Abstract
Dendritic cells (DC) were sorted on day 8 from cultures of CD34+ cells with stem cell factor/Flt-3 ligand/ granulocyte-macrophage colony-stimulating factor (GM-CSF)/tumor necrosis factor- (TNF-)/interleukin-4 (IL-4). Exposing immature CCR5+CXCR4lo/− DC to CCR5-dependent human immunodeficiency virus (HIV)-1Ba-L led to productive and cytopathic infection, whereas only low virus production occurred in CXCR4-dependent HIV-1LAI–exposed DC. PCR analysis of the DC 48 hours postinfection showed efficient entry of HIV-1Ba-L but not of HIV-1LAI. CD40 ligand- or monocyte-conditioned medium-induced maturation of HIV-1Ba-L–infected DC reduced virus production by about 1 Log, while cells became CCR5−. However, HIV-1Ba-L–exposed mature DC harbored 15-fold more viral DNA than their immature counterparts, ruling out inhibition of virus entry. Simultaneously, CXCR4 upregulation by mature DC coincided with highly efficient entry of HIV-1LAI which, nonetheless, replicated at the same low level in mature as in immature DC. In line with these findings, coculture of HIV-1Ba-L–infected immature DC with CD3 monoclonal antibody–activated autologous CD4+ T lymphocytes in the presence of AZT decreased virus production by the DC. Finally, whether they originated from CD1a+CD14− or CD1a−CD14+ precursors, DC did not differ as regards permissivity to HIV, although CD1a+CD14− precursor-derived immature DC could produce higher HIV-1Ba-L amounts than their CD1a−CD14+ counterparts. Thus, both DC permissivity to, and capacity to support replication of, HIV is primarily determined by their maturation stage.
DENDRITIC CELLS (DC) and Langerhans cells (LC) are presumed to play an important role in the natural history of human immunodeficiency virus (HIV) infection.1,2 At the earliest phase of infection, resident LC from the genital or rectal mucosae are assumed to be among the first target cells of the virus, and then to transport it by migrating to draining lymph nodes where, as interdigitating DC, they interact with T lymphocytes (TL) and initiate both the primary immune response to and productive infection of TL by HIV, leading to its systemic spreading.3-5 During the chronic phase of HIV infection, DC are probably involved in destruction of CD4+ TL either by transmitting virus to uninfected TL or by upregulating viral DNA transcription and subsequent virus production in latently infected cells.6-10 Conversely, DC may also contribute to long-term maintenance of an effective anti-HIV immune response and, thereby, to control of viremia.11,12 Finally, as in the murine LCMV model,13 a defect in DC function and/or decreased DC numbers might participate to the immune collapse of acquired immunodeficiency syndrome (AIDS).2 14
There is indeed evidence that LC and DC are susceptible to HIV.1,15 Epidermal LC as well as blood and spleen DC from HIV-infected patients harbor HIV DNA,16-18 express viral RNA and proteins,19-21 and even contain virions.19 In vitro, LC/DC isolated from the skin or blood, or differentiated from CD34+ hematopoietic progenitor cells (HPC) or monocytes, express CD4 and HIV coreceptors CCR3, CCR5, and CXCR422-28; and they harbor HIV DNA after exposure to CCR5-dependent (R5) or CXCR4-dependent (X4) strains.4,22,24,25,29-32 However, data regarding the capacity of DC/LC to support virus replication are conflicting, ranging from resistance to infection,33-35 nonproductive infection due to constitutive low capacity or inability to drive HIV promoter,31,36,37 to productive infection with R5 and X4 isolates.24,29,38-40 The reasons for these discrepancies are still unclear, but they may reflect different DC isolation procedures and culture methods resulting in heterogeneous populations as regards to both their activation/maturation stage and origin.41
The in vitro differentiation model of DC from cord blood CD34+ HPC represents a tool to reconcile these findings, inasmuch as it allows the independent differentiation of two distinct DC populations42: DC derived from CD1a+precursors are phenotypically and functionally close to LC, whereas DC derived from bipotent CD14+ DC-macrophage precursors resemble monocyte-derived and germinal center DC.42-46 In addition, depending on culture conditions—ie, with or without interleukin-4 (IL-4), CD40-ligand (CD40L), or monocyte-conditioned medium (MCM)—cells are obtained as either immature CD1a+CD83− or mature CD1alo/negCD83+ DC.45 47-50 Here we examined the susceptibility to HIV of such DC populations, and we show that their capacity to support replication of R5 or X4 laboratory strains is primarily determined by their activation/maturation stage, more than by their origin.
MATERIALS AND METHODS
Cord blood CD34+ HPC isolation, DC culture, and labeling.
Normal cord blood (Laboratoire Senders, Hôpital Saint-Vincent de Paul; Service de Gynécologie-Obstétrique, Hôpital Saint-Antoine, Paris, France) was collected according to institutional guidelines. After Ficoll-Paque (Pharmacia, Uppsala, Sweden) centrifugation, mononuclear cells were enriched into low-density cells by centrifugation on Percoll (density [d] =1.070; Pharmacia). CD34+ HPC were purified with CD34 monoclonal antibody (MoAb) 561-coated M-450 Dynabeads (Dynal, Oslo, Norway) as described,50 yielding 88% ± 7% pure viable CD34+ cells (n = 21), and they were cultured under reported conditions45 50 to promote DC differentiation. Briefly, 2 to 5 × 104/mL CD34+ HPC were cultured in 6-well plates (ATGC, Noisy le Grand, France) at 37°C in humidified 5% CO2, in RPMI 1640, 10% fetal calf serum (FCS; Dutscher, Brumath, France), 1% glutamine, 1% antibiotics (GIBCO-BRL, Paisley, UK), with the following human recombinant cytokines: 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (gift of Schering Plough, Kenilworth, NJ), 50 ng/mL stem cell factor (SCF; R&D Systems, Minneapolis, MN), 50 ng/mL Flt-3 ligand (FL; gift of Immunex, Seattle, WA), and 50 U/mL tumor necrosis factor-α (TNF-α; Genzyme, Cambridge, MA); 5 ng/mL of IL-4 (gift of Schering Plough) was added to cultures from day 5 onward.
DC precursors were sorted on culture day 5 with a FACStar Plus (Becton Dickinson, Mountain View, CA). Briefly, 5 to 106/mL washed cells were incubated for 30 minutes at 4°C with PE-CD14 (LeuM3; Becton Dickinson) and FITC-CD1a (Coulter, Coultronics, Margency, France) MoAb diluted 1:50. Cells were resuspended in phosphate-buffered saline (PBS), 2% FCS, and CD1a+CD14− and CD1a−CD14+ cells were sorted. Both populations (96% ± 2% pure, n = 20) were cultured for 3 more days before exposure to virus.
CD1a+ immature DC were sorted on culture day 8 with the RAM IgG1 CELLection kit (Dynal). Beads were washed in PBS, 0.1% bovine serum albumin (BSA), and incubated for 30 minutes with CD1a T6 MoAb (Coulter). They were washed again to remove unbound MoAb, mixed with the cells (107/mL) at a 5:1 ratio, and incubated for 15 minutes at 4°C on a rotor. Rosetted cells were separated with a magnet, and beads were detached with releasing buffer according to the manufacturer’s instructions. DC (97% ± 4% pure, n = 17) were then cultured for 18 to 24 hours with GM-CSF/TNF-α/IL-4 before exposure to virus.
For assessing CD4, CCR5, and CXCR4 membrane expression by DC derived either from CD1a+CD14− or CD1a−CD14+ precursors, cells were cultured as described previously but, because of high residual fluorescence after sorting, day 5 precursors were then sorted with the RAM IgG1 CELLection kit, after which they were labeled with PE-Leu3a/CD4 (Becton Dickinson), FITC-2D7/CCR5 and PE-12G5/CXCR4 (both from Pharmigen, San Diego, CA) MoAb before analysis with a FACScalibur (Becton Dickinson).
HIV strains and infection.
X4 HIV-1lai51 was purchased from Diagnostics Pasteur (Marne la Coquette, France), and R5 HIV-1Ba-L52 was a gift from B. Asjö(Bergen, Norway). Cells (0.5 to 1 × 106/mL) were incubated for 3 hours with 500 TCID50 (tissue culture infectious dose 50%) of DNAse-treated virus supernatant. Heat-inactivated (HI) virus (1 hour, 56°C) was used as negative control. Cells were washed twice and further cultured in RPMI 1640, 10% FCS, supplemented with GM-CSF/TNF-α/IL-4. Cytokines were added every 4 days together with fresh medium (20% of the volume), and viable cells were counted. The kinetics of virus production was followed by sequential measurement of viral p24 in supernatants by enzyme-linked immunosorbent assay (ELISA; Diagnostics Pasteur).
The effect of different activation/maturation signals on virus replication was assessed 2 days postinfection (PI). HIV-infected DC were harvested, washed twice to remove residual virions, and further cultured with the same cytokines but with or without 1 μg/mL soluble trimeric human CD40L (CD40LT; gift of Immunex), 2 ng/mL transforming growth factor-β1 (TGF-β1; Genzyme), 250 U IL-2 (gift of Chiron, Amsterdam, The Netherlands) or MCM at 25% final concentration.49
When the effect of recombinant macrophage inflammatory protein (MIP)-1α, MIP-1β, RANTES (regulated on activation normal T-cell expressed and secreted), or stromal cell–derived factor (SDF)-1α on virus entry was examined, 1 μmol/L of either chemokine was added 30 minutes before exposure to virus and maintained in culture after infection.
Detection of HIV DNA.
HIV DNA was detected by nested polymerase chain reaction (PCR) as described.30 Briefly, sorted DC (96% to 97% pure) were exposed for 90 minutes to 500 TCID50 of HIV-1Ba-L or HIV-1lai and analyzed for the presence of HIV pol DNA 2 days later. Cells (1 × 106/mL) were then washed and lysed in 10 mmol/L TRIS HCl, pH 8.3, 50 nmol/L KCl, 0.5% Tween 20 (Biorad, Hercules, CA), 0.5% Nonidet (Sigma, St Louis, MO). Proteinase K (20 μg/mL; Boehringer, Mannheim, Germany) was added, lysates were incubated at 56°C for 1 hour, and proteinase K was inactivated at 95°C for 10 minutes. Relative virus amounts in different samples were estimated by endpoint dilutions of the lysates in lysates of HIV− A301 cells (1 × 106/mL). Serially diluted samples (30 μL) were added to 0.5 μmol/L of each primer and 0.2 μmol/L of each dNTP (Pharmacia), 1.5 mmol/L MgCl2, and 1 UTaq DNA polymerase (Boehringer) in 50 μL final. After 5 minutes at 94°C, 35 cycles were performed in an automated DNA Thermal Cycler (Crocodile III; Appligene, Strasbourg, France), each consisting of 30 seconds at 94°C, annealing at 55°C, and extension at 72°C. Pol primers were P3 (TGGGAAGTTCAATTAGG AATACCAC) and P4 (CCTACATACAAATCATCCATGTATT).53 For the nested PCR, 2 μL of amplified products was submitted to another 35-cycle amplification under the same conditions using internal primers P5 (ATCAGTAACAGTACTGGATGTG) and P6 (GATAG ATAACTATGTCTGGATT). PCR sensitivity (1 copy/3 × 104 cells) was determined relative to serial dilutions of 8E5/LAV cells (1 copy/cell) in HIV− A301 parental cells. PCR was also performed with β globin primers PCO4 (CAACTTCATCCACGTTCACC) and GH2O (GAAGAGCCAAGGACAGGTAC) (Perkin Elmer, Foster City, CA) as amplification and DNA content controls. PCR products (15 μL) were electrophoresed onto 2% agarose, and stained with ethidium bromide for UV visualization.
Detection of chemokines in culture supernatants.
Supernatants were kept at −70°C until used. MIP-1α, MIP-1β, and RANTES levels were measured by ELISA according to the manufacturer’s instructions (R&D Systems).
Purification of cord blood CD4+ TL and coculture with HIV-infected DC.
After CD34+ HPC purification, autologous cord blood TL were enriched to ≥80% by sheep erythrocyte rosetting.54 CD4+ TL (94% ± 3% pure, n = 9) were then purified by negative selection: residual monocytes were depleted by adherence55 and CD8+ TL were depleted with CD8 MoAb ITI-5C2–coated M-450 Dynabeads. Cocultures were initiated by mixing 5 × 104 DC (96% ± 5% pure, n = 10) at 72-hours PI, with 5 × 105 resting or autologous CD4+ TL that had been activated with immobilized CD3 MoAb (UCHT1; Immunotech, Marseille, France), as described.56 Briefly, 10 μg/mL MoAb was added to wells of 6- or 12-well plates, and incubated for 90 minutes at 37°C. Wells were washed thrice with cold PBS, and CD4+ TL were added and cultured for 72 hours in RPMI 1640, 10% FCS. CD4+ TL were preincubated with azidothymidine (AZT; 10 μmol/L) for 2 hours before cocultures. These were conducted in RPMI 1640, 10% FCS, supplemented with IL-2 (100 U/mL; Chiron), in the continuous presence of 10 μmol/L AZT to block virus transmission to TL and to as yet uninfected DC. Due to use of AZT and the short 24- to 96-hour coculture period, virus production was assessed by quantifiying HIV RNA copies in supernatants with the AMPLICOR HIV-1 MONITOR assay (gift of Roche Diagnostic Systems, Branchburg, NJ). When cell-associated HIV RNA was assessed, cells were washed twice in PBS and trypsinized to eliminate residual and membrane-bound virions.
CD40-CD40L interactions in cocultures were assessed by preincubating CD3 MoAb-activated CD4+ TL with 10 μg/mL CD40L/CD154 MoAb M-90 (gift of Immunex) or irrelevant control mouse IgG1 (Sigma), 30 minutes before and during the whole coculture period.
RESULTS
Susceptibility of immature DC to R5 or X4 HIV strains.
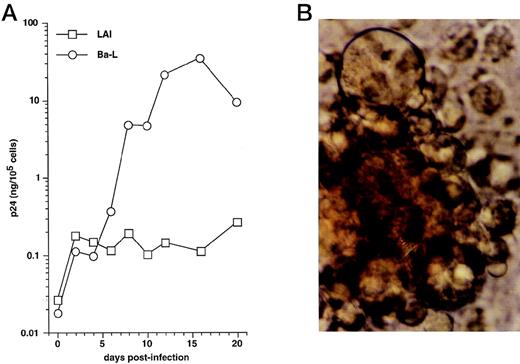
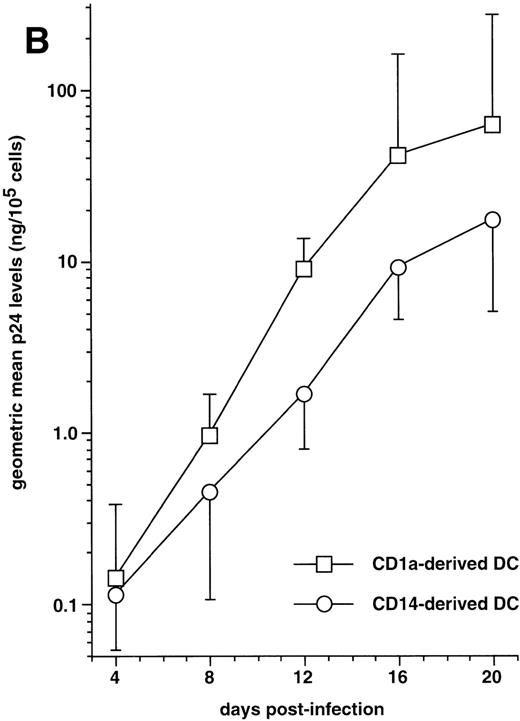
We first examined immature DC for permissivity to, and capacity to support, replication of R5 HIV-1Ba-L and X4 HIV-1LAI strains. CD34+ HPC were cultured for 8 days as reported46,50,57: ie, continuously with SCF/FL/GM-CSF/TNF-α, with IL-4 being added from day 5 onward. DC were then sorted and cultured further for 24 hours with GM-CSF/TNF-α/IL-4 (referred to as “standard condition” hereafter) before being exposed to virus. DC were then cultured under the same conditions, with fresh medium and cytokines being added every 4 days. To allow comparisons among different long-term DC cultures, p24 levels were assessed relative to numbers of viable cells remaining at each time point. In line with previous findings,30 HIV-1Ba-L–exposed DC replicated the virus, whereas no or more limited virus production occurred in HIV-1lai–exposed DC, with p24 levels reaching 9 to 118 ng/105 cells versus 0.25 to 19 ng/105 cells (n = 3) on day 20 PI, respectively (Fig 1A). Syncitia appeared from day 7-8 PI in HIV-1Ba-L–infected cultures (Fig 1B), but no cytopathicity was noted in HIV-1LAI–infected cultures. On day 20 PI, there remained ≤20% viable cells relative to initially HIV-exposed DC in both HIV-1Ba-L– and HIV-1lai–infected cultures as well as in cultures exposed to heat-inactivated virus, an indication that cell viability did not accurately reflect cytopathicity. Of note, absence of nonadherent CD14+ cells and of adherent macrophages for the whole culture period rules out participation of monocytes/macrophages to virus production in HIV-1Ba-L–infected cultures (data not shown).
HIV infection of immature DC. (A) Kinetics of p24 production: Culture day 8 sorted CD1a+ DC were exposed 1 day later to 500 TCID50 of HIV-1LAI (□) or HIV-1Ba-L (○), and further cultured with GM-CSF/TNF-/IL-4; virus production in supernatants is expressed as ng of p24/105 viable cells; p24 levels in heat-inactivated virus–exposed DC were always <0.1 ng/105 viable cells. (B) Morphologic examination of cells on day 8 PI with HIV-1Ba-L, showing that DC are involved in typical syncitia. Results of one of three experiments.
HIV infection of immature DC. (A) Kinetics of p24 production: Culture day 8 sorted CD1a+ DC were exposed 1 day later to 500 TCID50 of HIV-1LAI (□) or HIV-1Ba-L (○), and further cultured with GM-CSF/TNF-/IL-4; virus production in supernatants is expressed as ng of p24/105 viable cells; p24 levels in heat-inactivated virus–exposed DC were always <0.1 ng/105 viable cells. (B) Morphologic examination of cells on day 8 PI with HIV-1Ba-L, showing that DC are involved in typical syncitia. Results of one of three experiments.
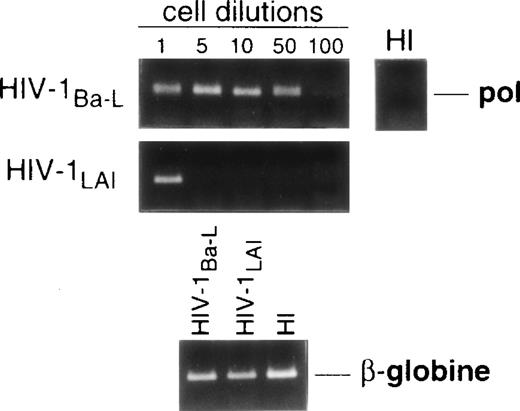
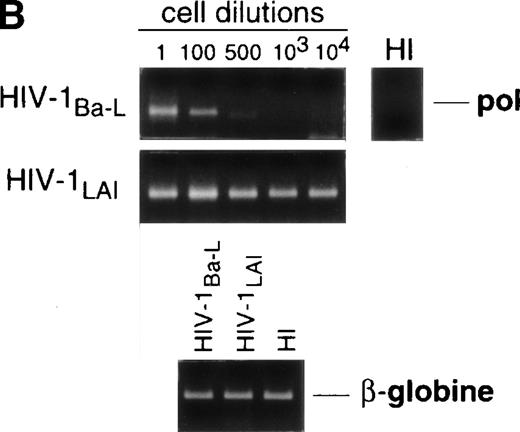
Limiting dilution analysis by nested PCR in DC obtained 48 hours PI confirmed these findings by showing greater amounts of viral DNA in HIV-1Ba-L– than in HIV-1LAI–exposed DC, geometric mean endpoint titers then being 7 and <1, respectively (Fig 2, and Table 1). In line with these findings, immature DC obtained under the same conditions homogeneously expressed CCR5, and only a minority were CXCR4lo (see Figs 4A and7A). At variance with another report,28fluorescence-activated cell sorter (FACS) analysis of such DC stained after saponine permeabilization did not disclose intracellular retention of CXCR4 (data not shown).
Nested PCR detection of viral DNA in HIV-infected immature DC. Culture day 8 sorted CD1a+ DC were exposed 1 day later to 500 TCID50 of HIV-1Ba-L or HIV-1LAI. Relative HIV DNA content was assessed 48 hours PI by limiting dilutions of infected cell lysates in uninfected A301 cell lysates. DC that had been exposed to heat-inactivated (HI) virus were used as negative controls and assayed undiluted. β-Globin DNA level was assessed as amplification and DNA content control. The PCR sensitivity was 1 copy/3 × 104 cells, as determined in parallel experiments by serial dilutions of 8E5/LAV cells (1 copy/cell) in HIV− A301 parental cells (data not shown). Results of one of four experiments.
Nested PCR detection of viral DNA in HIV-infected immature DC. Culture day 8 sorted CD1a+ DC were exposed 1 day later to 500 TCID50 of HIV-1Ba-L or HIV-1LAI. Relative HIV DNA content was assessed 48 hours PI by limiting dilutions of infected cell lysates in uninfected A301 cell lysates. DC that had been exposed to heat-inactivated (HI) virus were used as negative controls and assayed undiluted. β-Globin DNA level was assessed as amplification and DNA content control. The PCR sensitivity was 1 copy/3 × 104 cells, as determined in parallel experiments by serial dilutions of 8E5/LAV cells (1 copy/cell) in HIV− A301 parental cells (data not shown). Results of one of four experiments.
Semi-quantitative Endpoint Dilution PCR Analysis of HIV DNA Content in 48-Hour PI HIV-Infected DC Treated (mature DC) or Not Treated (immature DC) With CD40L
| HIV-1 Strain . | Immature DC (n = 4) . | Mature DC (n = 5) . | ||
|---|---|---|---|---|
| Ba-L . | LAI . | Ba-L . | LAI . | |
| Range of endpoint titers | 1-50 | 0-5 | 50-500 | 10-10,000 |
| LOG10 mean endpoint titers ± SD | 0.85 ± 0.61 | <0 | 2.02 ± 0.37 | 2.80 ± 1.17 |
| Geometric mean endpoint titers | 7 | <1 | 105 | 630 |
| HIV-1 Strain . | Immature DC (n = 4) . | Mature DC (n = 5) . | ||
|---|---|---|---|---|
| Ba-L . | LAI . | Ba-L . | LAI . | |
| Range of endpoint titers | 1-50 | 0-5 | 50-500 | 10-10,000 |
| LOG10 mean endpoint titers ± SD | 0.85 ± 0.61 | <0 | 2.02 ± 0.37 | 2.80 ± 1.17 |
| Geometric mean endpoint titers | 7 | <1 | 105 | 630 |
Relative amounts of viral DNA in different samples were estimated by endpoint dilutions of the DC lysates in lysates of HIV− A301 cells. Endpoint titers are expressed as the inverse of the last dilution giving a positive signal. The PCR sensitivity being 1 copy/3 × 104 cells, as determined in parallel experiments by serial dilutions of 8E5/LAV cells (1 copy/cell) in HIV− A301 parental cells (data not shown), one may estimate that the geometric mean endpoint titer of 7 corresponds to at least 2 copies/104 cells, the geometric mean endpoint titer of 105 to at least 35 copies/104 cells, and the geometric mean endpoint titer of 630 to at least 210 copies/104cells.
Maturation of HIV-1Ba-L–infected DC decreases virus production.
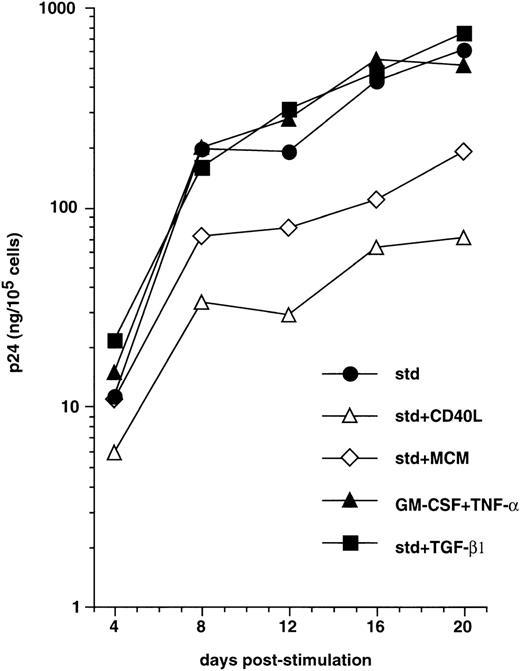
We next examined whether, as reported with monocyte-derived DC,58 maturation of CD34+ HPC-derived DC interfered with the capacity to support virus replication. CD40LT or MCM, both of which elicit DC maturation,45,49 60 were added to immature sorted DC on day 3 PI with HIV-1Ba-L, and cells were cultured further with these factors added to GM-CSF/TNF-α/IL-4. This resulted in strongly reduced virus production (Fig 3). For example, 12 days after induction of maturation (day 15 PI), p24 levels were 1.1 ± 0.3 Log and 0.8 ± 0.3 Log lower (n = 3) in the presence of CD40LT and MCM, respectively, than in cultures under standard conditions. Of note, that a similar effect was noted under both conditions indicates that inhibition of virus replication actually results from DC maturation per se, and not from the presence of chemokines in MCM or the possible direct interference of CD40LT with virus binding or entry into cells. Alternatively, culture of HIV-infected DC with TGF-β1 or IL-2 being added to GM-CSF/TNF-α/IL-4, or with GM-CSF/TNF-α only, did not affect virus production (Fig 3, and data not shown).
Effect of maturation on virus replication by HIV-infected DC. From 48 hours PI with HIV-1Ba-L, DC obtained as in Figs1 and 2 were cultured under standard conditions (std) with (▪) or without (•) TGFβ1, or they were induced to mature by adding CD40LT (▵) or MCM (◊) to GM-CSF/TNF-/IL-4, or they were cultured with only GM-CSF/TNF- (▴). Virus production is expressed as in Fig 1. Results of one of three experiments.
Effect of maturation on virus replication by HIV-infected DC. From 48 hours PI with HIV-1Ba-L, DC obtained as in Figs1 and 2 were cultured under standard conditions (std) with (▪) or without (•) TGFβ1, or they were induced to mature by adding CD40LT (▵) or MCM (◊) to GM-CSF/TNF-/IL-4, or they were cultured with only GM-CSF/TNF- (▴). Virus production is expressed as in Fig 1. Results of one of three experiments.
Thus, mature DC have reduced capacity to support HIV-1Ba-Lreplication, and this may result from reduced virus transmission to uninfected mature DC and/or decreased virus production by the DC already infected at the time when CD40LT or MCM were added.
Mature DC are permissive to both R5 HIV-1Ba-L and X4 HIV-1LAI.
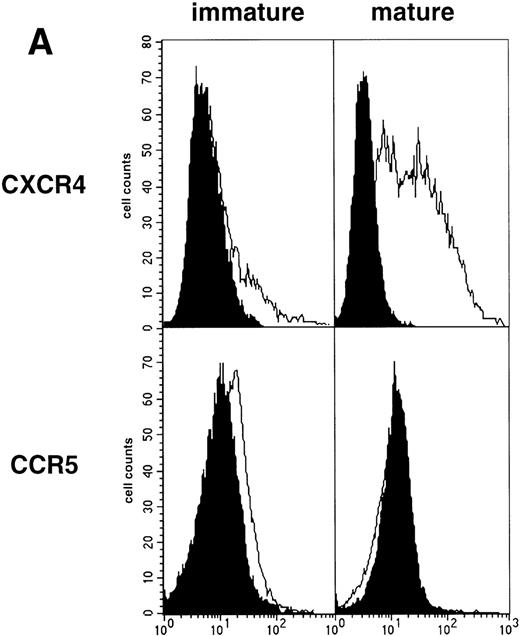
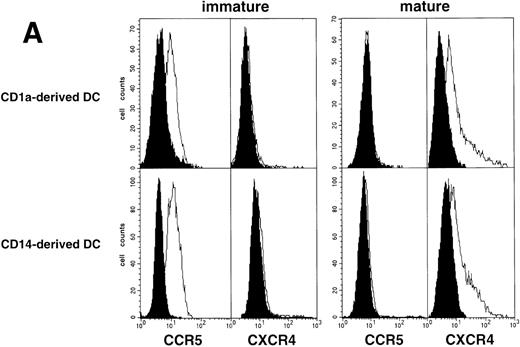
As an approach to investigate the mechanisms underlying this phenomenon, we analyzed expression of HIV coreceptors on DC by FACS, after 72-hour culture in the presence of CD40LT or MCM: CCR5 was then no longer detectable whereas CXCR4 expression strongly increased (Fig 4A). This led us to assess the permissivity of mature DC to HIV. Sorted DC were induced to mature for 72 hours with CD40LT and exposed to HIV-1Ba-L or HIV-1LAI. They were analyzed 48 hours later by nested PCR for viral DNA (Fig 4B, and Table 1). The geometric mean endpoint titer of 105 found in HIV-1Ba-L–exposed mature DC clearly indicated that CCR5 downmodulation did not affect virus entry in the cells. This suggested rather that HIV-1Ba-Linfection of mature DC could be independent of CCR5, but this was ruled out because 1 μmol/L RANTES or MIP-1β, but not CXCR4 ligand SDF-1α, inhibited infection (data not shown). Analysis of the same DC, exposed to HIV-1LAI under the same conditions, showed endpoint titers of up to 10,000, with a geometric mean titer of 630 (Table 1). HIV-1LAI infection was inhibited by 1 μmol/L SDF-1α, which argues for the role of CXCR4 in this process (data not shown).
Permissivity of mature DC to HIV. (A) Expression of HIV coreceptors: Culture day 8 sorted CD1a+ DC were cultured for 48 hours under standard conditions, with or without CD40LT, and labeled with FITC-CCR5 or PE-CXCR4 MoAbs; solid histograms: labeling with an irrelevant MoAb; open histograms: staining by the relevant MoAb; representative data of one of four experiments. (B) Nested PCR detection of viral DNA in HIV-infected mature DC: DC that had been cultured for 48 hours with GM-CSF/TNF-/IL-4 and CD40LT were exposed to HIV-1Ba-L or HIV-1LAI; cell lysates were prepared 48 hours PI. Comparative endpoint dilution analysis was performed as in Fig 2; results of one experiment of five.
Permissivity of mature DC to HIV. (A) Expression of HIV coreceptors: Culture day 8 sorted CD1a+ DC were cultured for 48 hours under standard conditions, with or without CD40LT, and labeled with FITC-CCR5 or PE-CXCR4 MoAbs; solid histograms: labeling with an irrelevant MoAb; open histograms: staining by the relevant MoAb; representative data of one of four experiments. (B) Nested PCR detection of viral DNA in HIV-infected mature DC: DC that had been cultured for 48 hours with GM-CSF/TNF-/IL-4 and CD40LT were exposed to HIV-1Ba-L or HIV-1LAI; cell lysates were prepared 48 hours PI. Comparative endpoint dilution analysis was performed as in Fig 2; results of one experiment of five.
These findings show that, in contrast to their immature counterparts, mature DC are permissive to both X4 HIV-1LAI and R5 HIV-1Ba-L.
CD40LT-induced DC maturation increases production of β-chemokines.
Because activation of DC via CD40 in a coculture system may induce MIP-1α production,47 we also investigated if CD40LT affected production of β chemokines MIP-1α, MIP-1β, and RANTES. Immature DC were cultured for 48 hours under standard conditions, with or without CD40LT, and chemokine levels in supernatants were measured. CD40 ligation increased levels of the chemokines by threefold to sixfold, but at concentrations far below those reported as being able to inhibit HIV infection61(Table 2).
Chemokine Levels in Supernatants of DC Cultured With or Without CD40LT for 48 Hours
| Culture Conditions . | RANTES . | MIP-1α . | MIP-1β . | |||
|---|---|---|---|---|---|---|
| Standard . | +CD40LT . | Standard . | +CD40LT . | Standard . | +CD40LT . | |
| Mean ± SD | 20 ± 11 | 108 ± 22 | 24 ± 5 | 130 ± 81 | 83 ± 17 | 222 ± 18 |
| P | .005 | .05 | .006 | |||
| n | 4 | 5 | 4 | |||
| Culture Conditions . | RANTES . | MIP-1α . | MIP-1β . | |||
|---|---|---|---|---|---|---|
| Standard . | +CD40LT . | Standard . | +CD40LT . | Standard . | +CD40LT . | |
| Mean ± SD | 20 ± 11 | 108 ± 22 | 24 ± 5 | 130 ± 81 | 83 ± 17 | 222 ± 18 |
| P | .005 | .05 | .006 | |||
| n | 4 | 5 | 4 | |||
Data are expressed in pg/mL. Sensitivity limit of the ELISA used was 5 pg/mL for RANTES, 6 pg/mL for MIP-1α, and 4 pg/mL for MIP-1β. Statistical analysis was performed using the paired Student’st-test.
Mature DC replicate HIV-1LAI at the same level as immature DC and to a greater extent than HIV-1Ba-L.
The fact that, like epidermal LC,28 mature CD34+ HPC-derived DC upregulate CXCR4 and become highly permissive to X4 HIV-1LAI, led us to examine their capacity to support virus replication. Immature DC were cultured for 72 hours with GM-CSF/TNF-α/IL-4 plus CD40LT, exposed to HIV-1LAIor HIV-1Ba-L, and cultured further under the same conditions. As shown in Fig 5, the DC exposed to HIV-1LAI produced more virus than those exposed to HIV-1Ba-L, although geometric mean p24 levels reached at day 20 PI were then only 1.23 and 0.33 ng/105 cells, respectively. By comparison, day 20 PI geometric mean p24 levels were found in independent experiments as being 2.12 and 33.4 ng/105 cells for immature DC infected with HIV-1LAI and HIV-1Ba-L (n = 3), respectively.
HIV-1LAI replication by mature DC. Culture day 8 sorted CD1a+ DC were cultured for 48 hours under standard conditions plus CD40LT, and they were then exposed to HIV-1Ba-L (○) or to HIV-1LAI (□). Virus production is expressed as geometric mean ± SD ng p24/105viable cells of three experiments. The p24 levels in HI virus-exposed DC were always <0.1 ng/105 viable cells; levels in HIV-1LAI– and HIV-1Ba-L–infected cultures were significantly different as assessed by two-way analysis of variance (P = .0015).
HIV-1LAI replication by mature DC. Culture day 8 sorted CD1a+ DC were cultured for 48 hours under standard conditions plus CD40LT, and they were then exposed to HIV-1Ba-L (○) or to HIV-1LAI (□). Virus production is expressed as geometric mean ± SD ng p24/105viable cells of three experiments. The p24 levels in HI virus-exposed DC were always <0.1 ng/105 viable cells; levels in HIV-1LAI– and HIV-1Ba-L–infected cultures were significantly different as assessed by two-way analysis of variance (P = .0015).
The fact that mature DC replicate HIV-1lai as well as immature DC and more efficiently than HIV-1Ba-L should be compared with Table 1 semi-quantitative PCR data which show that, 48 hours PI, mature DC harbor about sixfold more HIV-1lai than HIV-1Ba-L DNA copies. Thus, even if, relative to immature DC, mature DC may be more permissive to X4 than to R5 strains, they apparently display overall reduced capacity to support virus replication.
CD3 MoAb-activated autologous CD4+ TL modulate virus production by HIV-1Ba-L–infected immature DC.
We next questioned the physiological relevance of these findings by studying whether activated autologous TL affected virus replication by HIV-1Ba-L–infected immature DC. Because activated TL upregulate CD40L, produce high levels of cytokines, and induce DC maturation in cocultures (data not shown), one would expect that they reproduced the effect of CD40LT on virus replication by HIV-1Ba-L–infected DC.
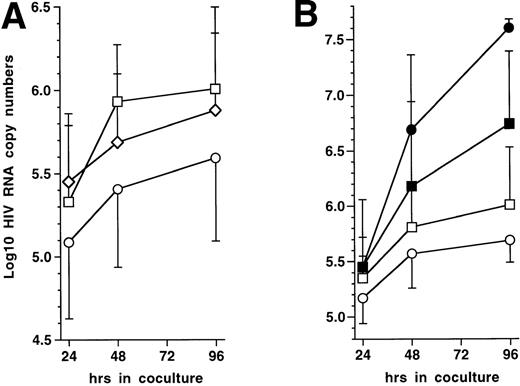
To test this prediction, 72 hours PI with HIV-1Ba-L, 5 × 104 immature DC were mixed with 5 × 105 autologous CD4+ TL, either resting or that had been activated with immobilized CD3 MoAb for 72 hours. Cocultures were conducted in the presence of 10 μmol/L AZT to prevent virus transmission to new cells. Indeed, preliminary experiments showed that 10 μmol/L AZT completely blocked HIV infection of DC as well as of CD3 MoAb-activated CD4+ TL (data not shown), which ensured us that virus was produced only by already infected DC in the cocultures with TL. Virus production, estimated as HIV RNA copy numbers, was indeed significantly reduced in supernatants of DC cocultured with CD3 MoAb-activated but not with resting CD4+ TL (Fig 6A). Because lower RNA copy numbers in supernatants could result from intracellular sequestration, we verified that cell-associated HIV RNA copy numbers were about 1 Log lower than, and paralleled those found in, supernatants under all conditions tested (data not shown). Despite decreased virus production in supernatants, HIV-infected DC still efficiently transmitted virus to activated CD4+ TL when AZT was omitted from cocultures (Fig 6B), as reported.1 These data show that activated autologous CD4+ TL downregulate virus production in supernatant by HIV-1–infected DC even though they are still able to transmit virus to the TL.
Effect on virus production of coculturing HIV-1Ba-L–infected immature DC with autologous CD4+ TL. (A) Effect of activated CD4+ TL (n = 9): 72 hours PI with HIV-1Ba-L, DC were cocultured or not (□) with resting (◊) or CD3 MoAb-activated (○) autologous CD4+ TL (T4L) in the presence of AZT; virus production is expressed as mean ± SD Log10 HIV RNA copy numbers; differences were not statistically significant (paired Student’s t-test) at any time point when comparing DC only versus DC + resting CD4+ TL; differences were significant when comparing DC + CD3-activated CD4+ TL versus DC + resting CD4+ TL (24 hours: P = .02; 48 hours: P= .02; 96 hours: P = .04), or versus DC only (24 hours:P = .05; 48 hours: P = .04; 96 hours: P = .02). (B) Virus transmission from DC to CD3-activated CD4+ TL (n = 4): 72 hours PI with HIV-1Ba-L, DC were cocultured (○/•) or not (□/▪) with CD3 MoAb-activated T4L in the presence (open symbols) or absence (black symbols) of AZT; differences between AZT+ and AZT− conditions were not significant (paired Student’st-test) as regards DC cultured alone; in cocultures, differences were significant at 48 hours (P = .04) and 96 hours (P = .001).
Effect on virus production of coculturing HIV-1Ba-L–infected immature DC with autologous CD4+ TL. (A) Effect of activated CD4+ TL (n = 9): 72 hours PI with HIV-1Ba-L, DC were cocultured or not (□) with resting (◊) or CD3 MoAb-activated (○) autologous CD4+ TL (T4L) in the presence of AZT; virus production is expressed as mean ± SD Log10 HIV RNA copy numbers; differences were not statistically significant (paired Student’s t-test) at any time point when comparing DC only versus DC + resting CD4+ TL; differences were significant when comparing DC + CD3-activated CD4+ TL versus DC + resting CD4+ TL (24 hours: P = .02; 48 hours: P= .02; 96 hours: P = .04), or versus DC only (24 hours:P = .05; 48 hours: P = .04; 96 hours: P = .02). (B) Virus transmission from DC to CD3-activated CD4+ TL (n = 4): 72 hours PI with HIV-1Ba-L, DC were cocultured (○/•) or not (□/▪) with CD3 MoAb-activated T4L in the presence (open symbols) or absence (black symbols) of AZT; differences between AZT+ and AZT− conditions were not significant (paired Student’st-test) as regards DC cultured alone; in cocultures, differences were significant at 48 hours (P = .04) and 96 hours (P = .001).
Finally, to evaluate the role of CD40 ligation in this system, cocultures of HIV-1Ba-L–infected DC with CD3 MoAb-activated CD4+ TL were conducted in the presence of CD40L/CD154 MoAb. The latter MoAb prevented decrease of virus production for the first 48 hours in four of five experiments, although differences did not reach statistical significance (mean Log10 HIV RNA copy numbers: 5.76 v 5.44; P = .1; paired Student’st-test). This, and the fact that at 96 hours of coculture HIV RNA copy numbers returned to control levels, indicate that other ligand-receptor interactions are also to be involved in this process.
HIV infection of CD1a+CD14−precursor-derived and CD1a−CD14+precursor-derived DC.
Because DC that differentiate from CD34+ HPC derive from either CD1a+CD14− or CD1a−CD14+ precursors, and display different phenotype, function, and susceptibility to apoptosis,42-46 we finally examined whether they also differed regarding susceptibility to HIV. CD1a+CD14− and CD1a−CD14+ precursors were sorted on day 5 from cultures conducted under standard conditions, and cultured further for 72 hours with GM-CSF/TNF-α/IL-4 with or without CD40LT. The two DC populations obtained then had comparable HIV coreceptor expression patterns either as immature or as CD40L-driven mature cells (Fig 7A). In line with these findings, nested PCR at 48 hours PI with HIV-1Ba-L or HIV-1LAI showed comparable HIV DNA amounts in immature as well as in mature DC derived from either CD1a+CD14− or CD1a−CD14+ precursors (data not shown).
HIV infection of DC derived from CD1a+CD14− or CD1a−CD14+ precursors. (A) Expression of HIV coreceptors by immature and mature DC of either population: CD1a+CD14− and CD1a−CD14+ DC precursors sorted on day 5 were cultured for 48 hours with SCF/FL/GM-CSF/TNF-/IL-4 with or without CD40LT, and labeled with FITC-CCR5 or PE-CXCR4 MoAbs; open and solid histograms are as in Fig 4A; representative composite results from two experiments of five. (B) Immature DC derived from either sorted CD1a+ CD14− (□) or CD1a−CD14+ (○) precursors were cultured for 48 hours under the standard condition before exposure to HIV-1Ba-L, the production of which is expressed as geometric mean ± SD ng p24/105 viable cells; differences were not statistically significant at any time point (n = 5).
HIV infection of DC derived from CD1a+CD14− or CD1a−CD14+ precursors. (A) Expression of HIV coreceptors by immature and mature DC of either population: CD1a+CD14− and CD1a−CD14+ DC precursors sorted on day 5 were cultured for 48 hours with SCF/FL/GM-CSF/TNF-/IL-4 with or without CD40LT, and labeled with FITC-CCR5 or PE-CXCR4 MoAbs; open and solid histograms are as in Fig 4A; representative composite results from two experiments of five. (B) Immature DC derived from either sorted CD1a+ CD14− (□) or CD1a−CD14+ (○) precursors were cultured for 48 hours under the standard condition before exposure to HIV-1Ba-L, the production of which is expressed as geometric mean ± SD ng p24/105 viable cells; differences were not statistically significant at any time point (n = 5).
The capacity of CD1a+CD14−precursor-derived and CD1a−CD14+precursor-derived immature DC to support HIV-1Ba-Lreplication was then compared by following p24 levels in supernatants. In five of seven experiments performed with different donors, CD1a+CD14− precursor-derived DC produced more virus than their CD1a−CD14+counterparts (Fig 7B): on day 20 PI, the geometric mean p24 level in supernatants of DC differentiated from CD1a+CD14− precursors was 61 ng/105 cells versus 17 ng/105 cells for CD1a−CD14+ precursor-derived DC; but, due to variability of the data, differences did not reach statistical significance (.1 < P > .05 by the paired Student’st-test) at any time point.
The effect of DC maturation on virus production by DC of each population, infected with HIV-1Ba-L, was then examined. On day 20 poststimulation, p24 levels was reduced by 1.40 ± 1.35 Log in supernatants of CD1a+CD14−precursor-derived DC and by 0.60 ± 0.35 Log (n = 3) in those from CD1a−CD14+ precursor-derived DC treated with CD40L, and by 1.20 ± 0.65 Log and 0.80 ± 0.25 Log (n = 4), respectively, in their MCM-treated counterparts.
Altogether, these data show that although immature CD1a+CD14− precursor-derived DC produce more virus than CD1a−CD14+precursor-derived DC, the capacity of CD34+ HPC-derived DC to support HIV replication is primarily determined by their maturation stage.
DISCUSSION
DC isolated from the skin or blood, or differentiated in vitro from CD34+ HPC or monocytes, express CD4 and HIV coreceptors CCR5 and CXCR4, and they are susceptible to HIV.22-30,32,59Although their capacity to directly support HIV replication has long been controversial,3,4,24,29,30,36,38,39,62,63 there is now agreement that DC at least transmit HIV to CD4+ TL with which they form large syncitia that strongly produce virus in vitro.3,4,35,64-67 Susceptibility of DC to HIV may actually vary according to their differentiation/maturation stage or their origin. This possibility is supported by findings that monocyte-derived DC are productively infected by R5 strains only when immature58 and that, in contrast to their blood or dermal counterparts, epidermal LC can be productively infected by HIV.24,40,65,68 69
Here we examined the susceptibility to HIV of DC differentiated in vitro from cord blood CD34+ HPC. This system allows concomitant differentiation of two major populations of DC, which derive from either CD1a+ or CD14+ precursors and differ as to their phenotype and function, and can be obtained as immature or mature DC depending on culture conditions.42-46We first found that sorted bulk immature CD1a+ DC, a mixture of the two populations, were permissive to R5 strain HIV-1Ba-L to a much greater extent than to X4 strain HIV-1LAI. These data agreed with HIV coreceptor expression pattern on these DC, which homogeneously expressed CCR5 but were CXCR4−, as reported.23,26,28 They also are in line with some reports,30,58 but at variance with others,29,39 showing that CD34+ HPC-derived immature CD1a+ DC can be productively infected by both X4 and R5 strains. These discrepancies probably relate to different experimental conditions, in particular use of nonsorted DC that are heterogeneous in that they comprise not only immature DC but also cells of the granulocyte lineage and CD13hiLin− intermediate precursors which are also susceptible to HIV.30 50
Maturation of HIV-1Ba-L–infected DC induced with CD40LT or MCM resulted in inhibition of virus production. This coincided with CCR5 downmodulation at the cell surface, in line with a previous report23 but at variance with another,26 which suggested that decreased virus production could result from inhibition of entry into cells. However, 48 hours PI, mature HIV-1Ba-L–exposed DC harbored about 15-fold more HIV DNA than their immature counterparts and, in line with other reports,22 26 RANTES or MIP-1β inhibited HIV-1Ba-L infection of mature DC, indicating that the virus used CCR5 even though it was undetectable by FACS. Simultaneously, mature DC upregulated membrane CXCR4, which then allowed efficient entry of X4 HIV-1LAI. However, HIV-1lai replicated at the same level in mature as in immature DC, though more efficiently than HIV-1Ba-L; but, overall, virus production was much lower than that of HIV-1Ba-L–infected immature DC. Permissivity of DC to X4 strains, if not the capacity to replicate the virus, appears thus as depending on their maturation stage.
As to the mechanisms responsible for low virus replication in mature DC, our data suggest that this should be due to a postentry block of the virus replication cycle and not to inhibition of HIV RNA retrotranscription, inasmuch as HIV-exposed mature DC harbored from 15 to >600-fold more HIV DNA than their immature counterparts.25,36,37,58 We also found that DC maturation was associated with increased production of β chemokines MIP-1α, MIP-1β, and RANTES, but at levels in supernatants that were far below those reported as being able to inhibit HIV infection.61Because it is possible that the immunoreactive β chemokine species detected by ELISA do not obligatorily represent all bioavailable species,70 increased β chemokine levels may still somehow contribute to limitation of HIV-1Ba-L spreading in cultures of mature DC.
Coculture of HIV-infected DC and TL have already been performed by other groups to evaluate the functional capacity of HIV-infected or -exposed DC, or to examine virus transmission to TL.2-4,33-35,63,66,67 71-73 Here we examined if, given the capacity of TL to induce DC maturation in cocultures, they may then affect virus production by HIV-infected DC. To this end, short-term assays were conducted in the presence of AZT to ensure that subsequent virus production was exclusively supported by the already infected DC. Indeed, autologous CD3 MoAb-activated CD4+ TL elicited decreased virus production by DC, stressing the physiological relevance of our findings on the effect of DC maturation on virus replication. However, from our experiments it could not be concluded that CD40L/CD40 interactions were exclusively responsible for the effect of activated TL in this system, indicating that other interactions are certainly involved. Interestingly, we also confirmed here that, despite reduced virus production in supernatant, mature DC still efficiently transmitted virus to TL if cocultures were conducted without AZT.
Finally, we examined whether susceptibility of DC to HIV is influenced by their origin. Indeed, both immature and mature DC derived from either CD1a+CD14− or CD1a−CD14+ precursors expressed equivalent levels of CXCR4 and CCR5, and harbored comparable HIV DNA amounts when exposed to HIV-1Ba-L or HIV-1LAI, which indicates that they probably do not differ as regards their permissivity to virus entry and retrotranscription. HIV-1Ba-L–infected immature DC derived from CD1a+CD14− precursors could produce more virus than their CD1a−CD14+precursor-derived counterparts, but the differences were limited and did not reach statistical significance. This observation could be compared with those suggesting that skin LC are apparently more permissive to HIV than derm or blood DC.24,40,65 69 In addition, inducing maturation with MCM or CD40LT of HIV-1Ba-L–infected DC of both origins reduced virus production in the same manner, indicating that the capacity to support HIV replication depends on the maturation stage rather than on the origin of DC.
In conclusion, our results confirm and extend other studies regarding the contribution of DC in the pathophysiology of HIV infection. That immature DC replicate better R5 than X4 strains is in line with the current view that DC from the vaginal or rectal mucosae select for R5 strains, accounting thus for the prominence of these strains during primary infection.58,59,74 In addition, by creating foci of infection in CD4+ TL-rich area of lymphoid organs, DC could be major contributors to the variants founder effect reported to occur there.75 The possible role of mature DC appears more complex: through CXCR4 upregulation, they could participate in the selection of X4 variants in vivo76-78; given their permissivity to both X4 and R5 strains and reduced capacity to support replication, they could serve as long-term virus reservoirs in chronically infected patients79; but their low capacity to support HIV replication, together with their increased β chemokine production, also suggests they could contribute to limit replication of R5 strains and promote local recruitment of helper CD4+ TL and CD8+ CTL, thus promoting maintenance of an effective immune response in patients.
ACKNOWLEDGMENT
We gratefully acknowledge the help of the following colleagues: Prof J. Milliez and his staff of the Service de Gynécologie-Obstétrique, Hôpital Saint-Antoine (Paris, France) for the gift of cord blood samples; Drs E. Thomas and E. Marakovski (Immunex, Seattle, WA) for their help and gift of recombinant Flt-3 ligand and CD40 ligand, and CD40L MoAb M-90; Dr D. Théophile (Roche Diagnostic Systems, Neuilly sur Seine, France) for the gift of AMPLICOR HIV-1 MONITORTM assay kits; Dr J. Maral (Chiron, Amsterdam, The Netherlands) for the gift of recombinant IL-2; Prof B. Asjö (University of Bergen, Norway) for the gift of the HIV-1Ba-L strain; and Schering Plough (Kenilworth, NJ) for the gift of recombinant GM-CSF and IL-4.
Supported by the Agence Nationale de Recherche contre le SIDA, Université Paris 6, the Centre national de la Recherche Scientifique, and the Association pour la Recherche sur les Déficits Immunitaires Viro-Induits (Paris, France).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Jean Claude Gluckman, MD, Laboratoire d’Immunologie, CERVI, hôpital de la Pitié-Salpêtrière, 83 Blvd de l’Hôpital, 75651 Paris Cedex 13, France; e-mail:jean-claude.gluckman@psl.ap-hop-paris.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal