Abstract
Lipopolysaccharide (LPS) is a mediator of inflammation and septic shock during bacterial infection. Although monocytes and macrophages are highly responsive to LPS, the biological effects of LPS in these cell types are only partially understood. We decided, therefore, to investigate the influence of LPS on macrophage pinocytosis and Fc receptor–mediated endocytosis, two prominent and related macrophage effector functions. We observed that LPS did not greatly influence endocytosis in either macrophages or monocytes, but did exert a dual action on pinocytosis: at lower concentrations (0.1 to 100 ng/mL), LPS caused a decrease in pinocytosis in both macrophages and monocytes, whereas at higher LPS concentrations, enhanced pinocytosis in macrophages was observed. Detoxified LPS was two orders of magnitude less potent in producing these effects. After inhibition of the LPS receptor CD14, the LPS-induced decrease in pinocytosis was absent, and stimulation of pinocytosis at lower LPS concentrations was unmasked. We conclude that LPS can influence pinocytosis via CD14-dependent and CD14-independent signaling pathways. Furthermore, as addition of LPS to macrophages effected pinocytosis but not Fc receptor–mediated endocytosis, these two processes are independently regulated in macrophages.
LIPOPOLYSACCHARIDE (LPS) is composed of O-specific polysaccharide side chains attached to a basal core oligosaccharide, which in turn is covalently bound to a lipid moiety known as lipid A.1 LPS is a component of the outer lipid bilayer of gram-negative bacteria and is an important inducer of host defense during bacterial infection. After its release from bacteria, LPS binds to a specific binding protein, known as LPS-Binding Protein (LBP).2,3 LBP transfers monomeric LPS to CD14, a protein from which both a soluble and membrane-bound form exist.4 The membrane-bound form of CD14, which is linked to the membrane by a glycosylphosphatidylinositol anchor, is highly expressed in phagocytes. This protein serves as a cellular receptor for LPS and mediates the LPS-induced cellular changes important for enhanced host defense, although the means by which binding of the LPS/LBP complex to CD14 results in the transfer of a signal across the cell membrane is still unclear. Further signaling includes activation of NF-κB, p38 MAP kinase, and stress-activated protein kinases, finally resulting in increased production of inflammatory cytokines (eg, tumor necrosis factor [TNF], interleukin-1β, interleukin-6, granulocyte-macrophage colony stimulating factor) and chemokines (eg, interleukin-8, macrophage inflammatory peptide-1α), generation of nitric oxide, and upregulation of surface antigen presentation.5 LPS is, therefore, an important regulator of monocyte and macrophage function in pathophysiology.
Among the most characteristic properties of monocytes and macrophages is the capacity of these cells to take up large volumes by fluid-phase pinocytosis and to ingest microbes and other particles by phagocytosis. Phagocytosis and pinocytosis require similar changes in the actin cytoskeleton, and both processes are sensitive to certain dominant negative mutants of the small ras-like GTPases of the Rho family6-8 and are blocked by inhibitors of phosphoinositide 3-kinase activity.9 It is assumed, therefore, that both processes are closely related with respect to the underlying molecular mechanism. Fluid phase pinocytosis by macrophages is a constitutive process, but may be greatly enhanced by phorbol esters or macrophage colony stimulating factor.10 Recently, we showed that TNF has a similar activity also.10a Phagocytosis can be initiated directly by direct binding of gram-negative bacteria to monocyte CD14,11 but in general, phagocytosis is initiated by binding of C3b or IgG to the appropriate receptors on the phagocyte surface. Some insight into the signal transduction mechanism leading from the activated Fc receptor to stimulation of phagocytosis was recently obtained by the observation that mouse macrophages lacking a functional copy of the gene coding for the Syk tyrosine kinase are deficient in Fc receptor–dependent phagocytosis.12Nevertheless, interaction between the endocytotic machinery and receptor signal transduction is poorly understood. Also, the possible modulation by LPS of TNF- and phorbol ester–stimulated pinocytosis or Fc receptor–stimulated endocytosis is unknown.
The above-mentioned considerations, as well as the general importance of LPS-dependent signaling in infection and immunity, prompted us to study the effects of LPS on pinocytosis and endocytosis in mouse macrophages and human peripheral blood monocytes. Use was made of the 4-4 clone of VN11 retrovirus–immortalized macrophages, recently generated in our laboratory from the spleen of a C57BI/6 mouse.13 We showed earlier that these cells display expression of the mature macrophage markers Mac-1 (CD11b), Mac-2, BM-8, F4-80, the transferrin receptor CD71, and the adhesion molecule CD18, whereas the immature macrophage marker ER-MP58 is not expressed.13 Furthermore, the cells show constitutive expression of the costimulatory ligands B7-1 (CD80) and B7-2 (CD86), and treatment of these cells with interferon γ readily induced strong expression of major histocompatibility class II (I-A) molecules.13 Functionally, these cells adhered strongly to plastic or glass surfaces, and antigen loading of these macrophages induced a proliferative T-cell response in vitro. Importantly, reintroduction of antigen-loaded 4-4 cells in syngeneic mice induced a primary T-cell response to the antigen and was not accompanied by myeloid leukemia.13 Apparently, apart from the capacity of the 4-4 clone to be maintained in vitro, these cells are phenotypically and functionally not different from primary isolated mature macrophages. Further analysis showed that these cells display high expression of the Fc γ receptor II (CD32) and that this receptor is coupled to the endocytotic machinery as 4-4 cells efficiently phagocytosed IgG-coated fluorescent microspheres and sheep erythrocytes.13 Finally, the cells expressed high levels of CD14, and addition of LPS to these cells induces secretion of interleukin-1, interleukin-6, interleukin-12, and TNF.13 We concluded that the 4-4 cells form a suitable model system to study the effects of LPS and decided to use these cells for investigating LPS action on macrophage phagocytosis and pinocytosis. In addition, we employed peripheral blood monocytes, isolated from healthy volunteers, as we have recently shown that these cells are highly responsive to LPS.
In this study, we show that LPS inhibits fluid phase pinocytosis at lower concentrations, but at higher concentrations stimulates pinocytosis. Interestingly, inhibition of CD14 with a specific antibody blocked the inhibitory influence of LPS on pinocytosis, and under these conditions, LPS-dependent stimulation of pinocytosis at lower concentrations became apparent. Finally, LPS did not effect Fc receptor–mediated phagocytosis. Therefore, these results not only identify CD14-dependent and CD14-independent effects on macrophage fluid phase pinocytosis, but also show that pinocytosis and Fc receptor–mediated endocytosis can be dissociated.
MATERIALS AND METHODS
Cell culture.
Isolation of the 4-4 clone of mouse macrophages has been described earlier in detail.13 For routine culture, cells were grown in RPMI 1640 (Life Technologies, Paisley, UK) and were supplemented with 7.5% fetal calf serum, 2 mmol/L L-glutamine, 100 U/mL penicilin, 100 μg/mL streptomycin, 1 mmol/L sodium pyruvate, and 40 μmol/L β-mercaptoethanol. Cells were seeded on six well dishes 48 hours before experimentation. Peripheral blood mononuclear cells were isolated from heparinized blood of healthy volunteers using Ficoll-Hypaque (Sigma Chemical Co, St Louis, MO) density gradient centrifugation (400g, 20 minutes). Subsequently, the cells residing at the interface were washed three times and resuspended at a concentration of 106 cells/mL in RPMI 1640 supplemented with 2% pooled human serum. After washing, cells were allowed to recover 30 minutes at 37°C, 5% CO2, before the onset of experimentation. The healthy status of the volunteers was confirmed by analysis of blood sedimentation.
[3H]Sucrose uptake.
If appropriate, cells were preincubated with a CD14-blocking antibody (Pharmingen, San Diego, CA) or irrelevant antibody (SUK5 antibody, directed against the tail domain of kinesin heavy chain; a kind gift of Dr K. de Vos, Flanders Institute for Biotechnology, Ghent, Belgium) for 15 minutes. For determining fluid phase uptake, the culture medium was supplemented with 1 μCi [3H]-sucrose (Amersham, Arlington Heights, IL) and the appropriate stimulus. Unless stated otherwise, the uptake of [3H]-sucrose was allowed to continue for 45 minutes at 37°C and 5% CO2. Subsequently, the cells were placed on ice, and the cells were washed six times with ice-cold phosphate-buffered saline. Afterwards, the cells were dissolved in 1% sodium dodecyl sulfate (SDS), and accumulated radioactivity was determined by scintillation counting. Parallel wells were incubated with [3H]-sucrose at 4°C to determine the contribution of nonendocytosed [3H]sucrose to total radioactivity. Other parallel wells were used to determine cell number per well. Using the specific activity of the [3H]-sucrose, fluid phase uptake was calculated and was always approximately 2 × 10−14L/min/cell, which is in agreement with the values reported by others and represents approximately 1% of the total cell volume. Solvent controls were without effect.
Measurement of pinocytosis and Fc receptor–mediated endocytosis using fluorescent human catalase.
Human catalase (Sigma) was fluorescently labeled using Fluorlink (Amersham). Subsequently, cells were incubated with the appropriate stimulus and 6.6 mg/mL fluorescent catalase. For determining Fc receptor–mediated endocytosis, human catalase was preincubated with a mix of the culture supernatants of three different hybridomas producing antihuman catalase antibodies (a description of antibody generation and their characteristics will be published elsewhere). Incubation of macrophages with human catalase was allowed to continue for 30′ minutes at 37°C and 5% CO2, after which the cells were placed on ice, washed four times with ice cold phosphate-buffered saline, and subsequently harvested using 2 mmol/L EDTA. Uptake of fluorescence was then determined using flow cytometry. To distinguish uptake from binding, parallel experiments were performed at 4°C. Determination of the pinocytotic activity in human peripheral blood monocytes was performed by incubating 106 freshly isolated mononuclear cells with 1 mg/mL fluorescently labeled (using Fluorlink from Amersham) human serum protein (Ig for intravenous injections; obtained from the Central Laboratory for Bloodtransfusion, Amsterdam; lot number 950315H60) for 45 minutes at 37°C and 5% CO2. Subsequently, cells were put on ice, washed twice, and analyzed by fluorescence-activated cell sorting (FACS). The scatter profile was used to identify monocytes, and average fluorescence per cell was determined. Cell types not identified as monocytes by the scatter profile displayed negligible accumulation of fluorescence.
LPS detoxification.
LPS was dissolved in a 0.1 mol/L NaOH solution in 95% ethanol and incubated for a prolonged time period (approximately 2 months) at room temperature. Subsequently, the solution was adjusted to pH 7.0 with acetic acid, and the LPS was spinned down and washed with ethanol. Subsequently, LPS was reconstituted in phosphate-buffered saline. Injection of 100 ng of thus-treated LPS into actinomycin D–sensitized mice was inactive, whereas untreated LPS was lethal at this concentration.
RESULTS
LPS inhibits or stimulates fluid phase pinocytosis depending on the LPS concentration involved.
To study fluid phase pinocytosis, [3H]-sucrose was used as a probe. It seemed that addition of [3H]-sucrose to the extracellular medium of 4-4 macrophages resulted in rapid accumulation of radioactivity in the cells and that this uptake remained in the semilinear phase for at least 60 minutes (Fig1). For further experiments, an assay time of 45 minutes was used. The effects of LPS on [3H]-sucrose uptake were twofold: at lower concentrations, LPS impaired pinocytosis, maximal inhibition being reached at 10 ng/mL (Fig 2A). At LPS concentrations in excess of 10 ng/mL, pinocytosis increased again, and in cells challenged with an LPS concentration of 10 μg/mL (the highest concentration used in this study), a clear stimulation of [3H]-sucrose uptake, as compared with unstimulated cells, was noted (Fig 2A). LPS exerts, therefore, different effects on macrophage fluid phase pinocytosis, depending on the LPS concentration involved.
Pinocytosis in macrophages. Murine clone 4-4 macrophages were incubated for various time intervals with [3H]-sucrose at 37°C. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, volume uptake was calculated and expressed as femtoliter per cell.
Pinocytosis in macrophages. Murine clone 4-4 macrophages were incubated for various time intervals with [3H]-sucrose at 37°C. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, volume uptake was calculated and expressed as femtoliter per cell.
Effects of LPS on macrophage fluid phase uptake. Murine clone 4-4 macrophages were stimulated with vehicle or different concentration of LPS for 45 minutes at 37°C and 5% CO2in the presence of [3H]sucrose. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute. The results show the average and standard error of three different experiments, each experiment being the average of three independent determinations. The statistical significance of the difference between the unstimulated and stimulated value is indicated by stars, one star indicating a P value <0.05 and two stars indicating a P value <0.01 (Pvalues were obtained using an unpaired two-tailed heteroscedastic Student’s t-test on the original nine values on which the graph is based.). (A) Effects of different LPS concentrations on macrophage pinocytosis. (B) Effect of different LPS concentrations on TNF (500 U/mL)-stimulated pinocytosis. (C) Effects of LPS in the presence of the CD14-blocking antibody rmC5-3. (D) Effects of LPS in the presence of an irrelevant antibody (SUK5).
Effects of LPS on macrophage fluid phase uptake. Murine clone 4-4 macrophages were stimulated with vehicle or different concentration of LPS for 45 minutes at 37°C and 5% CO2in the presence of [3H]sucrose. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute. The results show the average and standard error of three different experiments, each experiment being the average of three independent determinations. The statistical significance of the difference between the unstimulated and stimulated value is indicated by stars, one star indicating a P value <0.05 and two stars indicating a P value <0.01 (Pvalues were obtained using an unpaired two-tailed heteroscedastic Student’s t-test on the original nine values on which the graph is based.). (A) Effects of different LPS concentrations on macrophage pinocytosis. (B) Effect of different LPS concentrations on TNF (500 U/mL)-stimulated pinocytosis. (C) Effects of LPS in the presence of the CD14-blocking antibody rmC5-3. (D) Effects of LPS in the presence of an irrelevant antibody (SUK5).
TNF-stimulated pinocytosis is inhibited by LPS.
To obtain further insight into LPS action on pinocytosis, we investigated the effect of LPS under conditions that result in stimulated pinocytosis. To this end, cells were exposed to 500 U/mL TNF. As expected, this treatment resulted in a substantial increase in pinocytosis (Fig 2B). Subsequently, the effects of LPS on this TNF-stimulated [3H]-sucrose uptake were studied. It seemed that low concentrations of LPS strongly impaired TNF-stimulated fluid phase pinocytosis, a clear effect already being noted at an LPS concentration of 100 pg/mL (Fig 2B). At an LPS concentration of 10 ng/mL LPS, uptake of radioactivity was reduced to levels below those observed in unstimulated cells, similar to those observed after stimulation of macrophages with LPS in the absence of TNF. At LPS concentrations in excess of 10 ng/mL, fluid phase pinocytosis increased again, the absolute levels of [3H]-sucrose uptake closely resembling those observed in the absence of TNF (compare Fig 2A and B). We concluded that the LPS not only inhibited constitutive pinocytosis, but also TNF-stimulated pinocytosis, and thus, that LPS effects on pinocytosis are dominant to TNF effects with respect to this process.
CD14 mediates the LPS-dependent inhibition of macrophage pinocytosis.
Most, though not all, effects of LPS on cellular function are mediated by the LPS receptor CD14. We asked, therefore, to which extent the dualistic effects of LPS on macrophage pinocytosis involve CD14. To this end, 4-4 macrophages were incubated with a saturating amount of a monoclonal antibody directed against murine CD14 and subsequently challenged with different concentrations of LPS. Under these conditions, low concentrations of LPS stimulated, rather than reduced, pinocytosis (Fig 2C). Furthermore, at high concentrations of LPS (1 to 10 μg/mL), which already substantially enhance [3H]-sucrose uptake in the absence of the antibody, the presence of the anti-CD14 antibody even further increased pinocytosis (compare Fig 2A and C). As a control experiment, we incubated the cells with an irrelevant antibody (SUK5), but this antibody did influence neither the LPS-induced inhibition of pinocytosis at lower concentrations, nor the LPS-dependent stimulation at higher concentrations (Fig 2D). Interaction of LPS with CD14 requires LBP, a protein present in serum. Therefore, we washed cells with serum-free medium and stimulated cells in such medium. Under these conditions, an LPS-induced inhibition of fluid phase uptake was not detected, and stimulation of pinocytosis was enhanced (Fig3). We concluded that the LPS-induced inhibition of pinocytosis is mediated by a CD14-dependent mechanism, whereas the LPS-induced inhibition of pinocytosis is not.
Effects of LPS on macrophage fluid phase uptake in the absence of serum. Murine clone 4-4 macrophages were washed four times with serum-free medium and stimulated with LPS for 45 minutes at 37°C in the presence of [3H]sucrose. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute. The results show the average of three independent determinations.
Effects of LPS on macrophage fluid phase uptake in the absence of serum. Murine clone 4-4 macrophages were washed four times with serum-free medium and stimulated with LPS for 45 minutes at 37°C in the presence of [3H]sucrose. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute. The results show the average of three independent determinations.
LPS is known to activate members of the mitogen-activated protein (MAP) kinase family of proteins. Therefore, we investigated the roles of p42/44 MAP kinase and p38 MAP kinase in LPS effects on pinocytosis using the mitogen-activated protein kinase (MEK) inhibitor PD098059 and SB203580, a p38 MAP kinase inhibitor. PD098059 or SB203580, however, did not alter LPS effects (Fig 4), and LPS effects on fluid phase uptake are apparently mediated via alternative signaling events.
Effects of LPS on macrophage fluid phase uptake in the presence of (A) 1 μmol/L SB203580 or (B) 2 μmol/L PD098059. Murine clone 4-4 macrophages were preincubated with the inhibitors for 1 hour and stimulated with LPS for 45 minutes at 37°C in the presence of [3H]sucrose and the appropriate inhibitor. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute. The results show the average of three independent determinations.
Effects of LPS on macrophage fluid phase uptake in the presence of (A) 1 μmol/L SB203580 or (B) 2 μmol/L PD098059. Murine clone 4-4 macrophages were preincubated with the inhibitors for 1 hour and stimulated with LPS for 45 minutes at 37°C in the presence of [3H]sucrose and the appropriate inhibitor. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute. The results show the average of three independent determinations.
LPS acts specifically on fluid phase pinocytosis.
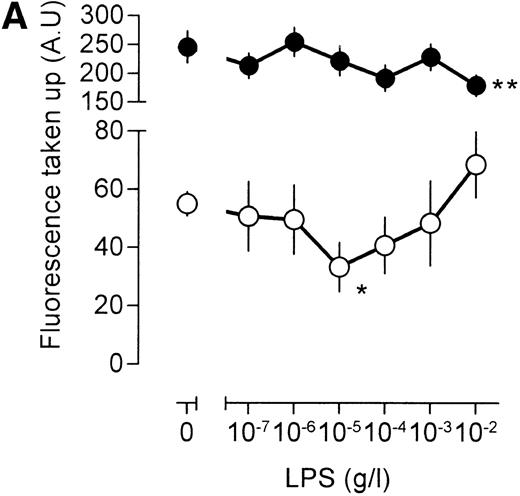
An important question is whether the effects of LPS on [3H]-sucrose reflect a general LPS action on macrophage endocytosis or whether these effects are associated specifically with the pinocytotic machinery. To this end, human catalase was fluorescently labeled. As mouse macrophages are unlikely to express specific receptors for human catalase, fluorescent catalase may only enter the cell by nonspecific mechanisms. If catalase is preincubated, however, with a mix of three mouse monoclonal antibodies directed against different epitopes of human catalase, aggregates of catalase and antibody will form, which may enter the cell by phagocytosis and/or receptor-mediated endocytosis after interaction with the macrophage Fc receptor. Indeed, cells incubated with antibody-catalase complexes accumulated approximately five times as much fluorescence as compared with cells that were incubated with fluorescent catalase alone (Fig 5A), showing the efficiency of antibody-directed uptake over nonspecific endocytosis. Low concentrations of LPS decreased uptake of nonantibody-bound catalase, maximal inhibition being reached at an LPS concentration of 10 ng/mL (Fig 5A). At higher concentrations of LPS, uptake of fluorescent catalase increased again, and at an LPS concentration of 10 μg/mL, a clear stimulation of cellular fluorescence, as compared with unstimulated cells, was apparent (Fig 5A). These results show, therefore, that the effects of LPS on fluid phase pinocytosis are not only observed using [3H]-sucrose as a probe, but are also observed with probes having a larger molecular size. No effect of LPS, however, was noted on the endocytosis of antibody-bound fluorescent catalase, except at the highest LPS concentration, at which a small decrease in uptake was noted (Fig 5A). Apparently, LPS has differential effects on antibody-directed endocytosis and fluid phase pinocytosis.
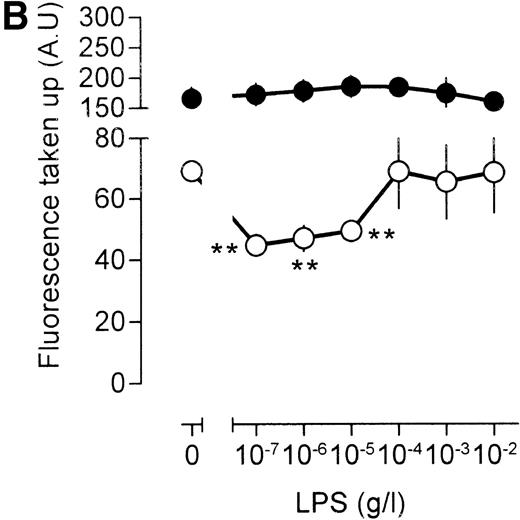
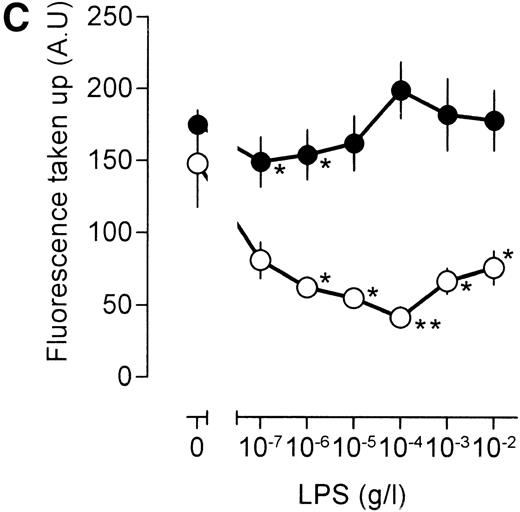
Influence of LPS on uptake of fluorescently labeled catalase. Murine clone 4-4 macrophages were stimulated with vehicle or different concentration of LPS for 45 minutes at 37°C and 5% CO2 in the presence of 6.6 mg/mL fluorescent catalase to assay pinocytosis (open circles) or fluorescent catalase and a mixture of three different supernatants of hybridomas producing antihuman catalase antibodies to assay Fc receptor–mediated uptake (filled circles). Uptake of fluorescence was then determined using FACS analysis. To distinguish uptake from binding, parallel experiments were performed at 4°C. The results show the average and standard error of four different experiments, each experiment being the average of two independent determinations. (A) Effects of different LPS concentrations. (B) Effects of different LPS concentrations in TNF (500 U/mL)-stimulated macrophages. (C) Effects of LPS on TPA (100 ng/mL)-stimulated macrophages.
Influence of LPS on uptake of fluorescently labeled catalase. Murine clone 4-4 macrophages were stimulated with vehicle or different concentration of LPS for 45 minutes at 37°C and 5% CO2 in the presence of 6.6 mg/mL fluorescent catalase to assay pinocytosis (open circles) or fluorescent catalase and a mixture of three different supernatants of hybridomas producing antihuman catalase antibodies to assay Fc receptor–mediated uptake (filled circles). Uptake of fluorescence was then determined using FACS analysis. To distinguish uptake from binding, parallel experiments were performed at 4°C. The results show the average and standard error of four different experiments, each experiment being the average of two independent determinations. (A) Effects of different LPS concentrations. (B) Effects of different LPS concentrations in TNF (500 U/mL)-stimulated macrophages. (C) Effects of LPS on TPA (100 ng/mL)-stimulated macrophages.
Similar results were obtained when the LPS action was investigated under conditions that result in enhanced fluid phase pinocytosis. Stimulation of the cells with TNF resulted in increased uptake of fluorescent catalase (Fig 5B), although uptake of antibody-bound catalase was somewhat lower as compared with cells that had not been treated with TNF (compare Fig 5A and B). Addition of LPS to TNF cells decreased uptake of fluorescence at lower LPS concentrations, and at higher concentrations of LPS, uptake of fluorescence increased again, in agreement with results using [3H]-sucrose as a probe. LPS, however, did not have a marked effect on the uptake of antibody-bound catalase. Addition of the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) to the macrophages resulted in a very large increase in the intracellular accumulation of fluorescent catalase (Fig 5C). If TPA, however, was added to the cells together with low concentration of LPS, this TPA-induced uptake of catalase was severely impaired (Fig 5C). At 10 to 100 ng/mL LPS, accumulation of fluorescence was lower than that observed in unstimulated cells and similar to the level observed in cells that had been stimulated with LPS in the absence of TPA (compare Fig 5A and C). Effects of LPS on the uptake of antibody-bound catalase were much less pronounced (Fig 5C). Furthermore, TPA-treated cells were less efficient in the uptake of antibody-bound catalase in comparison with untreated cells (compare Fig 5A and C). We concluded that LPS specifically influences fluid phase pinocytosis and is not a general regulator of endocytosis in macrophages.
LPS-dependent stimulation of pinocytosis is macrophage-specific.
Apart from macrophages, monocytes are also capable of measurable pinocytosis and phagocytosis. Furthermore, monocytes are highly responsive to LPS. Significant differences exist, however, between macrophages and monocytes with respect to the regulation of pinocytosis.14 We decided, therefore, to investigate the effects of LPS on phagocytosis and pinocytosis in freshly isolated peripheral blood monocytes. It seemed that LPS had little influence on phagocytosis of fluorescently labeled S pneumoniae (not shown). In contrast, LPS significantly inhibited pinocytosis of fluorescently labeled human serum protein by monocytes isolated from four different healthy volunteers (Fig6). The stimulation of pinocytosis at higher LPS concentrations, however, was not detected (Fig 6). We concluded that the CD14-mediated inhibition of fluid phase uptake is a general feature of LPS-induced signaling, but that the LPS-independent stimulation of pinocytosis at higher LPS concentrations is a macrophage-specific phenomenon.
Effects of LPS on fluid phase uptake of peripheral blood monocytes. Peripheral blood mononuclear cells were isolated from heparinized blood of healthy volunteers using Ficoll-Hypaque density gradient centrifugation. Subsequently, the cells residing at the interface were washed, allowed to recover from isolation, stimulated for 45 minutes at 37°C and 5% CO2 in the presence of 1 mg/mL fluorescently labeled human serum protein, and analyzed by FACS. The scatter profile was used to identify monocytes, and average fluorescence per monocyte was determined. Nonmonocyte cell populations did not accumulate significant amounts of fluorescence. The graph shows the curves from four different healthy volunteers.
Effects of LPS on fluid phase uptake of peripheral blood monocytes. Peripheral blood mononuclear cells were isolated from heparinized blood of healthy volunteers using Ficoll-Hypaque density gradient centrifugation. Subsequently, the cells residing at the interface were washed, allowed to recover from isolation, stimulated for 45 minutes at 37°C and 5% CO2 in the presence of 1 mg/mL fluorescently labeled human serum protein, and analyzed by FACS. The scatter profile was used to identify monocytes, and average fluorescence per monocyte was determined. Nonmonocyte cell populations did not accumulate significant amounts of fluorescence. The graph shows the curves from four different healthy volunteers.
Effects of detoxified LPS on pinocytosis.
The stimulatory effects of LPS on pinocytosis at higher concentrations (1 to 10 μg/mL) are somewhat surprising as lower concentrations of LPS are generally considered to maximally stimulate cells with respect to oxidative burst and cytokine production. It is therefore important to investigate if LPS acts on pinocytosis via a nonsignaling mechanism. We decided to test, therefore, the effect of detoxified LPS on macrophage pinocytosis. As evident from Fig7, detoxified LPS was much less potent in inducing changes in pinocytosis, inducing a rightward shift in the dose-response curve of two orders of magnitude. The inhibitory effects of LPS on pinocytosis seem, therefore, to be mediated by a bona fide LPS-induced signaling system. Although these data would also seem to support the notion that the stimulation of pinocytosis by LPS is signal transduction–dependent, it should be kept in mind that the residual activity of LPS (approximately 1%) may be sufficient to inhibit LPS stimulation of pinocytosis via a nonsignal transduction mechanism.
Effects of detoxified LPS on macrophage fluid phase uptake. Murine clone 4-4 macrophages were stimulated with LPS (open circles) or detoxified LPS (closed circles) for 45 minutes at 37°C and 5% CO2 in the presence of [3H]sucrose. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute.
Effects of detoxified LPS on macrophage fluid phase uptake. Murine clone 4-4 macrophages were stimulated with LPS (open circles) or detoxified LPS (closed circles) for 45 minutes at 37°C and 5% CO2 in the presence of [3H]sucrose. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute.
DISCUSSION
Monocytes and macrophages mediate many of the physiological effects of LPS. One of the most characteristic features of these cells is their capacity to endocytose extracellular material, either by pinocytosis, receptor-mediated endocytosis, or phagocytosis. Although a CD14-dependent pathway for phagocytosis of gram-negative bacteria has been characterized,11 the effects of LPS on fluid phase pinocytosis and Fc receptor–mediated endocytosis had not been studied in great detail. In the present study, we observed that LPS did not have a great effect on Fc receptor–mediated endocytosis, but did have a dual action on fluid phase pinocytosis: at lower LPS concentrations (0.1 to 10 ng/mL), pinocytosis was diminished in both macrophages and monocytes, whereas in macrophages at high LPS concentrations (10 μg/mL), this process was stimulated relative to untreated controls. In monocytes, stimulation of pinocytosis by LPS was not observed, and only inhibition of fluid phase uptake by LPS was noted. Interestingly, after treatment with a CD14-blocking antibody, the LPS-induced inhibition of fluid phase uptake was no longer discernible, and under these conditions, LPS-dependent stimulation of pinocytosis at lower concentrations was unmasked, suggesting that CD14 mediates inhibition of pinocytosis, whereas an LPS-induced CD14-independent mechanism acts stimulatory on this process. In agreement with this notion is our observation that in the presence of the CD14-blocking antibody, 10 μg/mL LPS produced even more fluid phase uptake as was observed at this concentration of LPS without the antibody. This shows that even at LPS concentrations facilitating pinocytosis, a CD14-dependent antipinocytotic influence is still present.
The molecular nature of the LPS-induced stimulation of fluid phase pinocytosis is unclear. Several groups have reported CD14-independent actions after stimulation with high concentrations of LPS,4,15-17 and recently, we showed in our own laboratory that a fraction of the CD14-negative population of mononuclear cells, isolated from the blood of healthy volunteers, displays nuclear translocation of NF-κB upon stimulation with 10 μg/mL LPS.17a In agreement, macrophages isolated from CD14 knockout mice display cytokine production after treatment with similar concentrations of LPS,18 and several molecules, including CD11b/CD18,19-21 CD11c/CD18,22,23 and L-selectin,24 have been proposed to act as low-affinity receptors for LPS, mediating its effects at higher concentrations. Uniquely, from our studies it emerged that the CD14-independent stimulation of fluid phase uptake may be detected at LPS concentrations as low as 1 to 10 ng/mL when a blocking antibody abrogates the CD14-dependent inhibitory influence of LPS on pinocytosis. We feel, however, that this observation per se does not indicate the existence of an alternative high-affinity LPS receptor, as such a receptor is not expected to yield the roughly linear stimulation of fluid phase uptake we observed over concentrations ranging from 1 ng/mL to 10 μg/mL. These data are probably more readily explained by a physical effect like, eg, altered membrane fluidity, although obviously further experimental work is needed to confirm this notion.
The effects of LPS on Fc receptor–mediated endocytosis were much less pronounced when compared with its effects on fluid phase uptake. Nevertheless, we did not fail to notice that at high concentrations of LPS, increased pinocytosis was associated with a reduction in uptake of antibody-bound material. Furthermore, the higher level of fluid phase pinocytosis in TNF-treated macrophages was also accompanied with lower Fc receptor–mediated endocytosis, whereas the strong stimulation of pinocytosis induced by the phorbol ester TPA resulted in a large reduction in the uptake of antibody-bound material. Together, these observations indicate that pinocytosis and Fc receptor–mediated endocytosis may compete for the availability of the endocytotic machinery. In agreement, both fluid phase pinocytosis and phagocytosis of IgG-opsonised erythrocytes are impaired by inhibitors of phosphatidylinositol-3-kinase,9 and also, dominant negative mutants of the p21ras-related small GTPase p21rac are reported to inhibit pinocytosis as well as phagocytosis,7,8 showing that similar signaling elements underlie both processes and that competition for these elements may exist. The molecular mechanism by which LPS binding to CD14 inhibits pinocytosis remains unclear. Among the signaling elements reported to be activated by LPS are p38 MAP kinase,22,25,26 p42/p44 MAP kinase,27,28stress-activated protein kinases,29,30 and NF-κB.31 These signaling elements, however, are also activated by TNF, which stimulates rather than inhibits pinocytosis, suggesting the involvement of an alternative LPS-specific molecular mechanism for inhibiting fluid phase pinocytosis. In agreement, we failed to detect an influence of p38 MAP kinase or p42/p44 MAP kinase inhibition on the LPS-induced changes in pinocytosis. Recently, it was reported that direct interaction of CD14 with LPS present in the outer membrane of gram-negative bacteria may directly induce bacterial phagocytosis.11 It is possible, therefore, that the induction of phagocytosis and the associated recruitment of signaling elements required for this phagocytosis may cause a diminished availability of such signaling elements for pinocytosis, and that this may explain the LPS-induced CD14-dependent inhibition fluid of phase uptake.
The present study has shown that LPS may have specific influence on pinocytosis, with a much less pronounced effect on Fc receptor–mediated endocytosis. It is often suggested that macrophage pinocytosis serves as mechanism for taking up extracellular antigen, which can subsequently be presented to CD4+ T cells.32 Phagocytosis, apart from its role in pathogen destruction, is well known to function in antigen presentation. Interestingly, we recently found that material taken up by pinocytosis and material taken up by Fc receptor–mediated endocytosis are routed to different intracellular compartments, suggesting that these processes have a specific function in antigen presentation and that LPS may influence antigen presentation via CD14-mediated inhibition of fluid phase uptake. Experiments exploring this possibility are currently under progress.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Maikel P. Peppelenbosch, Laboratory for Experimental Internal Medicine, G2-130 Academic Medical Centre, Meibergdreef 9, NL-1105 AZ Amsterdam, The Netherlands; e-mail:M.P.Peppelenbosch@AMC.UVA.NL.

![Fig. 1. Pinocytosis in macrophages. Murine clone 4-4 macrophages were incubated for various time intervals with [3H]-sucrose at 37°C. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, volume uptake was calculated and expressed as femtoliter per cell.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.4011/4/m_blod41129001x.jpeg?Expires=1769278838&Signature=26cRiYbRpBMC4p3rEaU9Mx1bIF5jqLQ55LPPk08E22dSgldeGlfEVdTqpbGeLZY-4lQoriBn-hD9ytzOY~nFTS07uPe~ykj5u1C5RBQ-P45EZZJnGVIiTkIDWtYP9B5f03zJ5C7pzWDHzslU9Oj5hX-1qLGsNxzeAA7379L8DaR1BGfyhY6P8Z2u1q6MxQRin1vXZbgzjlL~DZq9N5uhzXnBcP2GQ~t~H9XQEv9MwfzCkZ15~KBBgNxdjPBkkO4LjMx6X5KVtWlNPnJ8ulNLYqUaVjzj6Og7yPruPPiqKSUmKjltKzcV-cuQNrKVHd9RAUulupVvF5AtZ8zM~KIGHg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Effects of LPS on macrophage fluid phase uptake. Murine clone 4-4 macrophages were stimulated with vehicle or different concentration of LPS for 45 minutes at 37°C and 5% CO2in the presence of [3H]sucrose. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute. The results show the average and standard error of three different experiments, each experiment being the average of three independent determinations. The statistical significance of the difference between the unstimulated and stimulated value is indicated by stars, one star indicating a P value <0.05 and two stars indicating a P value <0.01 (Pvalues were obtained using an unpaired two-tailed heteroscedastic Student’s t-test on the original nine values on which the graph is based.). (A) Effects of different LPS concentrations on macrophage pinocytosis. (B) Effect of different LPS concentrations on TNF (500 U/mL)-stimulated pinocytosis. (C) Effects of LPS in the presence of the CD14-blocking antibody rmC5-3. (D) Effects of LPS in the presence of an irrelevant antibody (SUK5).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.4011/4/m_blod41129002ax.jpeg?Expires=1769278838&Signature=WUIwJx3DvhjSFxBgybgLrDqDFq5ZEL0e1xbjX-g6p8B-Zcpb0NPmAGRTVpDlzNsh7be0g7tuYDxNQhPB9QWsE3tmrlTMObuyEnfyllUXBFkzK0Roi5Cjmd8mfQa7u1oP9I2lj~-IqtsoPWv1dAuWBSAmnGomIp4Vl8wMUh08j5PsO0R1NIzSP5vV0vX4DTViPGonNgwQS3IRmoTtsoDvQ2HCOp~E3~NBl1VhE3i1prlGsXc37IiLlxIpRsNkkyTqF8Fi~ql6SRbPBNlcx3KgFUF5D66Qh~uJLx7jvMD1~L9a5NHLpn9-g32uGkQWrLA5yvq5c-sZFzBlNKIl1N~SCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Effects of LPS on macrophage fluid phase uptake. Murine clone 4-4 macrophages were stimulated with vehicle or different concentration of LPS for 45 minutes at 37°C and 5% CO2in the presence of [3H]sucrose. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute. The results show the average and standard error of three different experiments, each experiment being the average of three independent determinations. The statistical significance of the difference between the unstimulated and stimulated value is indicated by stars, one star indicating a P value <0.05 and two stars indicating a P value <0.01 (Pvalues were obtained using an unpaired two-tailed heteroscedastic Student’s t-test on the original nine values on which the graph is based.). (A) Effects of different LPS concentrations on macrophage pinocytosis. (B) Effect of different LPS concentrations on TNF (500 U/mL)-stimulated pinocytosis. (C) Effects of LPS in the presence of the CD14-blocking antibody rmC5-3. (D) Effects of LPS in the presence of an irrelevant antibody (SUK5).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.4011/4/m_blod41129002bx.jpeg?Expires=1769278838&Signature=TbnpO4FwkPBVxtvg5GRLxKNb2ET7XrBA8a7mDLyowZS1wk2h6gX-WVDmNZuUGR1D~OvlvtvwQ8fAW5kSdwrYBY~5mNM~ju0ZP6EBVKEnsk-SgMqZATCYqqj7j-zWU9HBzCPDM3wssZCP7~9D54LVluKbFsXPmcCyNowiwsLVrNkm2OLhHi1s4caeWXnxcjhas9I3ggMZRWchw45nj4bE26LcZ9UwP7hE~aAY6lS3sN3k4QbAEeNWUKXg3haMWBm-1tXEENfQTnFiGHAffYAkUAtrRtdMEROOBAXsf95DLwHLNshBvm6xaa~Ukx75q8X-eoqLVd4NRXIyAVbK7FUdIQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Effects of LPS on macrophage fluid phase uptake. Murine clone 4-4 macrophages were stimulated with vehicle or different concentration of LPS for 45 minutes at 37°C and 5% CO2in the presence of [3H]sucrose. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute. The results show the average and standard error of three different experiments, each experiment being the average of three independent determinations. The statistical significance of the difference between the unstimulated and stimulated value is indicated by stars, one star indicating a P value <0.05 and two stars indicating a P value <0.01 (Pvalues were obtained using an unpaired two-tailed heteroscedastic Student’s t-test on the original nine values on which the graph is based.). (A) Effects of different LPS concentrations on macrophage pinocytosis. (B) Effect of different LPS concentrations on TNF (500 U/mL)-stimulated pinocytosis. (C) Effects of LPS in the presence of the CD14-blocking antibody rmC5-3. (D) Effects of LPS in the presence of an irrelevant antibody (SUK5).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.4011/4/m_blod41129002cx.jpeg?Expires=1769278838&Signature=Bhmpxt0~MGThOSVIVvDWHJH7YV5YbusDkkAueEyGXYU2bbSI9QUCz0zmqaXdjDGsqwZjHz~dDny-sA7dpNv-0ateYVdgETklXG-GxJcbuAxnuNz2VFjSvX3HuNYJQSwrzpolWPS37Qj8jbaW9L5V9-zPA~07Zu0scZ2s1YpJmQm6GcuH3eJ~dwx-1ez4h44E~tAL~dnWg~D7UWNHq217A28nVWNcecltZzji0MViErHPjNKLvrgQJ0CoJFV39arJZaWSz4x~Pk2LnPlocIXL2KspMkGBEzkjiGkiP0xrEeCUCTCvTTjtae6C3-fchCuHDAoAv9f7NWiRDfvMgiolhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Effects of LPS on macrophage fluid phase uptake. Murine clone 4-4 macrophages were stimulated with vehicle or different concentration of LPS for 45 minutes at 37°C and 5% CO2in the presence of [3H]sucrose. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute. The results show the average and standard error of three different experiments, each experiment being the average of three independent determinations. The statistical significance of the difference between the unstimulated and stimulated value is indicated by stars, one star indicating a P value <0.05 and two stars indicating a P value <0.01 (Pvalues were obtained using an unpaired two-tailed heteroscedastic Student’s t-test on the original nine values on which the graph is based.). (A) Effects of different LPS concentrations on macrophage pinocytosis. (B) Effect of different LPS concentrations on TNF (500 U/mL)-stimulated pinocytosis. (C) Effects of LPS in the presence of the CD14-blocking antibody rmC5-3. (D) Effects of LPS in the presence of an irrelevant antibody (SUK5).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.4011/4/m_blod41129002dx.jpeg?Expires=1769278838&Signature=SKuMo9CglLHKHvYHDI~inokoND20qFYT4hWQJ0vhQcMKsaJpBCT993wgL5GD9F~9vuHDY4u9HedJi8k2M7-CHoglhAAP5Wbv15nXjoXXGE0sl-Ewd~Z9x5rD4v680dDu98G~f87BOSkfPO4aq7G8Xjnj8FVKCCW17Pm2X~ovY~f~GQxMAvC-WoSduhsmXbbCjniBwvn46U-t8f2FIDdscaULAJbDq09qGhk33UchqY9mrAs-q7TrIk5Dica-mESIBVCb58KXIOw3X~OPCEa5cimaFAbPHl8Zu~8uUzfuoFcYPlEFgicTpl4f63GhgWwOVETu3cUSMWhnmwJ1AlKasA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effects of LPS on macrophage fluid phase uptake in the absence of serum. Murine clone 4-4 macrophages were washed four times with serum-free medium and stimulated with LPS for 45 minutes at 37°C in the presence of [3H]sucrose. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute. The results show the average of three independent determinations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.4011/4/m_blod41129003x.jpeg?Expires=1769278838&Signature=HA9SLlVcvBaHWbT-fxrPJIfXLJEOdYxyDacyq14aACZ5JwvWyCghiBJBdARbkKabH8T8Dll2chNQs0fltaXxKKlpBVYVjxPzy9E2Pq93TwunUN0OQ28p-IKB3rVhTmJNr6YLujVWmygvqlC-12FR3-VVakE~qsYQMXT-HUhGLrL6BUna5czHrEtkHBqKh6nIKktxvwtiCVHHEpZxPgWkhycr6SZmYTfBGIKGKwW7n35tgitj-LeliYY90sMe0YhRJUFRF6PgWVIC2hGiN2wDnAuEcUvyhU4~7dlBVj1aiUKqvDsw-R2fa4f-YeU3HPkN8xDL9yJZACqAoiW5pUMF0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effects of LPS on macrophage fluid phase uptake in the presence of (A) 1 μmol/L SB203580 or (B) 2 μmol/L PD098059. Murine clone 4-4 macrophages were preincubated with the inhibitors for 1 hour and stimulated with LPS for 45 minutes at 37°C in the presence of [3H]sucrose and the appropriate inhibitor. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute. The results show the average of three independent determinations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.4011/4/m_blod41129004ax.jpeg?Expires=1769278838&Signature=DmapnvTcxc3XA5ibnk14QavHs8hYfPeMs9GSc70tz9VVNG7IAp-N6jVapccaaTBRuN392~M1HL86KiKJTk~0zVDGqaNd9NVniT7c6aMAl0n8611WP2CIt8KY5IhLVKNK0Fx7NhAvEtKUeP-X2BesvLxE6b7yZ3O4Fp~h0Bwr~mdZ8BL~ynxIw7L3-oXGO7mNW~vhgC1qYunfOOgKn-ZanYAI4vmNBqgVROp2UujMtuCIvZlH6d0~9EqsvlPYJEqEni0SFWnGRmRDgo4dvh9-0GC3BA1a7-CCXiIGJBQUIpjs6q9vGyXRxJTwFTAh9-4gY2ufGp8qW18SmpGuSlvLGQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effects of LPS on macrophage fluid phase uptake in the presence of (A) 1 μmol/L SB203580 or (B) 2 μmol/L PD098059. Murine clone 4-4 macrophages were preincubated with the inhibitors for 1 hour and stimulated with LPS for 45 minutes at 37°C in the presence of [3H]sucrose and the appropriate inhibitor. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute. The results show the average of three independent determinations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.4011/4/m_blod41129004bx.jpeg?Expires=1769278838&Signature=i6VJrSW4M9Di7~Bs~MSFEmRnYDTxBlVihMxVfTkt65qE0EaLXT09jyHcpdNF3NDGwSOReyUu8241dtnyJR2bT76NfCA6buvvE-2JYW1385bUPqEzswBuP0CzZtP3ltbwXeWAL9PNemsCiYrWwPgMtdQIRIPe-IQ1lId-IR1gy3Q8A-jl391KMop6wNVNdR1RM4rtEiIkRzyJ-4XlItbsqqNc3iqL~HGzfybwaRBRsvhnuu6pwX3vzgmwxdjRK6AQ0LKms2eQKG4iqPS-J41jcMPbjZ6AF3K3XUOpABYMhIM4ExYsYda6~MrQGP0eHDx6i6xlWDNguos-RdvW~hvqgw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 7. Effects of detoxified LPS on macrophage fluid phase uptake. Murine clone 4-4 macrophages were stimulated with LPS (open circles) or detoxified LPS (closed circles) for 45 minutes at 37°C and 5% CO2 in the presence of [3H]sucrose. Subsequently, the cells were placed on ice and washed, and accumulated radioactivity was determined by scintillation counting. Using the specific activity of the [3H]sucrose, fluid phase uptake was expressed as femtoliter per cells per minute.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.4011/4/m_blod41129007x.jpeg?Expires=1769278838&Signature=ZNPQMGZFJGhtkkd34ZDZYHp0zcSfwb8BY6VQCRVXGlepTkeJXhT7baSiCzzujO46wCNN4ojshV8PqLp-YnigVejoEJPrKxr8IfGxNX1sCKXQDbPLvquVdKcUB4ck2ej2LALJRJmir~fxUcdVgsprQCbtjYk7OHh4pT7w7XmxZWmougmqxcklqnhGQAuYXonY0QQA4v-7zE3J~lKefHzqXv3JFsiArO5-IpAmQCLsaEmviUJWymVUFQrX6o0ixDGctr663C5ZaDUu~HH2XNILtb3ZmMd67cmCp-KqirBc4rp1PmTMuEb2NsX9fWvDbXe~eE0~GTYzilV~rq2Uqi0sgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal