To the Editor:
Major intrinsic protein (MIP) is a highly conserved superfamily of membrane proteins that regulate, in eukaryotes and procaryotes, the transport of various small molecules.1,2 In all biological systems, water transport is a critical mechanism. Water crosses membranes by two pathways: either by diffusion or water-selective channels. Aquaporins (AQP) are members of the MIP superfamily that control osmolarity by limiting or promoting waterflow across cellular membranes. Nine distinct aquaporins have been cloned in mammals. Aquaporin monomer is a six-transmembrane domain protein of 28 kD. Both N- and C-terminal ends are located inside the cell.3-5
The first water channel (AQP1) was discovered on the red blood cell membrane.6 AQP1 is also expressed in numerous tissues, particularly in kidney in proximal tubule and descending limb of Henle epithelia and in vasa recta endothelia. Disruption of the AQP1 expression in mice showed only a mild growth retardation, and these mice are lethargic.7 Studies of renal functions indicated that AQP1 is required for the formation of a concentrated urine. The Colton blood group antigens (Coa/Cob) represent a polymorphism on this protein resulting from an alanine to valine change at residue 45 in the extracellular loop A.8The Colton-null or Co(a−b−) blood group corresponds to a lack of AQP1 expression. The frequency of this phenotype is extremely low and these patients appear clinically normal.9 10 Here we report a new example of a Co-null individual.
The patient (white female, born 1938) was first admitted in 1995 to the hospital with anemia (hemoglobin level, 7.8 g/dL) and rectal bleeding caused by hemorrhoids. Colon cancer was ruled out. She was not transfused and received only iron support. A potent antibody reacting with all cells from several panels and identified as anti-Co3 was discovered in her serum and she was shown to belong to the Co-null phenotype. In 1996, cararact surgery was performed. In February 1997, the patient was again admitted because of vaginal and rectal bleeding and a hemoglobin level of 4.2 g/dL. The patient was submitted to hemorrhoidectomy and hysterectomy with oophorectomy. A uterine high-grade uncommon sarcoma with ovarian metastasis was found. Although radiotherapy was performed, there was evolution of the gynecological disease and the patient had to be transfused with incompatible Co(a+b−) because of the shortage of Co-null blood. The direct antiglobulin test became positive and mild hemolytic reactions occurred. No renal impairment nor disseminated intravascular coagulation occurred. The patient died in December 1997.
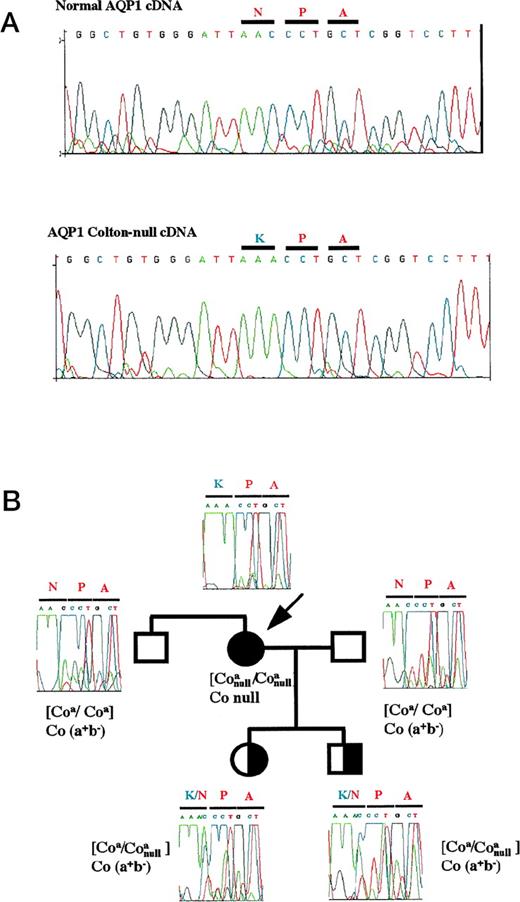
The AQP1 cDNA from this patient was amplified by reverse transcriptase-polymerase chain reaction (RT-PCR) and the PCR product was directly sequenced. The patient was homozygous for the Coa allele (C at nucleotide 134). In addition, the comparison with the normal AQP1 cDNA sequence showed a homozygous C to A transition at nucleotide 614 (AQP1 sequence6: HUMCHIP28A from Genbank data). This mutation affects the codon 192, resulting in an N192K amino acid substitution (Fig 1A).
Identification and inheritance of the Co-null mutation. (A) Direct sequence analysis of normal AQP1 (top) and AQP1 Co-null (bottom) cDNAs displaying a mutation in the codon 192 of NPA motif. Primers used for PCR: 5′-TCGGGCCAGGGCCTGGGCAT-3′ (sense, nucleotides 441 to 460) and 5′-CTACAGTATGGATTTCACCC-3′ (anti-sense, 911 to 930). (B): Inheritance of the Co-null mutation in the propositus (arrow) family. Genomic DNA from blood cells of the different members of the Colton-null family were isolated and direct sequence analysis was performed with the primers: 5′-CCCCTCCCTCTGTTTCTTTCCC-3′ (sense, intron B) and 5′-CTACAGTATGGATTTCACCC-3′ (anti-sense, 911 to 930 exon 4).
Identification and inheritance of the Co-null mutation. (A) Direct sequence analysis of normal AQP1 (top) and AQP1 Co-null (bottom) cDNAs displaying a mutation in the codon 192 of NPA motif. Primers used for PCR: 5′-TCGGGCCAGGGCCTGGGCAT-3′ (sense, nucleotides 441 to 460) and 5′-CTACAGTATGGATTTCACCC-3′ (anti-sense, 911 to 930). (B): Inheritance of the Co-null mutation in the propositus (arrow) family. Genomic DNA from blood cells of the different members of the Colton-null family were isolated and direct sequence analysis was performed with the primers: 5′-CCCCTCCCTCTGTTTCTTTCCC-3′ (sense, intron B) and 5′-CTACAGTATGGATTTCACCC-3′ (anti-sense, 911 to 930 exon 4).
Inheritance of the Colton-null mutation was further assessed by direct PCR sequencing with genomic DNA from blood cells of two generations. This study confirmed the homozygosity of the propositus and indicated the heterozygosity of the kindred (Fig 1B). This novel mutation fits with Colton-null phenotype and should be essential for channel function: indeed, it occurred in the NPA (asparagine-proline-alanine) motif found in all MIP proteins.2 Two NPA motifs are found in each half of the protein; in the case of AQP1, the first one is found at residues 76-78 (cytoplasmic B loop) and the second at residues 192-194 (extracellular E loop) near cysteine 189, site of inhibition of osmotic water permeability by mercurials. It has been shown, in vitro, that conservative substitution inside the second NPA motif (N192D or N192Q amino acid substitutions) leads to reduced Pf, absence of oligomerization, and failure of the protein AQP1 to reach the membrane. An hourglass structural model mediated by these NPA motifs has been proposed by Agre.11 12 The presence of a mutation inside an NPA motif in a patient presenting a Colton-null phenotype shows the functional importance of this motif in vivo.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal