The significant function of cytokines includes maintenance of cell survival as well as induction of cell differentiation and/or proliferation. We demonstrate here that interferon-γ (IFN-γ) plays a role for progression of Epstein-Barr virus (EBV)-infected natural killer cell leukemia (NK leukemia) through maintaining cell survival. NK leukemia cells obtained from 7 patients had clonal episomal forms of EBV, indicating that the leukemic cells were of clonal origin. Although normal NK cells constitutively expressed Bcl-2, the EBV-infected NK leukemia cells lacked endogenous Bcl-2 expression and were hypersensitive to apoptosis in vitro. The addition of IFN-γ to the culture significantly inhibited their spontaneous apoptosis without inducing cell proliferation or upregulation of Bcl-2. The NK leukemia cells constitutively secreted IFN-γ, and the patients’ sera contained a high concentration of IFN-γ, levels that were high enough to prevent NK leukemia cells from apoptosis. Bcl-XL was not involved in the IFN-γ–induced NK leukemia cell survival. These data suggest that the acquisition of IFN-γ–mediated autocrine survival signals, other than Bcl-2 or BCL-XL, might be important for the development of EBV-infected NK leukemia.
THE LYMPHOPROLIFERATIVE diseases of granular lymphocytes (LDGL) are classified into CD3+ LDGL and CD3− LDGL.1-3 The CD3+LDGL has been shown to clonally rearrange T-cell receptor (TCR) genes, indicating their clonal T-cell origin (T-LDGL). On the other hand, the CD3− LDGL belongs to the natural killer (NK) cell lineage (NK-LDGL); the surface antigens expressed in NK-LDGL cells include NK cell-associated antigens such as CD16, CD56, or CD57.
The NK-LDGL has been further classified into two categories according to clinical manifestations that are seen in specified endemic areas; the NK-LDGL is mainly observed in Asia and New Zealand and is an aggressive malignant disorder characterized by hepatosplenomegaly, pancytopenia, and disseminated intravascular coagulation due to systemic invasion of NK cells.4 On the other hand, NK-LDGL is prevalent in Europe or United States and mainly exhibits indolent chronic proliferation of NK cells (chronic NK lymphocytosis).5,6 Recently, several studies have demonstrated that cells from the former, but not from the latter, type of NK-LDGL frequently possesses Epstein-Barr virus (EBV) genomic DNA in a single episomal form.7 8 Accordingly, the agressive EBV-infected (EBV+) NK-LDGL are of clonal origin and can be diagnosed as NK leukemia.
EBV is capable of immortalizing B lymphocytes and epithelial cells of the nasopharynx, at least through inhibiting apoptosis of target cells.9 In these cases, EBV nuclear antigen-2 (EBNA-2) induces expression of EBV-associated latent membrane protein (LMP-1),10 and the LMP-1 transactivates bcl-2gene11 to promote survival of the infected cells.12 Enforced expression of EBNA-1 in B cells results in B-cell lymphoma in mice, indicating their oncogenic capacity.13 Therefore, it is reasonable to postulate that these EBV-related proteins are involved in transformation of NK cells or in reinforcement of NK leukemia cell survival.
NK leukemia is frequently associated with a systemic activation of the reticuloendothelial system called hemophagocytic lymphohistiocytosis (HLH).14-16 Macrophages and histiocytes in HLH are activated by macrophage-activating lymphokines such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α)17,18 that are released from CD2+ lymphocytes (ie, T cells and/or NK cells).19 Clonal proliferation of EBV-infected T cells has been found in HLH associated with EBV infection,20suggesting the potential role of EBV to induce production of cytokines such as IFN-γ and TNF-α in the EBV-infected T cells.21NK cells are also one of the major sources of IFN-γ and can produce a high amount of IFN-γ in response to interleukin-2 (IL-2), IL-12, and/or IL-15 in vitro.22,23 The total amount of IFN-γ released from NK cells reached a plateau within 3 days, because the NK cells undergo rapid apoptotic cell death after stimulation in vitro.23 This spontaneous apoptosis is induced at least by the TNF-α that is released from the activated NK cells themselves and might serve to limit the immune response.23 24 These data collectively suggest that the production of cytokines, including IFN-γ and/or TNF-α, is deregulated or that the cells have an altered response to these cytokines in the EBV+ NK leukemia.
These data led us to analyze the expression of EBV-related proteins and cytokines in the EBV+ NK leukemia. Unexpectedly, the EBV+ NK leukemia cells had reduced levels of endogenous Bcl-2 and spontaneously died in vitro. The EBV+ NK leukemia cells constitutively produced high amounts of IFN-γ, and IFN-γ prevented the spontaneous apoptosis of the EBV+ NK leukemia cells without inducing cell proliferation. EBV-related proteins such as LMP-1 and EBNA-2 do not appear to be involved in this process. We propose that the acquisition of survival response to IFN-γ in an autocrine fashion might be important for the progression of EBV+ NK leukemia.
MATERIALS AND METHODS
Patients.
Seven patients with aggressive NK leukemia (cases no. 1 through 7) and 1 with chronic NK lymphocytosis (case no. 8) were enrolled in this study (Table 1). NK leukemia cells bear a morphology of large granular lymphocytes (Fig 1A) and were positive for CD2 and CD56, but not CD16. Neither the TCRβ nor the TCRγ gene was rearranged in any of the 8 cases (data not shown). All 7 patients with NK leukemia presented with typical clinical features such as hepatosplenomegaly and pancytopenia and died from multiple organ failure within 6 months of diagnosis. There was prominent phagocytosis of hematopoietic cells by marrow histiocytes and macrophages in 3 of the 7 patients with NK leukemia (cases no. 2, 6, and 7). In these 3 cases, the clinical variables met the criteria for diagnosis as hemophagocytic lymphohistiocytosis.25 In case no. 8, the diagnosis of chronic NK lymphocytosis was established by persistent excess of blood NK cells according to the criteria proposed by Tefferi et al.6
Clinical and Phenotypic Characteristics of EBV+ NK Leukemia
| Case . | Diagnosis . | Age/Sex . | Peripheral Blood . | Surface Phenotypes . | Karyotypes . | Hepatosplenomegaly . | Survival (mo) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (/μL) . | LGL (% of MNC) . | CD2 . | CD3 . | CD7 . | CD16 . | CD56 . | ||||||
| 1 | NK leukemia | 18/M | 25,300 | 96% | 99.4 | 4.3 | 91.0 | 1.9 | 92.0 | 46XY | +++ | 5.0 |
| 2 | NK leukemia | 23/M | 5,300 | 98% | 98.2 | 4.4 | 79.6 | 1.1 | 88.3 | NE | +++ | 6.0 |
| 3 | NK leukemia | 75/M | 6,700 | 98% | 83.7 | 1.1 | 20.5 | 2.2 | 82.8 | +8,i17(q10) | ++ | 1.0 |
| 4 | NK leukemia | 16/F | 23,200 | 97% | 98.3 | 3.5 | 87.7 | 19.3 | 91.2 | NE | ++ | 0.5 |
| 5 | NK leukemia | 52/F | 12,600 | 98% | 96.5 | 1.5 | 2.3 | 16.1 | 96.6 | NE | ++ | 4.0 |
| 6 | NK leukemia | 65/M | 1,400 | 96% | 54.1 | 7.2 | 52.1 | 0.9 | 56.3 | 46XY | + | 1.5 |
| 7 | NK leukemia | 35/M | 12,800 | 89% | 93.4 | 6.1 | 79.0 | 3.0 | 89.8 | 46XY | +++ | 3.0 |
| 8 | Chronic NK lymphocytosis | 41/M | 5,200 | 47% | 76.5 | 21.0 | ND | 52.7 | 53.1 | NE | − | >48.0 |
| Case . | Diagnosis . | Age/Sex . | Peripheral Blood . | Surface Phenotypes . | Karyotypes . | Hepatosplenomegaly . | Survival (mo) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (/μL) . | LGL (% of MNC) . | CD2 . | CD3 . | CD7 . | CD16 . | CD56 . | ||||||
| 1 | NK leukemia | 18/M | 25,300 | 96% | 99.4 | 4.3 | 91.0 | 1.9 | 92.0 | 46XY | +++ | 5.0 |
| 2 | NK leukemia | 23/M | 5,300 | 98% | 98.2 | 4.4 | 79.6 | 1.1 | 88.3 | NE | +++ | 6.0 |
| 3 | NK leukemia | 75/M | 6,700 | 98% | 83.7 | 1.1 | 20.5 | 2.2 | 82.8 | +8,i17(q10) | ++ | 1.0 |
| 4 | NK leukemia | 16/F | 23,200 | 97% | 98.3 | 3.5 | 87.7 | 19.3 | 91.2 | NE | ++ | 0.5 |
| 5 | NK leukemia | 52/F | 12,600 | 98% | 96.5 | 1.5 | 2.3 | 16.1 | 96.6 | NE | ++ | 4.0 |
| 6 | NK leukemia | 65/M | 1,400 | 96% | 54.1 | 7.2 | 52.1 | 0.9 | 56.3 | 46XY | + | 1.5 |
| 7 | NK leukemia | 35/M | 12,800 | 89% | 93.4 | 6.1 | 79.0 | 3.0 | 89.8 | 46XY | +++ | 3.0 |
| 8 | Chronic NK lymphocytosis | 41/M | 5,200 | 47% | 76.5 | 21.0 | ND | 52.7 | 53.1 | NE | − | >48.0 |
Abbreviations: WBC, white blood cell count; LGL, large granular lymphocyte; MNC, mononuclear cell; −, negative; +, slight; ++, moderate; +++, huge; NE, no evaluable mitotic cells; ND, not done.
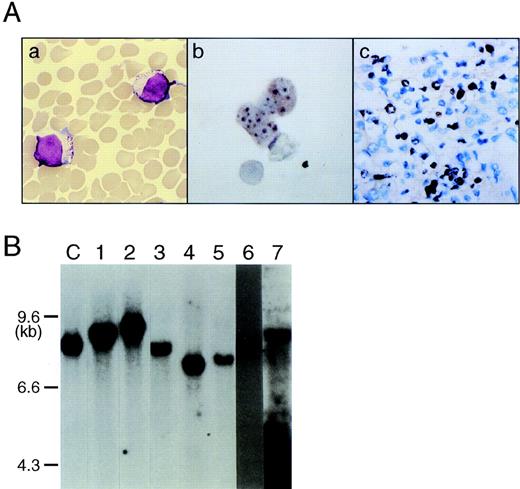
Clonal origin of EBV-infected NK cell leukemia. (A) NK leukemia cells in peripheral blood bear a morphology of large granular lymphocytes (a) (May-Grünwald-Giemsa staining; original magnification × 1,000); NK leukemia cells in peripheral blood (case no. 1) (b) and in the liver (case no. 1) (c) expressed EBER1 RNA on in situ hybridization, indicating that virtually all NK cells were infected with EBV. (B) Southern blot analysis of DNA extracted from NK-enriched PBMCs showed that each sample possessed a single joined terminal sequences of the EBV genome (EcoRI digestion), indicating their clonal origin from a single EBV-infected NK cell. The numbers on the top of each lane correspond to case numbers listed in Table 1. “C” is a positive control DNA from EBV-infected lymphoepithelioma that contains a single episomal from of EBV.
Clonal origin of EBV-infected NK cell leukemia. (A) NK leukemia cells in peripheral blood bear a morphology of large granular lymphocytes (a) (May-Grünwald-Giemsa staining; original magnification × 1,000); NK leukemia cells in peripheral blood (case no. 1) (b) and in the liver (case no. 1) (c) expressed EBER1 RNA on in situ hybridization, indicating that virtually all NK cells were infected with EBV. (B) Southern blot analysis of DNA extracted from NK-enriched PBMCs showed that each sample possessed a single joined terminal sequences of the EBV genome (EcoRI digestion), indicating their clonal origin from a single EBV-infected NK cell. The numbers on the top of each lane correspond to case numbers listed in Table 1. “C” is a positive control DNA from EBV-infected lymphoepithelioma that contains a single episomal from of EBV.
In all 8 patients, the serum EBV antibody titers showed that they had been infected by EBV, positive for IgG-viral capsid antigen (VCA) and EBV nuclear antigen, and negative for IgM-VCA. Antibodies to human T-cell leukemia virus type I were not detected in any of the cases.
Preparation of NK leukemia cells.
Heparinized peripheral blood samples were taken from patients after obtaining informed consent. Peripheral blood mononuclear cells (PBMCs) were separated by centrifugation on a Ficoll-Hypaque density gradient and incubated for 30 minutes in a plastic flask to remove monocytes, and the nonadherent PBMCs were incubated with anti-CD3 and anti-CD19 mouse IgG monoclonal antibodies (MoAbs). The CD3+ T cells and CD19+ B cells were removed by goat antimouse IgG-coated magnetic beads (Dynabeads; Dynal, Oslo, Norway). More than 95% of NK cell-enriched PBMCs were CD56+.
Phenotypic analysis and immunohistology.
Surface phenotypes was analyzed by flow cytometry using a FACScan (Becton Dickinson, Mountain View, CA) or a highly modified FACS vantage (Becton Dickinson).26 The MoAbs used were as follows: anti-CD2 (Leu5b), anti-CD3 (Leu4), anti-CD4 (leu3a), anti-CD5 (Leu1), anti-CD7 (Leu9), anti-CD8 (Leu2a), anti-CD10 (CALLA), anti-CD19 (leu12), anti-CD20 (Leu16), anti-CD34 (HPCA), anti-CD16 (Leu11) and anti-CD56 (leu19) were purchased from Becton Dickinson Imunocytometry Systems; anti-CD13 (MY7), anti-CD14 (MY4), and anti-CD33 (MY9) were from Coulter Immunology (Hialeah, FL); and anti-CD16 (OK-NK) was from Ortho Pharmaceutical Corp (Raritan, NJ). For detection of intracytoplasmic Bcl-2 and Bcl-XL proteins, cells were fixed in 2% paraformaldehyde, permeabilized with 0.03% saponin, and stained with either a fluorescein isothiocyanate (FITC)-labeled anti–Bcl-2 (DAKO Japan Co Ltd, Kyoto, Japan) or a purified anti–Bcl-XL MoAb27 (Transduction Laboratories, Lexington, KY). An FITC-labeled goat antimouse IgG1 antibody (Caltag, Burlingame, CA) was used to visualize Bcl-XL. Jurkat and HL-60 human leukemia cell lines were used for positive and negative controls for Bcl-XL staining, repectively. Cells are stained for intracytoplasmic IFN-γ staining according to the method reported by Buschle et al.28 Briefly, cells were incubated with RPMI media with 10% fetal calf serum (FCS) and 2 μmol/L of monensin for 3 hours and stained with an FITC-conjugated anti–IFN-γ antibody (Pharmingen, San Diego, CA).
To detect EBV-related proteins, cytocentrifuged preparations of NK cell-enriched PBMCs and frozen or paraffin-embedded tissue sections of liver biopsy specimens were stained with antibodies specific for EBNA2 and LMP-1 (DAKO) antibodies and with an avidin-biotin-alkaline phosphatase complex.14 Mouse IgG1 was used as a negative control.
Detection of EBV by Southern hybridization and in situ hybridization.
High molecular weight DNA was extracted from NK cell-enriched PBMCs and was digested with restriction enzyme EcoRI and BamHI (Takara Shuzou, Kyoto, Japan). The digested DNA was electrophoresed and transferred to nylon membranes. The presence of EBV-specific DNA sequences was determined by using BamHI-W (Bam W) fragments of EBV DNA (ENZO, Hudson, NY), and the polymorphic fused termini of the EBV genome were analyzed by using a probe containing the tandem terminal repeated (TR) sequences of EBV genome (kindly provided by Dr K. Hirai, Department of Virology and Immunology, Tokyo Medical and Dental University, Tokyo, Japan).14 29
In situ hybridization of EBV RNA was performed on the liver specimen or cytospin preparations using a 30-base oligonucleotide complementary to a portion of EBV-encoded small nonpolyadenylated RNAs (EBER1)-specific fragments, according to the method by Chang et al.30 The sequence of the oligonucleotide was 5′-AGACACCGTCCTCACCACCCGGGACTTGTA-3′.
Cell culture.
Purified NK leukemia cells (106/mL) were cultured in RPMI1640 (Flow Laboratories, Irvine, CA) containing 10% heat-inactivated FCS at 37°C in 5% CO2. The following cytokines were used: 10 U/mL IL-2 (Shionogi & Co, Osaka, Japan); 100 ng/mL IL-4 (Ono Pharmaceutical Co. Ltd., Osaka, Japan); 100 U/mL IL-1α, 50 ng/mL IL-6, 100 ng/mL stem cell factor (SCF), and 10 to 1,000 U/mL IFN-γ (Kirin Brewery Co, Tokyo, Japan); and 10 to 100 ng/mL of IL-12 (Genzyme Co, Cambridge, MA). A neutralizing anti–IFN-γ MoAb (Genzyme) was used at 200 ng/mL. At this concentration, the antibody can neutralize 200 U/mL of IFN-γ.
Detection of viability, apoptosis, and proliferation.
Cell viability was assessed using an MTT assay31 as well as a conventional trypan blue dye exclusion test. Briefly, 20 μL of 1 mg/mL MTT solution (Sigma Chemical Co, Ltd, Poole, UK) was added to 200-μL microcultures in a flat-bottomed 96-well microtiter plate, and the plate was incubated for 4 hours. The formazan crystals that formed were dissolved in 100 μL acid/alcohol (0.04 N HCl in isopropanol). The optical density (OD) was measured with a dual-beam multiplate reader (Titertek Multiscan, MCC; Flow Laboratories) using test and reference wave lengths of 540 and 620 nm, respectively. Cell viability was determined by calculating the change in the absorbance of the wells.
Apoptotic cells were identified by both light and electron microscopy. Cytocentrifuged cells were stained with May-Grünwald-Giemsa stain, and cells were scored as apoptotic if they had condensation of cytoplasm and chromatins and cytoplasmic fragmentation. In some cases, the apoptotic cell death was confirmed on an electronmicroscopy. Fragmentation of DNA was also determined as reported previously.32 DNA synthesis of NK leukemia cells was evaluated by measuring [3H]thymidine([3H]TdR) incorporation.33
Measurement of cytokines.
The concentrations of cytokines were measured in serum samples obtained from all patients and in cultured supernatants of NK leukemia cells harvested after plating at a concentration of 106 cells/mL and incubating for 24 hours at 37°C with 5% CO2. The concentrations of IFN-γ, IL-2, and TNF-α were measured by using radioimmunoassay kits that could detect human IFN-γ, IL-2, and TNF-α (Ire-Medgenix, Fleurus, Belgium), respectively. IL-12 p70 was measured by using PREDICTA IL-12p70 enzyme-linked immunosorbent assay (ELISA) kit (Genzyme). These assay systems could detect concentrations as low as 0.1 U/mL IFN-γ, 0.1 U/mL IL-2, 5 pg/mL TNF-α, and 2 pg/mL IL-12.
RESULTS
EBV+ NK leukemia cells are of clonal origin.
The Bam W sequence of EBV was detected in all 7 cases of NK leukemia (cases no. 1 through 7) by Southern blot analysis, but not in case no. 8, the patient with chronic NK lymphocytosis (Table 2). The liver specimens showed a diffuse aggressive infiltration of large granular lymphocytes in all 3 cases studied (cases no. 1, 2, and 4). In situ hybridization analysis to detect EBER1 RNA showed that virtually all NK leukemia cells expressed EBER1 in all 5 cases studied (Table 2 and Fig 1A). Southern blot analysis of the joined terminal repeats of the EBV genome contained in the NK cell-enriched PBMCs showed a single band byEcoRI digestion in all 7 cases (Fig 1B), indicating that NK leukemia cells contained a single episomal form of EBV. These data indicate that EBV+ NK leukemia cells were originated from a single EBV-infected NK cell in all 7 cases.
The Expression of EBV-Related Proteins in NK Leukemia
| Case No. . | Sample . | EBER . | EBV-DNA Southern Blot . | EBNA2 . | LMP1 . | Bcl-2 . | |
|---|---|---|---|---|---|---|---|
| Flow . | IH . | ||||||
| 1 | PB | + | + | − | − | − | − |
| Liver Bx | + | ND | − | − | ND | − | |
| 2 | PB | + | + | − | − | − | − |
| Liver Bx | + | ND | − | − | ND | − | |
| 3 | PB | ND | + | ND | ND | − | ND |
| 4 | PB | + | + | − | − | − | ND |
| Liver Bx | + | ND | − | − | ND | − | |
| 5 | PB | + | + | − | − | − | ND |
| 6 | PB | ND | + | ND | ND | − | ND |
| 7 | PB | + | + | − | − | − | ND |
| 8 | PB | ND | − | ND | ND | + | + |
| Case No. . | Sample . | EBER . | EBV-DNA Southern Blot . | EBNA2 . | LMP1 . | Bcl-2 . | |
|---|---|---|---|---|---|---|---|
| Flow . | IH . | ||||||
| 1 | PB | + | + | − | − | − | − |
| Liver Bx | + | ND | − | − | ND | − | |
| 2 | PB | + | + | − | − | − | − |
| Liver Bx | + | ND | − | − | ND | − | |
| 3 | PB | ND | + | ND | ND | − | ND |
| 4 | PB | + | + | − | − | − | ND |
| Liver Bx | + | ND | − | − | ND | − | |
| 5 | PB | + | + | − | − | − | ND |
| 6 | PB | ND | + | ND | ND | − | ND |
| 7 | PB | + | + | − | − | − | ND |
| 8 | PB | ND | − | ND | ND | + | + |
Bcl-2 expression was examined by flow cytometric analysis (Flow) and imunohistochemical staining (IH).
Abbreviations: PB, peripheral blood; liver Bx, liver biopsy; ND, not done.
To determine the possible role of EBV-related proteins, we analyzed the expression of these proteins by immunohistochemical stainings. The EBV+ NK leukemia cells did not express LMP-1 and EBNA-2 in all 5 cases studied (Table 2).
EBV+ NK leukemia cells lack endogenous Bcl-2 expression.
Because the infection of EBV has been known to induce Bcl-2 in B cells,12 we evaluated the cytoplasmic Bcl-2 expression in the EBV+ NK leukemia cells. Normal NK cells constitutively expressed Bcl-2 at levels that are detectable upon immunohistochemical and flow cytometric analyses (Fig 2), as reported previously.34 NK cells from 1 patient with chronic NK lymphocytosis (case no. 8) also expressed Bcl-2. However, unexpectedly, cytoplasmic Bcl-2 was undetectable in all EBV+ NK leukemia cases; circulating NK leukemia cells did not express detectable levels of Bcl-2 on both flow cytometric and immunohistochemical assays in all 7 cases (Fig 2 and Table 2). Bcl-2 could also not be detected in the NK leukemia cells that infiltrated into the liver in all 3 cases studied (Table 2). Bcl-XL was undetectable by a flow cytometry in normal controls as well as in all 4 cases with NK leukemia studied (cases no. 1, 2, 4, and 5; data not shown). These data indicate that the expression of Bcl-2 is impaired in the EBV+ NK leukemia cells.
Impaired expression of endogenous Bcl-2 in the EBV-infected NK leukemia cells. Analysis of Bcl-2 expression in either normal NK cells, NK leukemia cells, or NK cells from chronic NK lymphocytosis on immunohistochemical (upper panels) and flow cytometric (lower panels) analyses. On both assays, endogenous Bcl-2 was undetectable in NK leukemia cells, although NK cells from normal controls and a case of chronic NK lymphocytosis constitutively expressed Bcl-2. On the immunohistochemical method, Bcl-2 is visualized as diffuse red staining in cytoplasm in normal NK cells and chronic NK lymphocytosis (upper left and right panels). Results are summarized in Table 2.
Impaired expression of endogenous Bcl-2 in the EBV-infected NK leukemia cells. Analysis of Bcl-2 expression in either normal NK cells, NK leukemia cells, or NK cells from chronic NK lymphocytosis on immunohistochemical (upper panels) and flow cytometric (lower panels) analyses. On both assays, endogenous Bcl-2 was undetectable in NK leukemia cells, although NK cells from normal controls and a case of chronic NK lymphocytosis constitutively expressed Bcl-2. On the immunohistochemical method, Bcl-2 is visualized as diffuse red staining in cytoplasm in normal NK cells and chronic NK lymphocytosis (upper left and right panels). Results are summarized in Table 2.
EBV+ NK leukemia cells are hypersensitive to apoptosis in vitro.
The absence of Bcl-2 in the NK leukemia cells strongly suggests that these cells might be sensitive to apoptosis. To evaluate this, we cultured the cells in vitro and over 3 days determined the percentages of viable cells as well as apoptotic cells. As shown in Fig 3A, NK leukemia cells rapidly lost their viability as determined by a trypan blue dye exclusion test; approximately 40% of cells died as early as 24 hours after incubation and approximately 60% of cells died after 48 hours. The spontaneous apoptotic death of NK leukemia cells was most prominent in the first 24 hours, but almost reached its plateau after 48 hours. The decrease in viable NK leukemia cells during the culture was correlated with the gradual increases in the apoptotic cells (Fig 3B) that were characterized by condensed and fragmented nuclei and a loss of cell volume (Fig 4A). The mean percentage of viable NK leukemia cells evaluated 48 hours after the initiation of culture by a trypan blue dye exclusion test (42%) was almost equal to that of viability determined on an independent MTT assay (44%; Fig 5). The DNA also became more fragmented during the culture of NK leukemia cells (Fig 4B). In contrast, greater than 95% of NK cells from 6 normal donors were viable and did not undergo apoptosis even 72 hours after the initiation of culture (Fig3). NK cells from 1 patient with chronic NK lymphocytosis (case no. 8) also did not undergo significant apoptosis during the culture (data not shown).
Sequential analysis of spontaneous apoptosis of EBV-infected NK leukemia cells in vitro. NK leukemia cells (NKL; •) from cases no. 1 through 7 and normal NK cells from 6 normal controls (□) were cultured in vitro, and percentages of viable cells (A) and percentages of apoptotic cells (B) were sequentially determined on a trypan blue dye exclusion test and on morphology under an microscope, respectively. The NK leukemia cells underwent apoptotic cell death immediately after initiation of the culture. Note that apoptotic cell death reached a plateau approximately 48 hours after initiation of the culture. The addition of IFN-γ (100 U/mL) to the culture significantly inhibited the progression of spontaneous apoptosis of NK leukemia cells on both tests (NKL+IFNγ; ○). Data are shown as the mean ± SD (error bars).
Sequential analysis of spontaneous apoptosis of EBV-infected NK leukemia cells in vitro. NK leukemia cells (NKL; •) from cases no. 1 through 7 and normal NK cells from 6 normal controls (□) were cultured in vitro, and percentages of viable cells (A) and percentages of apoptotic cells (B) were sequentially determined on a trypan blue dye exclusion test and on morphology under an microscope, respectively. The NK leukemia cells underwent apoptotic cell death immediately after initiation of the culture. Note that apoptotic cell death reached a plateau approximately 48 hours after initiation of the culture. The addition of IFN-γ (100 U/mL) to the culture significantly inhibited the progression of spontaneous apoptosis of NK leukemia cells on both tests (NKL+IFNγ; ○). Data are shown as the mean ± SD (error bars).
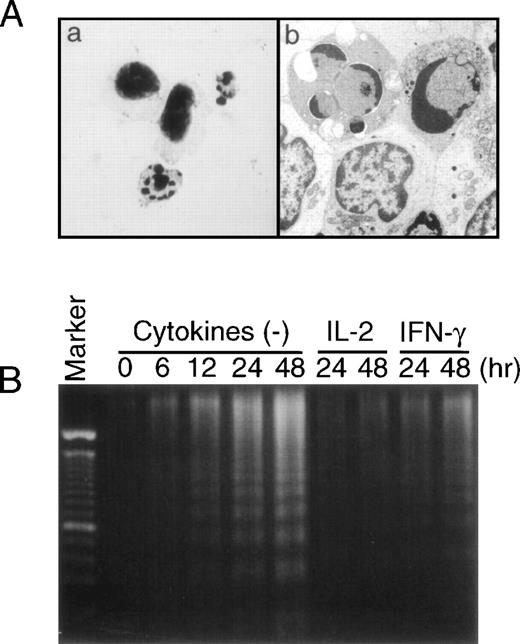
Evidences of apoptotic cell death in EBV-infected NK leukemia cells. (A) Apoptotic NK leukemia cells 48 hours after the in vitro culture. Morphology of the apoptotic cells with May-Grünwald-Giemsa staining (original magnification × 1,000) (a) and with an electron microscopy (original magnification × 15,000) (b). (B) Fragmentation of DNA from NK leukemia cells (case no. 1) cultured in vitro. DNA ladder gradually became evident during the cultures in the absence of cytokines. The addition of either IFN-γ or IL-2 significantly inhibited the amounts of DNA fragmentation.
Evidences of apoptotic cell death in EBV-infected NK leukemia cells. (A) Apoptotic NK leukemia cells 48 hours after the in vitro culture. Morphology of the apoptotic cells with May-Grünwald-Giemsa staining (original magnification × 1,000) (a) and with an electron microscopy (original magnification × 15,000) (b). (B) Fragmentation of DNA from NK leukemia cells (case no. 1) cultured in vitro. DNA ladder gradually became evident during the cultures in the absence of cytokines. The addition of either IFN-γ or IL-2 significantly inhibited the amounts of DNA fragmentation.
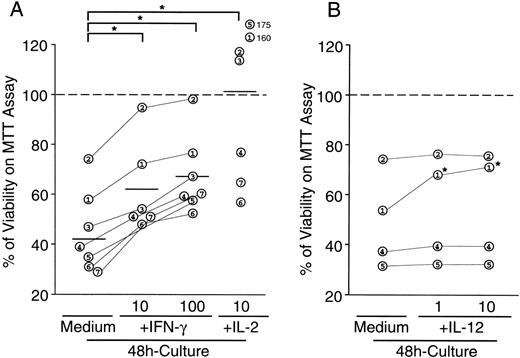
Effects of IFN-γ, IL-2, and IL-12 on viability of EBV-infected NK leukemia cells. Cells (106/mL) were cultured in the presence or absence of either IFN-γ (10 and 100 U/mL), IL-2 (10 U/mL), or IL-12 (10 ng/mL), and viability of cells was determined on MTT assay 48 hours after the initiation of cultures. Data are shown as percentages of viability determined by OD after the culture/OD before the culture. Numbers in open circles correspond to case numbers in Table 1. (A) The addition of IFN-γ to the culture significantly increased viablity of NK leukemia cells in a dose-dependent manner. The antiapoptotic effects of 1,000 U/mL of IFN-γ did not significantly differ from those of 100 U/mL of IFN-γ (data not shown). Percentages of viability reached more than 100% in some cases cultured with IL-2, which was due to the cell proliferation during the culture with IL-2, whereas IFN-γ did not stimulate proliferation of NK leukemia cells (see text). *A significant difference between the groups (P < .05) determined on Wilcoxon signed-rank tests. (B) The addition of IL-12 inhibited spontaneous apoptosis of NK leukemia cells in only 1 of 4 cases studied. *A significant difference (P < .05) determined on Student’s t-tests.
Effects of IFN-γ, IL-2, and IL-12 on viability of EBV-infected NK leukemia cells. Cells (106/mL) were cultured in the presence or absence of either IFN-γ (10 and 100 U/mL), IL-2 (10 U/mL), or IL-12 (10 ng/mL), and viability of cells was determined on MTT assay 48 hours after the initiation of cultures. Data are shown as percentages of viability determined by OD after the culture/OD before the culture. Numbers in open circles correspond to case numbers in Table 1. (A) The addition of IFN-γ to the culture significantly increased viablity of NK leukemia cells in a dose-dependent manner. The antiapoptotic effects of 1,000 U/mL of IFN-γ did not significantly differ from those of 100 U/mL of IFN-γ (data not shown). Percentages of viability reached more than 100% in some cases cultured with IL-2, which was due to the cell proliferation during the culture with IL-2, whereas IFN-γ did not stimulate proliferation of NK leukemia cells (see text). *A significant difference between the groups (P < .05) determined on Wilcoxon signed-rank tests. (B) The addition of IL-12 inhibited spontaneous apoptosis of NK leukemia cells in only 1 of 4 cases studied. *A significant difference (P < .05) determined on Student’s t-tests.
EBV+ NK leukemia cells avoid apoptotic cell death by constitutive secretion of IFN-γ.
Various cytokines such as IFN-γ, TNF-α, and IL-2 are involved in the activation and subsequent spontaneous apoptotic cell death in normal NK cells.23 We evaluated the effect of IFN-γ and IL-2 on the spontaneous apoptotic cell death of NK leukemia cells in vitro. As shown in Fig 3, the addition of 100 U/mL of IFN-γ in the culture resulted in the significant increase in percentages of viable cells and the significant decrease in the percentages of apoptotic cells, evaluated by a tripan-blue exclusion test and a morphology-based apoptotic cell determination, respectively. The spontaneous DNA fragmentation was inhibited by the addition of either IL-2 (10 U/mL) or IFN-γ (100 U/mL; Fig 4B). Furthermore, the evaluation of viability of the NK leukemia cells on an MTT assay also showed that the addition of either IFN-γ (10 to 1,000 U/mL) or IL-2 (10 U/mL) in these cultures significantly promoted their viability in both 24-hour (data not shown) and 48-hour cultures (Fig 5A). This survival-promoting effect of IFN-γ on NK leukemia cells was dose-dependent (Fig 5A) and reached its plateau at 100 U/mL. IFN-γ did not induce cell proliferation in vitro, because the absolute numbers of cells remained unchanged, and3H-thymidine was not incorporated during the cultures in all 7 cases cut (Table 3). In contrast, IL-2 induced proliferation of NK leukemia cells as well as cell survival, because numbers of cells significantly increased after the 48-hour culture, and the 3H-thymidine was significantly incorporated during the culture (Table 3).
DNA Synthesis of NK Leukemia Cells in the Presence of Cytokines
| Case No. . | [3H]Thymidine Incorporation (cpm) . | |||||
|---|---|---|---|---|---|---|
| Control . | IFN-γ (U/mL) . | IL-2 (U/mL) . | ||||
| 10 . | 100 . | 1 . | 10 . | 100 . | ||
| 1 | 82 | 80 | 95 | 184 | 2,9613-150 | 5,3253-150 |
| 2 | 38 | 81 | 78 | 9233-150 | 1,0133-150 | 2,4523-150 |
| 3 | 50 | ND | 69 | ND | ND | 3,1903-150 |
| 4 | 54 | 96 | 206 | 2,7403-150 | 2,5613-150 | 6,6723-150 |
| 5 | 43 | 57 | 44 | 68 | 2153-150 | 5623-150 |
| 6 | 75 | ND | 87 | ND | ND | 2,9883-150 |
| 7 | 37 | ND | 57 | ND | ND | 1,2203-150 |
| Case No. . | [3H]Thymidine Incorporation (cpm) . | |||||
|---|---|---|---|---|---|---|
| Control . | IFN-γ (U/mL) . | IL-2 (U/mL) . | ||||
| 10 . | 100 . | 1 . | 10 . | 100 . | ||
| 1 | 82 | 80 | 95 | 184 | 2,9613-150 | 5,3253-150 |
| 2 | 38 | 81 | 78 | 9233-150 | 1,0133-150 | 2,4523-150 |
| 3 | 50 | ND | 69 | ND | ND | 3,1903-150 |
| 4 | 54 | 96 | 206 | 2,7403-150 | 2,5613-150 | 6,6723-150 |
| 5 | 43 | 57 | 44 | 68 | 2153-150 | 5623-150 |
| 6 | 75 | ND | 87 | ND | ND | 2,9883-150 |
| 7 | 37 | ND | 57 | ND | ND | 1,2203-150 |
Results are shown as the mean value in triplicate cultures.
Abbreviation: ND, not done.
P < .05 on Student’s t-tests.
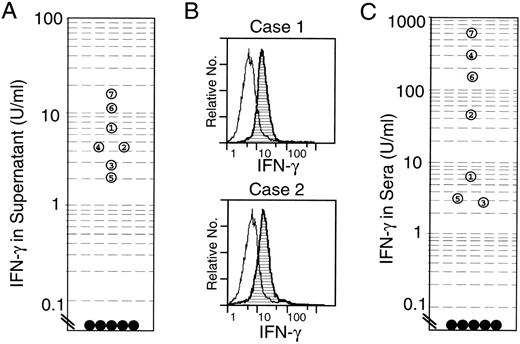
Figure 6 shows the production of these cytokines from the NK leukemia cells. Significant production of IFN-γ was seen in the 24-hour culture supernatants of the NK leukemia cells in all cases (2.0 to 18 U/mL; Fig 6A), whereas neither TNF-α nor IL-2 was detectable (data not shown). Intracytoplasmic stainings of cytokines showed that a majority of NK leukemia cells possessed intracytoplasmic IFN-γ (Fig 6B), but not IL-2 in all 4 cases studied (cases no. 1, 2, 4, and 5). However, IL-2 further promoted the production of IFN-γ from NK leukemia cells in vitro in all 3 cases studied (cases no. 1, 2, and 5; Table 4), suggesting that IL-2 maintained cell survival at least by inducing high amounts of IFN-γ from NK leukemia cells themselves.
Constitutive expression of IFN-γ in the EBV-infected NK leukemia cells. (A) Concentrations of IFN-γ in 24-hour culture supernatants of NK leukemia cells. The culture supernatants of NK leukemia cells (open circles 1 through 7) contained significant levels of IFN-γ, whereas those of normal NK cells did not (•). (B) Intracytoplasmic staining of IFN-γ in the NK leukemia cells on flow cytometric analysis. The cells were incubated with monensin (2 μmol/L) for 3 hours before the analysis. The majority of NK leukemia cells expressed intracytoplasmic IFN-γ protein. (C) Concentrations of IFN-γ in patients’ sera. Significant levels of serum IFN-γ were seen in all NK leukemia patients (○), whereas in normal controls (•), levels of IFN-γ were below detectable levels (<0.1 U/mL).
Constitutive expression of IFN-γ in the EBV-infected NK leukemia cells. (A) Concentrations of IFN-γ in 24-hour culture supernatants of NK leukemia cells. The culture supernatants of NK leukemia cells (open circles 1 through 7) contained significant levels of IFN-γ, whereas those of normal NK cells did not (•). (B) Intracytoplasmic staining of IFN-γ in the NK leukemia cells on flow cytometric analysis. The cells were incubated with monensin (2 μmol/L) for 3 hours before the analysis. The majority of NK leukemia cells expressed intracytoplasmic IFN-γ protein. (C) Concentrations of IFN-γ in patients’ sera. Significant levels of serum IFN-γ were seen in all NK leukemia patients (○), whereas in normal controls (•), levels of IFN-γ were below detectable levels (<0.1 U/mL).
In contrast, NK cell-enriched PBMCs from normal controls do not secrete detectable levels of either IFN-γ or IL-2. These antiapoptotic effects on NK leukemia cells were not observed in other cytokines, including IL-1α, IL-4, IL-6, and SCF (data not shown).
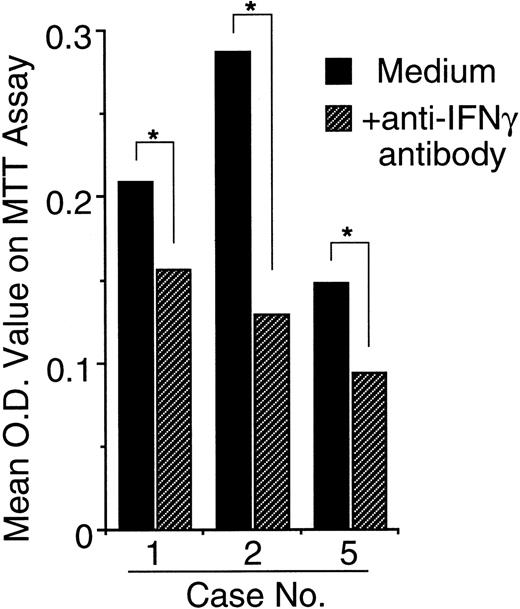
Because 24 hours after initiation of culture the NK cell leukemia cells secreted high enough levels of IFN-γ to prevent apoptosis, the apoptosis of the cells in vitro might be at least partially prevented by the IFN-γ released from NK leukemia cells themselves. To confirm this, we added neutralizing anti–IFN-γ antibodies to the culture. As expected, in all 3 cases studied (cases no. 1, 2, and 5), the anti–IFN-γ antibodies significantly promoted the spontaneous apoptosis of NK leukemia cells, resulting in significant decreases in OD value on MTT assays evaluated 24 hours after initiation of the culture (Fig 7).
Neutralization of IFN-γ in culture media accelerates spontaneous apoptosis of EBV-infected NK leukemia cells in vitro. Data are shown as mean OD values on MTT assay 24 hours after the culture that correlate with viability of the cells. In all 3 cases studied, the addition of neutralizing anti–IFN-γ antibodies to the culture media resulted in an accelerated loss of viability of NK leukemia cells in vitro. *A significant difference between the groups (P < .05) determined on Student’s t-tests.
Neutralization of IFN-γ in culture media accelerates spontaneous apoptosis of EBV-infected NK leukemia cells in vitro. Data are shown as mean OD values on MTT assay 24 hours after the culture that correlate with viability of the cells. In all 3 cases studied, the addition of neutralizing anti–IFN-γ antibodies to the culture media resulted in an accelerated loss of viability of NK leukemia cells in vitro. *A significant difference between the groups (P < .05) determined on Student’s t-tests.
IL-12 is not involved in the IFN-γ–mediated autocrine survival loop of NK leukemia cells.
We were interested in whether IL-12 is involved in the IFN-γ–mediated autocrine survival loop of NK leukemia cells, because IL-12 is known to be a potent inducer of IFN-γ in normal NK cells. Figure 5B and Table 4 show the effect of IL-12 on the NK leukemia cell survival and on the production of IFN-γ or IL-2 from NK leukemia cells, respectively. In only 1 of 4 cases studied (case no. 1), the addition of IL-12 to the culture significantly promoted the survival of NK leukemia cells (Fig 5B). IL-12 also stimulated IFN-γ production from NK leukemia cells only in case no. 1, but not in the other 2 cases studied (Table 4). Furthermore, NK leukemia cells did not produce detectable levels of IL-12 in the absence or presence of either IL-2 or IFN-γ in all 3 cases studied including the case no. 1 (Table 4). Accordingly, although responsiveness to IL-12 (to produce IFN-γ) might be preserved in some cases of NK leukemia, IL-12 does not play a role in the IFN-γ–mediated autocrine survival loop of NK leukemia cells.
Concentration of IFN-γ and IL-12 in Culture Supernatants of NK Leukemia Cells
| Case No. . | No Cytokines . | +IL-2 (10 U/mL) . | +IL-12 (10 ng/mL) . | +IFN-γ (100 U/mL) . | ||||
|---|---|---|---|---|---|---|---|---|
| IFN-γ (U/mL) . | IL-12 (pg/mL) . | IFN-γ (U/mL) . | IL-12 (pg/mL) . | IFN-γ (U/mL) . | IL-12 (pg/mL) . | IFN-γ (U/mL) . | IL-12 (pg/mL) . | |
| 1 | 5.3 | <2.0 | 155.0 | <2.0 | 160.3 | NE | NE | <2.0 |
| 2 | 4.4 | <2.0 | 14.9 | <2.0 | 4.2 | NE | NE | <2.0 |
| 5 | 6.2 | <2.0 | 87.8 | <2.0 | 9.3 | NE | NE | <2.0 |
| Case No. . | No Cytokines . | +IL-2 (10 U/mL) . | +IL-12 (10 ng/mL) . | +IFN-γ (100 U/mL) . | ||||
|---|---|---|---|---|---|---|---|---|
| IFN-γ (U/mL) . | IL-12 (pg/mL) . | IFN-γ (U/mL) . | IL-12 (pg/mL) . | IFN-γ (U/mL) . | IL-12 (pg/mL) . | IFN-γ (U/mL) . | IL-12 (pg/mL) . | |
| 1 | 5.3 | <2.0 | 155.0 | <2.0 | 160.3 | NE | NE | <2.0 |
| 2 | 4.4 | <2.0 | 14.9 | <2.0 | 4.2 | NE | NE | <2.0 |
| 5 | 6.2 | <2.0 | 87.8 | <2.0 | 9.3 | NE | NE | <2.0 |
Data are shown as mean in triplicate cultures.
The sera from patients with EBV+ NK leukemia contains high concentrations of IFN-γ, but not IL-2 or IL-12.
Because IFN-γ maintains survival of NK leukemia cells in an autocrine fashion in vitro, we measured concentrations of IFN-γ in NK leukemia patients’ sera to test whether serum IFN-γ prevent circulating NK leukemic cells from apoptosis in vivo. As shown in Fig 6C, all 7 NK leukemia sera showed an increased levels of IFN-γ (2.8 to 620 U/mL; mean, 156.2 U/mL). On the other hand, IFN-γ was undetectable in sera from 5 healthy volunteers and 1 patient from chronic NK lymphocytosis (case no. 8). Neither IL-2, IL-12, nor TNF-α was detectable in all sera from EBV+ NK leukemia cases as well as those from normal controls.
Antiapoptotic effect of IFN-γ on EBV+ NK leukemia cells does not involve upregulation of either Bcl-2 or Bcl-XL.
Some cytokines have been shown to directly upregulate endogenous Bcl-2 to maintain survival of target cells; for example, we have shown that a principal role of IL-7 for T-cell development is to maintain cell survival through upregulating (at least) Bcl-2.26 To clarify a mechanism of survival-promoting effect of IFN-γ on the EBV+ NK leukemia cells, we evaluated intracytoplasmic Bcl-2 and Bcl-XL levels after 24 hours of incubation with either IFN-γ (100 U/mL) or IL-2 (10 U/mL) by flow cytometry. Although both cytokines could block the spontaneous apoptosis of NK leukemia cells, both Bcl-2 and Bcl-XL remained undetectable in the IFN-γ– or IL-2–treated NK leukemia cells in all 4 cases studied (cases no. 1, 2, 4, and 5; data not shown). Accordingly, the survival-promoting effects of IFN-γ and IL-2 on EBV+ NK leukemia cells might be independent of either Bcl-2 or Bcl-XL.
DISCUSSION
NK cells are innate immune effector cells. The production of IFN-γ from NK cells plays a pivotal role for the initiation of an immune response to various infections through activating monocytes to eliminate pathogens. However, this immune response of NK cells is self-limiting; NK cells undergo apoptotic cell death approximately 3 days after activation, probably through producing apoptosis-inducing cytokines (ie, TNF-α) in an autocrine fashion.23 24 In the present report, we demonstrated that this self-limited process of activation of NK cells is disrupted in the EBV+ NK leukemia cells.
The EBV+ NK leukemia cells were hypersensitive to apoptosis when they were transferred into liquid culture. The majority of NK leukemia cells constitutively secreted IFN-γ. These features of NK leukemia cells closely resemble those of normal NK cells activated by IL-2 and IL-12.23 However, in NK leukemia, the addition of IFN-γ at the initiation of liquid cultures significantly inhibited the spontaneous apoptosis. The EBV+ NK leukemia cells gradually ceased to undergo apoptosis in the liquid culture without adding IFN-γ, because the relative numbers of apoptotic cells reached the plateau after 48 hours. This probably results from the accumulation of IFN-γ released from the NK leukemia cells, because concentrations of IFN-γ in the 24-hour culture supernatant had already reached levels that protected NK leukemia cells from apoptosis, and neutralization of the secreted IFN-γ significantly promoted the apoptosis of NK leukemia cells in vitro. The NK leukemia cells probably receive this survival signal from IFN-γ in vivo, because the concentration of IFN-γ in patients’ sera raise to the effective levels. Neither IL-2, IL-12, nor TNF-α was detectable in either culture supernatants or patients’ sera. Thus, the NK leukemia cells might become responsive to IFN-γ to maintain cell survival through the transformation process, resulting in the acquisition of an autocrine loop for survival. IL-12 seems not to be involved in the autocrine loop. It is possible that lack of TNF-α-mediated apoptosis-inducing signals23 24 may augment the survival-promoting effect of IFN-γ.
Another significant intracellular change in NK leukemia cells is an impaired expression of the survival protein, Bcl-2.11 This might account for the hypersensitivity to apoptosis of the NK leukemia cells. Because normal NK cells do not downregulate Bcl-2 after they are activated by IL-2 and IL-12,23 the reduction of Bcl-2 expression in the NK leukemia cells might result from EBV-related transforming events. The aquisition of the autocrine loop via IFN-γ might be required to compensate the loss of Bcl-2 in NK leukemia cells. Bcl-XL was not detected in normal NK cells or NK leukemia cells, and neither IFN-γ nor IL-2 could induce Bcl-XL in both populations. Therefore, the survival-promoting effect of IFN-γ on the NK leukemia cells is independent of Bcl-2 or Bcl-XL.
The impairment of Bcl-2 expression and the autocrine survival-promoting loop via IFN-γ are exclusively seen in EBV+ NK leukemia, but not in EBV− chronic NK lymphocytosis or normal NK cells. Furthermore, the aggressive clinical course in EBV+NK leukemia markedly differs from the relatively indolent clinical features in EBV− chronic NK lymphocytosis.6 Accordingly, it is reasonable to assume that EBV may cause alterations of intracellular events specific for EBV+ NK leukemia, such as constitutive expression of IFN-γ, loss of Bcl-2 expression, and IFN-γ–mediated survival response, and that the acquisition of these alterations might lead to aggressive phenotypes in EBV+ NK leukemia. It is curious that hypersensitivity to apoptosis with loss of Bcl-2 was seen in EBV+ NK leukemia cells; EBV-infection in Hodgkin’s lymphoma cells and B cells has been shown to induce bcl-212and/or bcl-2 homologue, BHRF-1,35,36 to protect the cells from apoptosis. Because EBNA-2 and LMP-1 were undetectable in EBV+ NK leukemia cells, it appears that these EBV-related proteins do not have anything to do with the prolonged survival of EBV+ NK leukemia cells in response to IFN-γ. This was not unusual, because both EBNA-2 and LMP-1 are largely undetectable in other EBV-related neoplasms such as Burkitt’s lymphoma, nasopharyngeal carcinoma,37 and T-cell neoplasms.38
Other similarities between EBV+ NK leukemia and Burkitt’s lymphoma are that both are hypersensitive to apoptotic cell death39 and have an impaired expression of Bcl-2.40 Because EBNA-1 is reportedly expressed in Burkitt’s lymphoma,41 further studies of other EBV-related proteins or genes, including EBNA-1, concerning the regulation of Bcl-2 and/or IFN-γ expression and the modulation of downstream events of IFN-γ receptors are warranted in Burkitt’s lymphoma as well as EBV+ NK leukemia.
SCF has been shown to maintain survival of NK cells through upregulating Bcl-2.42 However, SCF is not involved in the maintenance of NK leukemia cells, because the NK leukemia cells did not express the SCF receptor, c-Kit, and the spontaneous apoptosis was not inhibited in the presence of SCF in vitro. IL-2 could induce both proliferation and survival of NK leukemia cells. The survival-promoting effect of IL-2 on NK leukemia cells is independent of Bcl-2 or Bcl-XL and might be mediated by IFN-γ released from proliferating NK leukemia cells, as shown in vitro (Table 4). Although IL-2 was not detected in either culture supernatants or patients’ sera, it is possible that localized production of IL-2 from cells other than NK leukemia cells, ie, normal T cells, plays an important role for the stimulation of proliferation of the NK leukemia cells at the sites of invasion such as liver and spleen. Other NK cell-stimulating cytokines, including IL-12 and IL-15, may be involved in the proliferation of NK leukemia cells, because activated monocytes can produce IL-1243 and IL-15.22 However, our study showed that NK leukemia cells retain responsiveness to IL-12 in only a minority of cases.
To clarify the oncogenesis of EBV to NK cell lineage, the initial target cells of EBV infection in EBV+ NK leukemia is needed to be determined. Although CD21, a receptor for the EBV envelope protein, was reportedly expressed in a EBV+ NK cell line established from lymphoblastic lymphoma44 and a case of EBV+ nasal lymphoma with an activated NK cell phenotype,45 de novo EBV+ NK leukemia cells have not been demonstrated to express CD21.7 In agreement with previous reports, we could not detect CD21 expression in all 3 cases analyzed (data not shown). It is of interest to examine susceptibility to EBV infection in NK cell progenitors, including lymphoid-restricted common lymphoid progenitors46,47 as well as a minority of mature NK cells that reportedly express CD21.45
The autocrine loop for maintenance of cell survival in EBV+NK leukemia demonstrated here may not directly connect the oncogenesis of the disease, but might be related to its aggressive phenotype. The maintenance of cell survival is one of the important functions of cytokines,26,48,49 and the conversion into aggressive phenotypes or the accumulation of malignant cells could result from the acquisition of prolonged cell survival in some hematological malignancies.50-52 In the case of EBV+ NK leukemia, although malignant cells lack endogenous Bcl-2 expression and are susceptible apoptotic cell death, the cells maintain survival by a positive autocrine loop via IFN-γ. The high levels of IFN-γ released from NK leukemia cells might also contribute to activation of macrophage and histiocytes, triggering the occurrence of fatal HLH.19,45 Therefore, this study demonstrates that clinical maneuvers directed at inhibition of IFN-γ in vivo may be one of the potential therapeutic strategies for patients with this disease. It is important to evaluate the role of responsible survival proteins other than Bcl-2 and Bcl-XL, such as Bcl-Xγ53 on the IFN-γ–mediated survival loop in NK leukemia cells. The participation of EBV-related genes for the oncogenesis of EBV+ NK leukemia remains unclear. Understanding the effect of EBV-related genes other than LMP-1 and EBNA-2 to NK cells on the regulation of Bcl-2 and IFN-γ expression and/or on the modulation of the IFN-γ signaling pathway will help to clarify developmental mechanisms of EBV+ NK leukemia.
ACKNOWLEDGMENT
The authors are indebted to Drs Akazawa Kouhei and Naoko Kinukawa (Department of Medical Informatics, Faculty of Medicine, Kyushu University) for statistical analysis of the data. We thank Drs T. Okamura, Y. Takamatsu, T. Eto, Y. Ohno, and H. Gondo for providing samples of NK leukemia cells and Dr A. Schlageter for critically reviewing this manuscript.
Supported by a Grant-in-aid from the Ministry of Education, Science and Culture and in part by the Jose Carreras International Leukemia Foundation to K.A.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Koichi Akashi, MD, Departments of Pathology, B-261 Beckman Center, Stanford Univeristy School of Medicine, Stanford, CA 94305; e-mail: Akashi@Darwin.Stanford.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal