% | 0% | 3% | 33% |
| 12 | 0% | 0% | 0% | 3% |
| 134-150 | 86% | 0% | 26% | 0% |
| 15 | 0% | 0% | 0% | 48% |
| 17 | 0% | 0% | 0% | 22% |
| 18 | 0% | 43% | 6% | 24% |
| X | 25%4-151 | 0% | 32%4-151 | 6%‡ |
| Y | 0% | 0% | 0% | 0% |
Results are expressed as a percentage of trisomic/monosomic cases for MM patients.
Chromosomes in which the incidence of numeric aberrations was different between PCL and MM (P < .05).
Calculated only in females (0% in males).
Calculated only in males (0% in females).
The PCL patient in whom two PC subsets with different DNA content were identified had a complete FISH study, and two subpopulations of clonal PCs were also found, one displaying tetrasomy for all chromosomes analyzed and another one in which monosomy 13 and trisomy 18 were detected.
Response to treatment and outcome.
Within the PCL group, 29% of cases achieved an OR to treatment and 8% a PR, while 50% showed progressive disease and 13% died before the response could be evaluated. In contrast, in MM patients, there was a complete response (negative immunofixation) in 4%, OR in 37%, PR in 22%, and stable disease in 9%, with only 13% of patients displaying progressive disease. The remaining MM patients died before the response was evaluated. Overall, patients with primary PCL achieved a significantly lower response rate than patients with MM (38% v63%, P = .01). Nevertheless, the frequency of complete response plus OR was not significantly different between the two groups of patients (41% v 29%, P > .05). Among primary PCL patients, the response tended to be worse in cases treated with MP (17% of responses OR + PR, with only 8% OR) versus cases that received polychemotherapy (OR + PR, 50%; OR, 47%), although these differences did not reach statistical significance, probably due to the low number of patients.
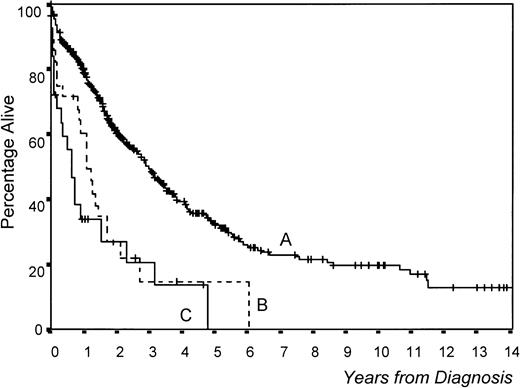
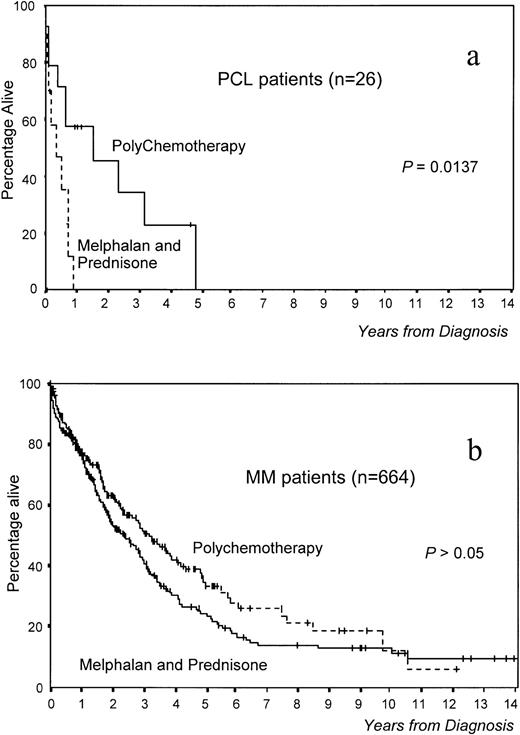
Overall survival was significantly worse in PCL versus MM patients (median survival, 8 v 36 months, respectively,P < .0001; Fig 1). Interestingly, survival was significantly better in patients with primary PCL treated with polychemotherapy versus MP (18 v 3 months, P = .0137; Fig 2a). In MM, although survival was also better with polychemotherapy, the differences were not statistically significant (Fig 2b). However, this difference in therapeutic results between MM and PCL patients could be due to the existence of different prognostic features within both groups. To assess this aspect, we selected a group of 28 MM patients matched with patients from the PCL group (high percentage of PCs in S phase, high β2-microglobulin, anemia, hypercalcemia, and stage III). No differences in survival were observed between these two groups of patients (Fig 1). Moreover, in analyzing the influence of the type of treatment in 28 poor-prognosis MM patients (MP, n = 11; polychemotherapy, n = 17), it was found that the percentage of ORs was also higher in those treated with polychemotherapy (56%) versus the MP group (9%, P < .05), although this does not translate into a significantly different survival.
Survival differences between MM and primary PCL: (A) 664 MM patients (mean survival, 36 months), (B) 28 MM patients with poor prognostic features (S-phase PCs >3%, β2-microglobulin >6 mg/mL, and stage III) (mean survival, 13 months), and (C) 26 PCL patients (mean survival, 8 months). 664 MM versus 26 PCL, P < .0001; 28 poor-prognosis MM versus 26 PCL, P = .2989.
Survival differences between MM and primary PCL: (A) 664 MM patients (mean survival, 36 months), (B) 28 MM patients with poor prognostic features (S-phase PCs >3%, β2-microglobulin >6 mg/mL, and stage III) (mean survival, 13 months), and (C) 26 PCL patients (mean survival, 8 months). 664 MM versus 26 PCL, P < .0001; 28 poor-prognosis MM versus 26 PCL, P = .2989.
Survival according to treatment in (a) 26 primary PCL patients and (b) 664 MM patients.
Survival according to treatment in (a) 26 primary PCL patients and (b) 664 MM patients.
The response duration was slightly shorter in PCL versus MM patients (9v 20 months, P = .0613). Due to the low number of patients with primary PCL who achieved a response with MP (n = 2), it was not possible to compare its duration with the duration observed in primary PCL patients treated with polychemotherapy.
Only one PCL patient received intensive therapy followed by stem cell transplantation as first-line therapy. She was 45 years old and achieved an OR after six courses of VCMP/VBAP, and then she received high-dose (200 mg/m2) melphalan followed by autologous stem cell transplantation. At the time of the data collection, this patient was alive and free from progression 18 months after diagnosis.
Analysis of prognostic factors.
Ten variables were identified as having an unfavorable prognostic influence (P < .05) on the survival of primary PCL cases (serum β2-microglobulin ≥6 mg/L, S-phase BM PCs ≥4.5%, ECOG ≥2, serum LDH ≥460 U/L, serum creatinine ≥2 mg/mL, calcemia ≥11.0 mg/mL, serum C-reactive protein ≥6 mg/dL, platelet count ≥100 × 109/L, MP therapy, and absolute peripheral blood PC count ≥4 × 109/L). Cox regression in 21 primary PCL patients showed that the β2-microglobulin serum level and percentage of S-phase PCs were the only parameters with independent prognostic value for predicting the outcome in these patients (Table 5).
Analysis of Prognostic Factors for Overall Survival in PCL Patients
| Variable . | No. of Patients . | Median Survival (d) . | Univariate P . | Multivariate P . |
|---|---|---|---|---|
| β2-Microglobulin | ||||
| <6 mg/L | 8 | 560 | .0252 | .0037 |
| ≥6 mg/L | 15 | 138 | ||
| S-phase plasma cells | ||||
| <4.5% | 12 | 266 | .0459 | .0200 |
| ≥4.5% | 9 | 184 | ||
| ECOG scale | ||||
| <2 | 11 | 1,157 | .0014 | NS |
| ≥2 | 15 | 138 | ||
| Protein C reactivity | ||||
| <6 mg/dL | 7 | 1,157 | .0051 | NS |
| ≥6 mg/dL | 11 | 62 | ||
| Platelets | ||||
| <100 × 109/L | 12 | 116 | .0014 | NS |
| ≤100 × 109/L | 13 | 560 | ||
| Therapy | ||||
| Polychemotherapy | 14 | 560 | .0137 | NS |
| MP | 12 | 116 |
| Variable . | No. of Patients . | Median Survival (d) . | Univariate P . | Multivariate P . |
|---|---|---|---|---|
| β2-Microglobulin | ||||
| <6 mg/L | 8 | 560 | .0252 | .0037 |
| ≥6 mg/L | 15 | 138 | ||
| S-phase plasma cells | ||||
| <4.5% | 12 | 266 | .0459 | .0200 |
| ≥4.5% | 9 | 184 | ||
| ECOG scale | ||||
| <2 | 11 | 1,157 | .0014 | NS |
| ≥2 | 15 | 138 | ||
| Protein C reactivity | ||||
| <6 mg/dL | 7 | 1,157 | .0051 | NS |
| ≥6 mg/dL | 11 | 62 | ||
| Platelets | ||||
| <100 × 109/L | 12 | 116 | .0014 | NS |
| ≤100 × 109/L | 13 | 560 | ||
| Therapy | ||||
| Polychemotherapy | 14 | 560 | .0137 | NS |
| MP | 12 | 116 |
DISCUSSION
The data presented in this report show that patients with primary PCL display a wide range of clinical and biologic differences compared with MM patients, some of which concern the intrinsic characteristics (immunophenotype, DNA cell content, and cytogenetics) of PCs and are probably responsible for the variability in the treatment response and the clinical behavior pattern.
PCs from PCL displayed a more immature phenotype than MM as assessed by the expression of the CD20 antigen, which is usually absent in MM.20 In addition, PCs from PCL frequently lacked the CD56 antigen, which has been considered to have an important role in anchoring PCs to the BM stroma.21,22 Nevertheless, the phenotypic differences do not allow a complete discrimination between PCL and MM. The phenotypic characteristics could also help to explain the differences in survival, since CD56 antigen expression has been associated with a good prognosis21 while the CD20 antigen has been associated with a shorter survival.7
To the best of our knowledge, only a few cases have been reported with data for DNA cell content.17,23 Our study shows that all PCL patients have a DNA index of 1 or less. This clinical picture is completely different from that found in MM, which usually displays hyperdiploidy—DNA index greater than 1.1 Moreover, MM patients with a DNA index of 1 or less usually have a poor prognosis.17,24,25 With the same laboratory approach used to assess DNA cell content, the distribution of PCs along the different cell-cycle phases can also be measured,15 and clonal PCs from primary PCL cases displayed a higher proliferative capacity (S-phase cells) versus MM. There are no other reports in which the proliferative rate of PCs from PCL has been analyzed, but our observation would explain why previous reports showed that PCL is frequently associated with high serum LDH and aggressive behavior.4 6 In addition, we have also found in PCL that the proliferation of normal BM cells (residual cells in S phase) is markedly blunted. This could explain why the degree of anemia and thrombocytopenia is much higher in PCL versus MM, which would be difficult to explain based only on the tumor burden.
We have detected a very high incidence of chromosome 13 monosomies (85%) in PCL, in contrast to the low incidence observed in MM (26%). This chromosomal abnormality has been associated with a short survival in MM treated with either conventional chemotherapy26 or high-dose therapy.27 In this PCL series, trisomies of chromosomes 6 and 9 were absent, whereas they were frequent in MM cases, with statistically significant differences. Other chromosomal aberrations repeatedly found in MM, like trisomies for many chromosomes (3, 7, 11, 15, and 17),18,28-30 were not present in our PCL cases. Interestingly, some of these trisomies have been found to be associated with a good prognosis in MM, such as trisomies 6, 9, and 17.26 Dimopoulos et al6 have reported nine PCL cases in which conventional cytogenetic analysis was available, and showed similar data for the presence of monosomy 13 (45% in nine cases), numeric chromosome 1 changes (45%), and +18 (22%). However, conventional cytogenetics showed that, apart from these results, many other chromosomal aberrations can be observed in PCL cases that form highly complex karyotypes.
The clinical data observed in our series are concordant with previous reports3,31-33 showing that primary PCL patients usually have more extramedullary disease, anemia, thrombocytopenia, hypercalcemia, increased LDH and β2-microglobulin serum levels, and impaired renal function. These findings can be easily explained not only by the presence at presentation of more extensive disease in primary PCL versus MM, but also by the presence of a high proliferative ratio of neoplastic cells and adverse cytogenetic data. All of these data represent a unique array of adverse prognostic factors that explain the poor outcome generally described for patients with primary PCL. An additional observation in concordance with previous reports4 6 is the poor response to MP compared with polychemotherapy. Although such a difference has not been observed in MM, it should be noted that in MM, treatment comparisons have generally not been restricted to a cohort of patients with such adverse prognostic features. In the present study, we selected a group of MM patients with prognostic features matched to the group of PCL patients, but the therapeutic results in the former group did not differ according to the treatment administered. These findings indicate that upon comparing different treatment approaches, PCL patients seem to display a real difference in chemosensitivity compared with MM patients.
In summary, our study illustrates that PCs from PCL display a singular phenotype, a DNA cell content and cytogenetic characteristics that are responsible for a different disease evolution versus MM. In addition, our data confirm that primary PCL requires not only different clinical management but also different treatment.
ACKNOWLEDGMENT
The authors thank Mark Anderson for technical assistance.
Supported in part by grants from the Spanish Fondo de Investigaciones Sanitarias de la Seguridad Social (FIS-SS 96/1233), Dirección General de Investigación Cientı́fica y Tecnológica (DGICYT PB93-0614), Areces Foundation (1997), Dirección General de Enseñanza Superior (DGES PM97-0161), and a grant from the LAIR Foundation (1998 to J.A.P.-S.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Prof J.F. San Miguel, MD, PhD, Department of Hematology, University Hospital of Salamanca, Paseo de San Vicente, 58-182, Salamanca, 37007 Spain; e-mail: <sanmigiz@gugu.usal.es>

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal