The hematopoietic growth factor erythropoietin (Epo) triggers changes in the expression of genes that encode important regulators of erythroid cell growth and differentiation. We now report that Epo markedly upregulates chop (gadd153) expression and that this transcription factor plays a role in erythropoiesis. Using a differential hybridization assay, we isolated a full-length cDNA ofchop as an Epo upregulated gene in Rauscher murine erythroleukemia cells. RNase protection assays demonstrated that Epo or dimethyl sulfoxide induction increased steady-state mRNA levels 10- to 20-fold after 24 to 48 hours. Western blot analysis confirmed a marked increase in CHOP protein. Among the other c/ebp family members, only c/ebp β was also upregulated during erythroid differentiation. Among normal hematopoietic cells examined, steady-state mRNA levels were highest in erythroid cells, with levels peaking during terminal differentiation. Transient overexpression ofchop in Rauscher cells resulted in a significant increase in Epo- or dimethyl sulfoxide (DMSO)-induced hemoglobinization, further linking chop upregulation to erythroid differentiation. Artificial downregulation of chop in normal murine bone marrow cells with antisense oligodeoxynucleotides inhibited colony-forming unit-erythroid (CFU-E)–derived colony growth in a concentration-dependent manner. Burst-forming unit-erythroid (BFU-E)–derived colony growth was not affected. Using a Far Western type of analysis, we detected several potential CHOP binding partners among the nuclear proteins of Rauscher cells. Importantly, the number and relative abundance of these proteins changed with differentiation. The results strongly suggest that CHOP plays a role in erythropoiesis, possibly through interactions with both C/EBP and non-C/EBP family members.
THE INTRACELLULAR signaling network triggered by the hematopoietic growth factor erythropoietin (Epo) modulates the expression of genes encoding important regulatory proteins. For example, the association of Epo with its receptor triggers activation of phospholipase-C-γ and protein kinase Cε.1 This signaling pathway is required for Epo’s upregulation of the transcription factor c-myc2-4and is associated with Epo’s mitogenic effect. Docking of Epo with its receptor also results in the activation of the JAK/STAT pathway. JAK2 kinase associates with the receptor’s cytoplasmic domains and phosphorylates STAT5.5 The activated STAT5 dimerizes and translocates to the cell’s nucleus. Erythroid differentiation also requires the downregulation of c-myb.6,7 In some experimental systems, artificial downregulation of Myb protein in the absence of erythropoietin is sufficient to induce hemoglobinization.8 Other regulatory proteins important to erythroid differentiation include GATA-19,10 and NF-E2.11 These transcription factors regulate globin gene expression in a coordinate fashion; however, it is not known which signal transducers activate them. One approach to understanding how Epo-induced signaling leads to the establishment of erythroid-specific patterns of gene expression is to compare the species of mRNAs present before and after Epo treatment.
In the present study, we used a differential hybridization technique to identify Epo-regulated genes. We report that, in Rauscher murine erythroleukemia cells, both Epo and dimethyl sulfoxide (DMSO) markedly upregulate chop (gadd153), a member of the c/ebp family of transcription factor genes.12 13 We further show that an increase in chop transcript levels is associated with terminal differentiation of normal erythroid cells in vivo and that antisense chop oligodeoxynucleotide treatment of bone marrow cells inhibits colony-forming unit-erythroid (CFU-E) but not burst-forming unit-erythroid (BFU-E) colony growth. In addition to the possibility of CHOP-C/EBP interactions, our data also suggest that there may be other, non-C/EBP proteins in erythroid cells with which CHOP interacts. Moreover, these potential alternative binding partners change during erythroid differentiation.
CHOP was first isolated as growth arrest andDNA-damage inducible gene 153 (gadd153).12,14 CHOP was also isolated on the basis of dimerization with C/EBPβ.13CHOP and the other gadd genes are induced by a wide variety of treatments that cause metabolic stress or cessation of mitosis. Some of these inducers are DNA alkylation, nutritional deprivation, oxidative stress, and treatments that perturb endoplasmic reticulum function.15-18 These treatments also tend to induce cessation of mitosis. Cessation of mitosis also occurs when cells undergo terminal differentiation: in fact, direct expression of CHOP can lead to cell cycle arrest.19 CHOP mRNA increases dramatically when dividing 3T3-L1 fibroblasts are induced to differentiate into amitotic, lipid-rich adipocytic cells13,15,16 and during keratinocyte differentiation.20 However, because CHOP is ubiquitously expressed (see Results), it is likely that it plays a role in the differentiation of many tissues.
Other members of the C/EBP family have been studied more extensively in terms of the role they play in differentiation. In adipocytic differentiation, the α, β, and δ C/EBP isoforms are expressed with complex kinetics.21 These C/EBP protein isoforms may interact in specific hierarchical patterns that change as differentiation proceeds.22 Differentiating monomyelocytic cells have their own distinct pattern of C/EBP isoform expression.23 Similarly, C/EBP α levels fluctuate during differentiation of hepatoma cells,24 ovarian follicles,25 and gut epithelium,26 as do levels of C/EBPβ levels during B-cell27 and eosinophilic differentiation.28 Expression of the recently described C/EBPε may be particularly important in the development of granulocytic cells.29 CHOP is an unusual member of this family, in that it does not appear to form homodimers and it has a noncanonical DNA-contact domain.30 Hence, its function and mechanism may be substantially different than other C/EBPs.
MATERIALS AND METHODS
Cell culture.
Construction and screening of the cDNA library.
A Rauscher cell cDNA library was prepared by Clontech (Palo Alto, CA) using the λ DR2 vector. RNA was isolated from cells treated with Epo (50 U/mL) and cycloheximide (10 μg/mL) for 1 hour using the acid guanidinium thiocyanate method of Chomczynski and Sacchi,33except that lithium precipitation was not performed. The cDNA library was screened using differential plaque hybridization. Approximately 40,000 clones were plated in 10-cm dishes at 8 pfu/cm2. Nitrocellulose filters (Millipore, Bedford, MA) were lifted off each plate in duplicate. One set of nitrocellulose lifts was hybridized to 32P-labeled cDNA prepared from untreated Rauscher cells. The duplicate set was hybridized to32P-labeled cDNA prepared from cells treated with Epo (50 U/mL) and cycloheximide (10 μg/mL) for 1 hour. The cDNA was prepared using Superscript reverse transcriptase (GIBCO-BRL, Gaithersburg, MD) according to the manufacturer’s instructions. Hybridizations were performed in 50% formamide, 6× SSC, 5× Denhardt’s, 0.5% sodium dodecyl sulfate (SDS), 100 μg/mL salmon sperm ssDNA at 42°C. Filters were washed three times in 1× SSC, 0.5% SDS at 60°C for 1 hour. DNA sequencing was performed using a USB DNA sequencing kit (USB, Cleveland, OH).
RNA analysis.
RNase protection assays were performed essentially as directed by the Ambion RNase Protection Assay kit (Ambion, Inc, Austin, TX) except that 20 U RNase T1 and 0.5 U RNase A were used in a sample volume of 100 μL. The antisense probes protected the chop cDNA sequence from nucleotide 470 to 782; the glyceraldehyde 3-phosphate dehydrogenase (gapdh) probe protected nucleotide 1120 to 1228. Northern blots were performed on poly A+ RNA isolated from 1 mg of total RNA using standard methods. Electrophoresis was through formaldehyde-agarose gels. Washes were 20 minutes each of 2× SSC, 1× SSC, and 0.1× SSC, all with 0.1% SDS at 60°C.
Expression of recombinant CHOP.
For expression of CHOP protein in Escherichia coli,BamHI and EcoRI restriction sites were introduced into the chop cDNA 5′ and 3′ ends, respectively, by polymerase chain reaction (PCR) using the following two primers, 5′-TTAAGGGATCCCAGCTGAGTCCCTG-3′ and 5′-TTCGGAATTCCTATGTGCAAGCCGA-3′. The PCR product was cloned into the BamHI and EcoRI sites of the pTrc-His expression vector (InVitrogen, Carlsbad, CA) in the reading frame, resulting in a histidine tagged CHOP protein when expressed in E coli. The histidine-tagged protein was purified using Ni-NTA resin (Qiagen, Valencia, CA). Briefly, cells from 3 L of culture were collected by centrifugation and resuspended in sonication buffer (50 mmol/L sodium phosphate, 300 mmol/L NaCl, pH 8.0). The cells were disrupted by sonication, and the sonicated mixture was centrifuged at 10,000g for 20 minutes. The supernatant was collected, and 6 mL of 50% slurry of Ni-NTA resin, previously equilibrated in sonication buffer, was added and stirred at 4°C for 60 minutes. The resin was poured into a 1.5-cm diameter column and washed with sonication buffer followed by 50 mmol/L sodium phosphate, 300 mmol/L NaCl, pH 6.0, until the A280 of the effluent was less than 0.01. The proteins were eluted with a pH gradient from 6 to 3.5. The fractions containing CHOP protein were collected, and the pH was adjusted to 7.4 with phosphate buffer.
For expression of the CHOP-glutathione-S-transferase (GST) fusion protein, the chop PCR product was cloned into the pGEX-3x vector (Amersham Pharmacia Biotech, Inc, Piscataway, NJ). CHOP-GST protein was purified following the manufacturer’s procedure (Pharmacia). Briefly, cells were centrifuged, resuspended in phosphate-buffered saline (PBS) buffer (140 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4, 1.8 mmol/L KH2PO4, pH 7.3), disrupted by sonication, and centrifuged. Glutathione Sepharose 4B equilibrated with PBS buffer was added to the supernatant of the sonicated mixture, and the mixture was incubated with gentle agitation at room temperature for 1 hour. The mixture was then packed into a small column and washed with PBS buffer until the A280 of flow-through was less than 0.01. The CHOP-GST fusion protein was eluted with 10 mmol/L reduced glutathione in 50 mmol/L Tris-HCl, pH 8.0.
Western blot analysis.
Cells were harvested by centrifugation, washed once with ice-cold Dulbecco’s PBS, and lysed directly in SDS sample buffer. A 10:1 ratio of sample buffer to cell pellet was used. After boiling the samples for 5 minutes, they were passed repeatedly through a 23-gauge needle and syringe to fragment the DNA. Cell lysate protein (100 μg/lane) was electrophoresed on a 13% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gel and was transferred electrophoretically (100 mA for 2 hours at 4°C) onto polyvinylidene fluoride (PVDF) filters (Millipore). The filters were allowed to dry after transfer and then were rewet with methanol. The blots were blocked overnight with 5% nonfat dry milk in PBS, 0.1% Tween 20.
Rabbit polyclonal antiserum against His-CHOP was prepared by Organon Teknika (Durham, NC) using purified CHOP protein as antigen. The polyclonal antibody was affinity purified using His-CHOP as the ligand immobilized on Affi-Gel 15 (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. For antibody purification, 150 mL of anti-CHOP rabbit serum diluted in an equal volume of buffer A (10 mmol/L Tris-HCl, 0.1 mol/L NaCl, pH 7.5) was applied to the affinity column by circulation overnight. The column was washed sequentially with buffer A and buffer B (10 mmol/L Tris-HCl, 4 mol/L NaCl, pH 7.5) sequentially and the eluates were collected. The antibodies were eluted finally with 4 mol/L MgCl2 followed by 4 mol/L guanidine HCl in buffer A. The elution was monitored by A280. The collected fractions were dialyzed, concentrated by ultrafiltration, and stored at −20°C.
Antisense oligodeoxynucleotide experiments.
To demonstrate a role for CHOP in normal erythropoiesis, loss-of-function experiments were performed. Mouse bone marrow cells were treated with chop antisense (GGACTCAGCGCCATGAC) or missense (CAGTACCGTCGACTCAGG) oligodeoxynucleotides (oligos; Oligos Etc, Wilsonville, OR). BFU-E– and CFU-E–derived colony growth was studied as follows. Bone marrow cells were flushed from the femurs of 7-week-old female C57BL6/J mice (Jackson Laboratories, Bar Harbor, ME) with Alpha medium (GIBCO-BRL) using a 23-gauge needle and syringe. To obtain a single-cell suspension, the cells were twice drawn through the same needle. The cells were washed twice in fresh Alpha medium, counted, and resuspended in Alpha medium/3% FBS (Hyclone Laboratories, Logan, UT) at 1 × 106 cells/mL. The cells were incubated in the absence or presence of specified concentrations ofchop antisense or missense oligos before plating. Further additions of oligos were made according to the schedules described below. All experiments were performed in duplicate.
For CFU-E growth, on day 0 the oligos were added to 0.6 mL of the cell suspension and 2.7 mL MethoCult M3330 medium (Stem Cell Technologies, Inc, Vancouver, British Columbia, Canada). After 4 hours of incubation at 37°C in a humidified 5% CO2 incubator, 1.1 mL of the mixture was plated into each of two 35-mm dishes. On day 1, the cultures received an addition of oligos equal to 25% of the initial concentration. On day 2, the cultures were scored. Each 35-mm dish was placed within a gridded 60-mm dish, and the number of CFU-E–derived colonies in 2 quadrants of the dish was determined using an inverted microscope. The counts from duplicate dishes were averaged.
For BFU-E growth, on day 0 the oligos were added to 0.3 mL of the cell suspension and 3.0 mL MethoCult M3434 medium (Stem Cell Technologies) and the cells were incubated overnight at 37°C in a humidified 5% CO2 incubator. Cell suspensions (1.1 mL each) were plated into replicate 35-mm dishes on day 1. The cultures received an addition of oligos equal to 25% of the initial concentration on day 1 and on each successive day. All dishes were scored on day 8 for BFU-E–derived colonies. The counts for duplicate dishes were averaged.
Isolation of hematopoietic cells.
Populations of T cells, B cells, and erythroid cells were isolated using antibody-coated plates using standard methods.34 35Antibody-coated plates were prepared using polystyrene bacteriological petri plates. Antibodies were diluted to 100 μg/mL in 50 mmol/L Tris, pH 9.5. Ten milliliters was poured into 100 × 15 mm plates and allowed to incubate overnight at 4°C. Plates were then washed 4× in PBS. B cells were recovered using antimouse Ig. Nonadherent cells were then panned on plates coated with an antithymocyte antibody. Cells negative to both selections were harvested as erythroid cells.
Ten murine spleens were prepared as a single-cell suspension in RPMI 1640 with 5% FBS at 2 × 107 cells/dish. Cells were allowed to incubate for 30 minutes. Nonadherent cells were removed by decanting, and adherent cells were then washed 5 times in PBS with 1% FBS. Cells were removed by repeated pipetting with a pasteur pipette using the wash buffer. Erythroid precursors were isolated and fractionated as described.36 Spleen cells were isolated from Epo-treated mice and washed in Hank’s Balanced Salt Solution without magnesium or calcium (GIBCO). Spleens were crushed and filtered through a mesh filter to obtain a single-cell suspension. The cell suspension was sedimented through Ficoll-Hypaque (Pharmacia) to remove lymphocytes. The pellet from the Ficoll-Hypaque was then layered onto a discontinuous Percoll gradient that consisted of 6-mL layers of 45%, 65%, 70%, 77%, and 90% Percoll in magnesium- and calcium-free Hank’s Balanced Salt Solution. Approximately 1 × 108cells were loaded onto the 30-mL gradient and were subjected to centrifugation at 5,000g for 20 minutes at 4°C. After centrifugation, fractions of cells were collected using a peristaltic pump and then washed in DMEM. Cells were counted and characterized by microscopic examination and benzidine staining.37Differential counts were performed before and after the separation. Cell numbers were quantified using a hemacytometer.
Cell transfection.
One day before transfection, Rauscher cells were plated at a density of 1 × 105 cells/mL in 10-cm tissue culture dishes in DMEM/10% FBS. Cells were harvested by centrifugation, suspended in DMEM without FBS at a density of 3 × 106 cells/mL, and transfected with pSVK3-chop or pSVK3 alone (20 μg DNA/transfection) by electroporation using a GenePulser II (Bio-Rad) according to the manufacturer’s instructions. Cells were grown for 48 hours after transfection and analyzed. The transfection efficiency ranged from 10% to 20% in several experiments, as monitored by transfection of pCMV-βgal control plasmid.
Detection of CHOP binding partners.
A variation on the nuclear extraction procedure of Dignam et al38 was used. Extract preparation was performed at 4°C. Cells were harvested by centrifugation, washed in ice-cold PBS, resuspended in a small volume of PBS, and recentrifuged in microfuge tubes. Five packed-cell volumes of Dignam’s buffer A were added to the pellet, which was gently resuspended by low-speed vortexing. The cells were then incubated at 0°C for 10 minutes. The cells were pelleted at low speed in a microfuge, and the supernatant was discarded. Five volumes of buffer A were added, and the cells were broken by vigorous vortexing. The nuclei were pelleted, and four original cell volumes of Dignam’s buffer C were added. The nuclei were lysed with 20 strokes of a Dounce homogenizer (type B pestle). The lysate was stirred for 30 minutes at 0°C. The nuclear extract was obtained by centrifugation of the broken nuclei (10 minutes in a microfuge at 4°C). Five hundred micrograms of nuclear protein extract was loaded onto a 8-mm wide lane of a 13%/0.35% acrylamide/bis gel and electrophoresed. Molecular weights were assigned by comparison to prestained molecular weight markers (GIBCO-BRL). The proteins were transferred electrophoretically to a PVDF membrane (Millipore Immobilon P; 0.45 μm) for 90 minutes at 100 mA in 39 mmol/L glycine, 48 mmol/L Tris-HCl, 0.037% SDS, 20% methanol. After blotting, each lane was cut into two strips, with half being used to test for either control (GST) or experimental (CHOP-GST fusion) protein binding (modified from the procedure of Ferrell and Martin39). The PVDF strips were incubated in 7 mol/L guanidine HCl, 50 mmol/L Tris-HCl, 50 mmol/L dithiothreitol (DTT), 2 mmol/L EDTA, pH 8.0, at room temperature with gentle rocking for 1 hour. Blots were rinsed with and then incubated with 1% bovine serum albumin, 250 mmol/L KCl, 20 mmol/L potassium phosphate, 2 mmol/L DTT, 0.2 mmol/L EDTA, pH 7.4 (4°C overnight with gentle rocking). Blots were then blocked for 3 hours with 5% bovine serum albumin, 50 mmol/L KCl, 20 mmol/L potassium phosphate, 2 mmol/L DTT, 0.2 mmol/L EDTA, pH 7.9. To each blocked strip was added either GST protein or CHOP-GST fusion protein (15 μg/mL in blocking buffer), and the proteins were allowed to bind overnight. The blots were washed twice for 10 minutes in blocking buffer. Goat anti-GST antibody (Pharmacia) diluted 1/100 in blocking buffer was added and allowed to bind for 1 hour at room temperature with gentle rocking. The blots were washed twice for 10 minutes in blocking buffer. The second antibody (peroxidase-conjugated rabbit antigoat IgG, 1/1,000; Cappell-Organon Teknika 55358) was added. The blots were again allowed to incubate at room temperature for 1 hour and were washed as described before. ECL (Amersham Pharmacia Biotech, Inc) chemiluminecent detection of the bound complexes was performed according to the manufacturer’s instructions.
RESULTS
Identification of Epo-regulated genes.
To identify genes regulated by Epo, we performed a differential screening process. We constructed a λ phage cDNA library from Epo-induced Rauscher murine erythroleukemia cells and prepared duplicate nitrocellulose lifts. One lift was hybridized to [32P]cDNA prepared from uninduced Rauscher cell mRNA. The other was hybridized to [32P]cDNA prepared from cells induced with Epo (50 U/mL) in the presence of cycloheximide (10 μg/mL) for 1 hour. After washing and autoradiography, careful visual comparison of the duplicates showed members of the library that corresponded to candidate Epo-regulated genes. Approximately 40,000 clones were screened initially.
To verify the authenticity of the Epo-upregulation, the cDNA inserts of the tentative positives were isolated and used as probes in Northern blot assays of RNA prepared from Rauscher cells induced with Epo but without cycloheximide. Three of the initial tentative positives were authentically Epo-upregulated. DNA sequencing showed that one of the Epo-upregulated cDNAs bore exact identity to the transcription factorchop.12 13 It contained the entire coding region and complete 3′ untranslated region. The 815-bp chop cDNA hybridized to an mRNA of approximately 1.1 kb (not shown). The other two genes were novel and will not be discussed further.
chop is upregulated during erythroid differentiation of Rauscher cells.
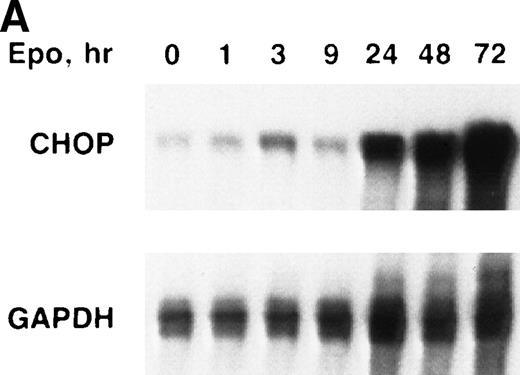
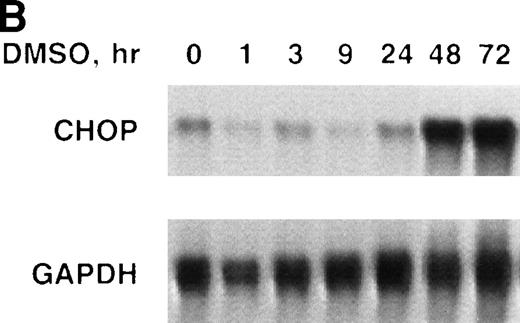
We determined the steady-state levels of chop mRNA in Rauscher cells after induction with Epo or DMSO using nuclease protection assays. Rauscher cells were induced with either Epo (100 U/mL) or DMSO (0.7%) for 0 to 72 hours. During this interval, the cells differentiated as evidenced by hemoglobin synthesis. Both Epo and DMSO induction resulted in a marked increase in steady-state levels ofchop mRNA (Fig 1A and B). In some experiments, chop mRNA increased modestly (50% to 100%) after 1 to 3 hours of induction. However, in all experiments, chopmRNA increased 10- to 20-fold after 24 to 48 hours and remained at these high levels throughout the 72-hour induction. Similar results were obtained in several replicate experiments.
chop is upregulated during erythroid differentiation of Rauscher cells. (A) Epo induction. (B) DMSO induction. RNase protection assays for chop and gapdh(glyceraldehyde-3 phosphate dehydrogenase) were performed as described in Materials and Methods and Results.
chop is upregulated during erythroid differentiation of Rauscher cells. (A) Epo induction. (B) DMSO induction. RNase protection assays for chop and gapdh(glyceraldehyde-3 phosphate dehydrogenase) were performed as described in Materials and Methods and Results.
The Epo- or DMSO-induced upregulation of chop mRNA was unrelated to cell density or to nutrient depletion. The same basal level of chop was detected in uninduced cells (0 time) and in cells maintained in standard culture conditions for 72 hours without Epo or DMSO. Moreover, when cells were induced with Epo or DMSO but maintained at subconfluent densities (by dilution with Epo-or DMSO-supplemented media to 0.5 × 106 cells/mL),chop upregulation was equally striking. In contrast to results reported with adipocytic cell lines,16 reduced glucose levels did not effect chop induction. Rauscher cells cultured in glucose 2 mmol/L showed the same pattern of chop induction as cells cultured in 10 mmol/L glucose (data not shown).
CHOP protein expression.
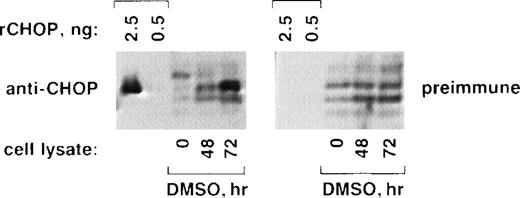
We confirmed that CHOP protein levels increase markedly during Rauscher cell differentiation (Fig 2). Purified, bacterially expressed recombinant CHOP (rCHOP; 2.5 or 0.5 ng) or SDS-solubilized protein extracts from DMSO uninduced (0 hours) or induced (48 or 72 hours; 100 μg/lane) were subjected to SDS-PAGE and were analyzed by Western blot using affinity-purified rabbit anti-CHOP antibodies (left panel) or preimmune rabbit IgG (right panel). The anti-CHOP antibodies specifically recognized 2.5 ng but not 0.5 ng of rCHOP. Preimmune IgG did not recognize rCHOP. Both anti-CHOP and preimmune IgG cross-reacted nonspecifically with several minor protein species from Rauscher cells (lanes 0, 48, and 72). However, despite this background, only the anti-CHOP antibodies identified a prominent band in differentiating cells (left panel, 72 hours) that migrated to the same position as rCHOP. This increase in CHOP protein correlates well with the increase in CHOP mRNA (Fig 2).
CHOP protein increases during erythroid differentiation of Rauscher cells. Purified recombinant CHOP protein (rCHOP) or lysates from uninduced (0 hours) or DMSO-induced (48 and 72 hours) cells were subjected to SDS-PAGE and electrophoretic transfer. They were probed with either affinity-purified anti-CHOP antibodies (left panel) or purified preimmune IgG (right panel). Note the prominent increase in CHOP protein detected after 72 hours of induction (left panel, 72 hours).
CHOP protein increases during erythroid differentiation of Rauscher cells. Purified recombinant CHOP protein (rCHOP) or lysates from uninduced (0 hours) or DMSO-induced (48 and 72 hours) cells were subjected to SDS-PAGE and electrophoretic transfer. They were probed with either affinity-purified anti-CHOP antibodies (left panel) or purified preimmune IgG (right panel). Note the prominent increase in CHOP protein detected after 72 hours of induction (left panel, 72 hours).
Expression of c/ebp isoforms during Rauscher cell differentiation.
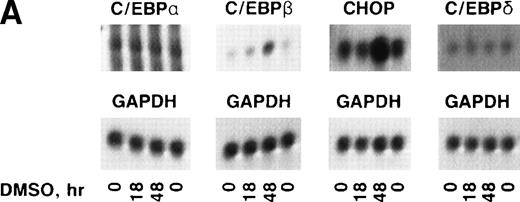
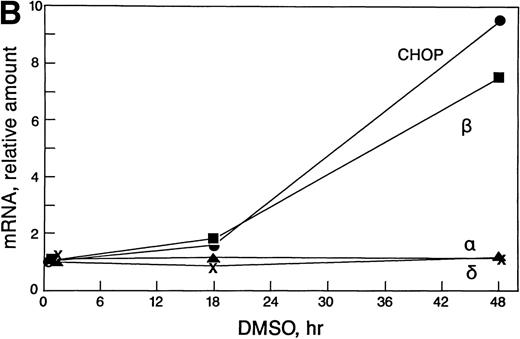
In some cell types, the C/EBP isoforms are known to heterodimerize and to have changing interactions during differentiation.23-25 Therefore, it was important to determine which c/ebp isoforms are expressed in erythroid cells and whether their expression changes during erythropoiesis. Northern blots were prepared with polyA+ RNA from uninduced and DMSO-induced Rauscher cells and were probed with c/ebp α, c/ebp β, c/ebp δ, or chop (Fig 3A and B). The blots were probed subsequently with gapdh cDNA.c/ebp α and c/ebp δ mRNA levels were extremely low and did not change during differentiation. In contrast, c/ebp β and chop mRNA increased 8.5- and 9.5-fold, respectively, during induction, as determined by laser densitometry.
Expression of c/ebp isoforms during erythroid differentiation. (A) Autoradiograms of Northern blot analyses. (B) Densitometric analysis of data in (A) normalized to 0 hours. Note the increase in both chop and c/ebpβ. In contrast,c/ebp and c/ebpδ were unchanged.
Expression of c/ebp isoforms during erythroid differentiation. (A) Autoradiograms of Northern blot analyses. (B) Densitometric analysis of data in (A) normalized to 0 hours. Note the increase in both chop and c/ebpβ. In contrast,c/ebp and c/ebpδ were unchanged.
These blots were also used to approximate the levels of the differentc/ebp isoforms relative to each other. In uninduced Rauscher cells, chop mRNA was fivefold to 10-fold more abundant thanc/ebp β mRNA (based on the use of equal amounts of probe with equal specific activity, assaying equal amounts of polyA+mRNA). The levels of c/ebp α and c/ebp δ were conspicuously lower than chop and c/ebp β. Thus, at all times during induced differentiation, chop steady-state mRNA levels were in large excess over the other c/ebps.
Tissue distribution of chop mRNA.
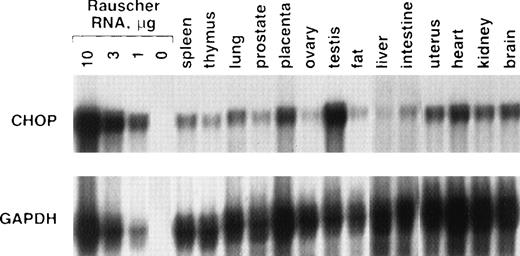
To determine whether fully differentiated cells in other tissues express chop, RNase protection assays were performed from a variety of adult mouse tissues (Fig 4). Although chop mRNA was detectable in all tissues, its abundance varied greatly. Uninduced Rauscher cells had chop mRNA levels 10- to 30-fold higher than most of the other tissues. Among the 14 tissues tested, only testis had conspicuously high chop mRNA levels, approaching that found in Rauscher cells.
chop expression in adult mouse tissues. RNase protection assay for chop or gapdh was performed as described in the text. Ten micrograms of RNA was used for each tissue.
chop expression in adult mouse tissues. RNase protection assay for chop or gapdh was performed as described in the text. Ten micrograms of RNA was used for each tissue.
CHOP expression in normal hematopoietic cells.
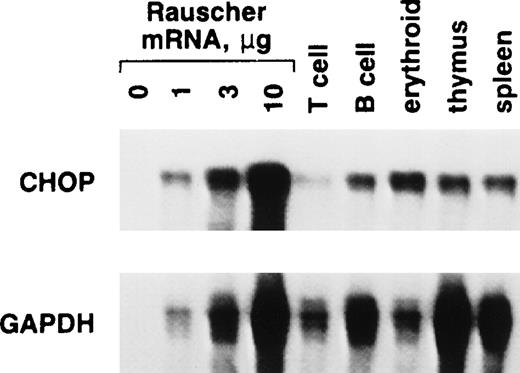
Next, we compared chop expression in Rauscher cells with that seen in normal murine hematopoietic cells (Fig 5). Among the normal cells studied, erythroid cells isolated from the spleens of Epo-treated mice had the highest level of chop mRNA. This level was not as high as that seen in Rauscher cells. However, the splenic erythroid population is heterogeneous, consisting of cells at various stages of differentiation. This analysis was complicated by the rather large differences in gapdh mRNA seen among these various cell types despite even loading of the gels demonstrated by ethidium bromide staining. Interestingly, the chop/gapdh ratio seen in normal erythroid cells was as high as that seen in Rauscher cells.
chop mRNA expression in Rauscher cells and a variety of normal murine hematopoietic cells. Splenic T, B, and erythroid cells were isolated using antibody-coated plates, as described.
chop mRNA expression in Rauscher cells and a variety of normal murine hematopoietic cells. Splenic T, B, and erythroid cells were isolated using antibody-coated plates, as described.
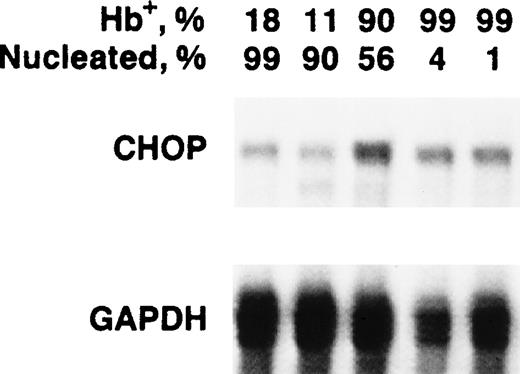
Because the pronounced upregulation of chop in Rauscher cells correlated with induction of hemoglobinization, we measuredchop mRNA levels in differentiating normal murine erythroid precursors. As erythroid precursors mature, they decrease in cell volume and undergo chromatin condensation and enucleation. These changes result in increasing cell density, a characteristic that can be used as the basis of a separation technique. Normal splenic erythroid precursor cells were separated on a 5-step discontinuous Percoll gradient. The cells from each were evaluated for chopexpression by Northern blot, for their degree of hemoglobinization by benzidine staining, and for whether they had differentiated to the point that they had condensed and extruded their nucleus by microscopic examination. The least dense fraction contained the least mature population of precursors. There were only 18% hemoglobinized (Hb+) cells and virtually all (99%) retained their nuclei.chop mRNA levels were relatively low in this least dense fraction. The third of the five fractions contained 90% Hb+ cells, of which 56% retained their nuclei. Importantly, this stage of terminal differentiation was associated with a significant increase in chop mRNA, which is very similar to the association of chop upregulation and hemoglobinization of Rauscher cells. The increase was fourfold to fivefold over the least dense fraction. The two most dense fractions contained 99% Hb+ cells and 4% and 1% nucleated cells, respectively. chop mRNA began to decrease in these more mature erythroid cells (Fig 6).
chop mRNA levels in normal murine erythroid precursors. Cells were separated by discontinuous Percoll density fractionation. Cells in each fraction were characterized for the percentage of hemoglobinized cells and the percentage of nucleated cells.
chop mRNA levels in normal murine erythroid precursors. Cells were separated by discontinuous Percoll density fractionation. Cells in each fraction were characterized for the percentage of hemoglobinized cells and the percentage of nucleated cells.
Ectopic expression of chop in Rauscher cells.
We obtained evidence of a functional role for chop in erythropoiesis by increasing its expression through transient transfection (Table 1). chopcDNA was subcloned into a pSVK3 expression vector and was transfected into Rauscher cells. After 24 hours, cells were incubated in the absence or presence of Epo or DMSO for 48 hours, and differentiating cells were identified by staining for hemoglobin. Nontransfected cells induced with Epo or DMSO for 48 hours were 34% ± 4% and 49% ± 5% hemoglobinized (Hb+), respectively. In contrast, cells transfected with and overexpressingchop were increased significantly to 43% ± 5% and 62% ± 5% Hb+, respectively, strongly suggesting that increased chop enhanced erythroid differentiation (P< .02). Overexpression of CHOP protein was confirmed by Western blot analysis (not shown). Cells transfected with vector alone had responses to Epo or DMSO virtually identical to those of nontransfected cells. It is important to note that Rauscher cells are relatively resistant to transfection. Only 10% to 20% of cells were transfected on average as monitored by Gal-4 transfection. The absolute 9% to 13% increase in hemoglobinization seen in chop-transfected cells correlates well with the transfection efficiencies achieved.
Effect of Ectopic chop Expression on Epo- or DMSO-Induced Rauscher Cell Differentiation
| Rauscher Cells . | Hb+ Cells (%)* . | |
|---|---|---|
| Epo . | DMSO . | |
| Nontransfected | 34 ± 4 | 49 ± 5 |
| chop-transfected | 43 ± 5 | 62 ± 5 |
| Vector-transfected | 32 ± 5 | 47 ± 5 |
| Rauscher Cells . | Hb+ Cells (%)* . | |
|---|---|---|
| Epo . | DMSO . | |
| Nontransfected | 34 ± 4 | 49 ± 5 |
| chop-transfected | 43 ± 5 | 62 ± 5 |
| Vector-transfected | 32 ± 5 | 47 ± 5 |
Cells were induced for 48 hours with 100 U Epo/mL or 0.7% DMSO. Hemoglobinized cells were qualified by benzidine staining. Noninduced cells exhibited less than 5% Hb+ cells.
Artificial downregulation of chop with antisense oligodeoxynucleotides inhibits CFU-E colony growth.
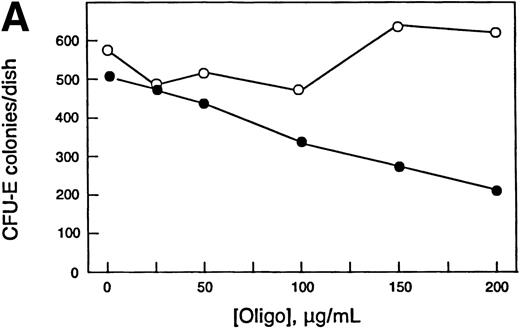
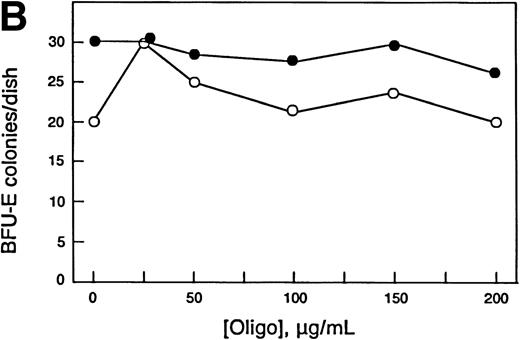
Increased chop expression correlates with erythroid differentiation, and ectopic expression of chop increased Epo- and DMSO-induced differentiation of Rauscher cells. Therefore, we reasoned that downregulating chop levels should result in reduced differentiation. In the experiment shown in Fig 7, normal murine bone marrow cells were grown in methyl cellulose culture in the absence or presence of specified concentrations of antisense or missense chop oligos using conditions required for growth of CFU-E– or BFU-E–derived colonies. Further additions of oligos were made daily. CFU-E colonies were scored after 2 days, and BFU-E colonies were scored after 8 days. Antisense chop oligos inhibited CFU-E colony growth in a concentration-dependent manner. The maximum antisense oligo concentration used (200 μg/mL) reduced CFU-E colony numbers to 42% of control values. Importantly, missense chop oligo treatment had no effect on CFU-E growth, providing evidence for the specificity of the antisense effect. In other, less detailed experiments, sense chop oligo treatment also had no effect on CFU-E growth. In contrast to the inhibitory effect seen on CFU-E colony growth, neither antisense nor missense (nor in pilot experiments, sense) chop oligos inhibited BFU-E colony growth. Similar results were obtained in other experiments.
Effect of antisense chop oligodeoxynucleotides on normal murine erythropoiesis in vitro. (A) CFU-E–derived colony growth. (B) BFU-E–derived colony growth. (•) Antisense oligos; (○) missense oligos. Note the specific concentration-dependent inhibition of CFU-E colony formation by antisense chop oligos. Each point is the mean of duplicate determinations.
Effect of antisense chop oligodeoxynucleotides on normal murine erythropoiesis in vitro. (A) CFU-E–derived colony growth. (B) BFU-E–derived colony growth. (•) Antisense oligos; (○) missense oligos. Note the specific concentration-dependent inhibition of CFU-E colony formation by antisense chop oligos. Each point is the mean of duplicate determinations.
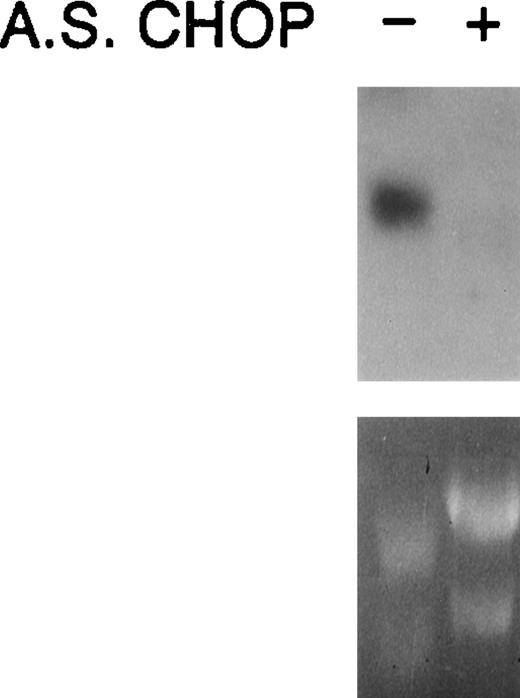
Because of technical difficulties in quantifying choptranscript levels in erythroid colonies grown in methyl cellulose, we used Rauscher cells grown in suspension culture to verify the action of antisense chop oligos (Fig 8). Rauscher cells were incubated in the absence or presence of 100 μg antisense chop oligo/mL for 24 hours. RNA was extracted and a Northern blot analysis was performed. Antisense chop oligo treatment caused a marked reduction in chop steady-state transcript levels. No change was observed using missense oligos (not shown).
Northern analysis confirms downregulation of chopby antisense oligodeoxynucleotides.
Northern analysis confirms downregulation of chopby antisense oligodeoxynucleotides.
CHOP binding partners during erythroid differentiation.
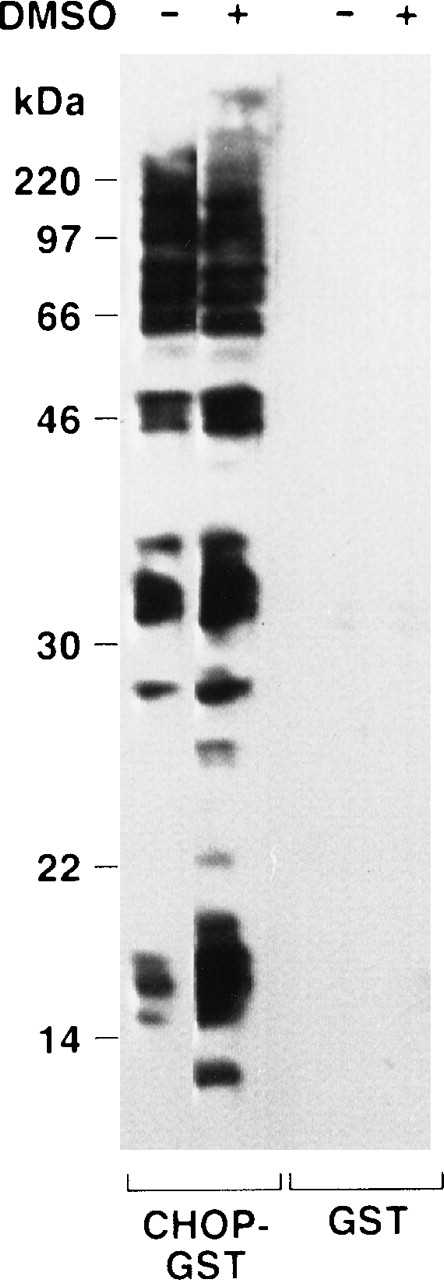
Because of reports that C/EBP isoforms can interact with non-C/EBP proteins,40-42 we used a protein-renaturation method to identify potential alternative CHOP binding partners and to determine whether they changed during erythropoiesis. Nuclear extracts from uninduced and induced cells (0.7% DMSO, 48 hours) were prepared, subjected to denaturing electrophoresis, transferred to blotting membranes, and then treated with a denaturing guanidine buffer. The denaturing buffer was washed off, and the membrane-bound proteins were allowed to renature overnight. The membranes were then probed with CHOP-GST fusion protein or GST alone (Fig9). The CHOP-GST fusion protein interacted strongly with a number of the nuclear proteins, whereas the GST protein binding was negligible. The relatively large number of potential alternative binding partners was surprising. Nonetheless, similar observations were made in repeat experiments. Interestingly, the nuclear extract from induced cells had a number of new electrophoretically distinct species that bound CHOP-GST. In six experiments, CHOP-GST bound to proteins of 14.3, 17.5, 38.5, and 54 kD that were unique to the nuclear extracts from induced (differentiating) cells. There was also a number of CHOP binding proteins in uninduced cells, the abundance of which increased markedly during differentiation. They had molecular weights of 16, 27, 32, and 34 kD. Two proteins of approximately 66 and 95 kD, respectively, appeared to decrease in abundance during differentiation.
Far Western analysis shows numerous potential CHOP binding partners in erythroid cells and changes in them during erythropoiesis. See Results.
Far Western analysis shows numerous potential CHOP binding partners in erythroid cells and changes in them during erythropoiesis. See Results.
In addition to nuclear extracts, we also examined cytosolic fractions of both uninduced and induced cells for CHOP binding partners. There were a few proteins of very low abundance that appeared to bind CHOP-GST or GST proteins weakly. There were no differences between induced and uninduced cells (data not shown).
DISCUSSION
In this study, we have identified the transcription factor chopas an Epo-regulated gene and have obtained evidence that it plays a role in erythroid differentiation. c/ebpβ was also upregulated during erythropoiesis. We found that chop message levels in Rauscher cells increased 10- to 20-fold after treatment with natural or chemical inducers of erythroid differentiation. Distinct signal transduction pathways are activated in response to EPO or DMSO,2 but treatment with either inducer results in upregulation of chop. This suggests that inducers of erythroid differentiation converge on chop upregulation. We also found that chop message is particularly abundant in erythroleukemia cells, relative to terminally differentiated adult tissues, and that its abundance in normal erythroid cells is modulated during terminal differentiation. Furthermore, manipulation of CHOP levels perturbs normal erythropoiesis. This regulated expression is suggestive of a significant functional role for chop in red blood cell development.
In normal hematopoietic cells, the highest levels of CHOP were found in erythroid cells, with levels peaking during terminal differentiation. Rauscher cells have attributes of late BFU-E and early CFU-E.43 Upon induction with Epo or DMSO, they resemble differentiating CFU-E. This later point in Rauscher cell differentiation is when chop levels were determined to be the highest. We demonstrated that CFU-E colony growth was inhibited by antisense chop treatment, whereas BFU-E colony growth was unaffected. The inhibition of CFU-E colony development by antisensechop is consistent with the absolute Epo requirement for CFU-E differentiation and strongly supports a role for CHOP in the terminal differentiation of erythroid cells. This finding compliments those described with Epo and EpoR-null mice, ie, there is an essential role for Epo in regulating the survival and terminal differentiation of CFU-E.44 Because we have identifiedchop as an Epo-upregulated gene, it would be expected that its downregulation would block differentiation at the CFU-E stage, as we have shown. However, the apparent lack of antisense chop oligo effect on BFU-E colony numbers and morphology is less easily understood. Epo addition to BFU-E cultures can be delayed somewhat without affecting BFU-E colony development significantly. However, ultimately, Epo addition is essential for the final appearance of mature BFU-E–derived colonies. If BFU-E differentiation in vitro passes through a CFU-E stage identical to that characterized by CFU-E progenitors harvested from the bone marrow, then it would be expected that antisense chop oligos should inhibit terminal differentiation of this CFU-E–like cell, resulting in small and less robust BFU-E–derived colonies (even if actual colony numbers were not affected). Our results are consistent with the hypothesis that BFU-E differentiation in culture may exhibit characteristics unique to the in vitro environment. This may also include relative resistance to oligo uptake by in vitro CFU-E–like cells. Taken together, our studies of Rauscher and normal erythroid cells show that chop expression is most robust in terminally differentiating CFU-E progeny and that CHOP plays an important role in the regulation of erythroid differentiation.
CHOP’s action is not the same in all cell types. Increasedchop facilitates erythroid differentiation. This effect is the opposite of that seen in adipogenic differentiation, in which increasedchop expression attenuates differentiation and interferes with the induction of c/ebp α and β.45 In adipocyte systems, chop is induced by nutrient depletion in the culture medium and may slow down differentiation in response to metabolic stress (particularly the condition of low glucose). In contrast, chop expression in Rauscher cells was unaffected by altered glucose conditions; also, its induction was not related to cell density. CHOP expression can also be induced by treatments that adversely affect function of the endoplamic reticulum.18 46
At present, the precise mechanism of CHOP’s action in erythroid differentiation is unknown, as is the mechanism by which Epo upregulates chop’s expression. Of note is the existence of a possible GATA-1 binding site at nucleotide −415 in the hamsterchop gene14 and of nucleotide −435 in the human chop gene.47 The human site is a perfect, prototypical GATA site, and the hamster site conserves the GATA-1 core. Indeed, the Epo receptor itself,48 known to be transactivated by GATA-1,9 49 contains a rather nonconforming TTATCT sequence. Also of obvious interest (in view of Epo’s chop upregulation concurrent with c/ebpβupregulation) are the numerous NF-I16 (C/EBPβ) sites in bothchop genes.
The coordinate upregulation of both chop and c/ebpβduring erythroid differentiation is suggestive of a mechanistic relationship. In this regard, it has recently been shown that C/EBPβ participates in regulating the pro-B cell-specific enhancer (PBE).50 In this system, CHOP and C/EBPβ interact in a developmental stage-specific pattern. These factors associate and form an inactive complex in mature B-cell lines, whereas pro-B–cell lines have active complexes of C/EBP proteins, but no detectable CHOP protein. This is reminiscent of our results in erythroid cells. CHOP levels increase as the cells mature. It may well be that the CHOP-C/EBPβ has a negative regulatory function in some contexts. Earlier experiments using cell lines suggested that C/EBPβ activates transcription of the IL-6 promoter.51 However, results from the c/ebpβ knock-out mouse show lymphoproliferative and myeloproliferative histologies similar to mice overexpressing IL-6.52 This in vivo result suggests that C/EBPβ might negatively regulate the IL-6 gene. Differentiation requires restriction in developmental potential, and downregulation of the canonical CCAAT/enhancer sites may be part of this process. Global transcription that is characteristic of the undifferentiated state can be suppressed by increasing the amount of repressors such as CHOP, LIP, and, possibly, C/EBPβ. Once physiologically relevant targets of CHOP regulation are defined, the exact role of CHOP in erythroid differentiation can be addressed, ie, whether it acts as a dominant-negative regulator of C/EBP binding, directing CHOP-C/EBP heterodimers away from canonical C/EBP binding sites, or whether CHOP activates transcription from other classes of target genes.
Interactions between the different C/EBP proteins (including CHOP) may be critical to their mechanism and function during differentiation. All of the family members have a well-conserved C-terminus that includes five heptad repeats that form an amphipathic helix. Dimerization of these amphipathic helices allows formation of a leucine zipper. All of the C/EBP family members contain this motif, and most can form homodimers and heterodimers. CHOP is an unusual member of this family in that it does not form homodimers and it has a noncanonical DNA-contact domain. In some instances, CHOP acts as a dominant negative regulator of other C/EBPs.13 Through heterodimerization, it inhibits the binding of its partner to the canonical C/EBP target sequence. In other instances, the CHOP/C/EBP heterodimer can recognize and activate noncanonical DNA binding sites.30 Although a prefered DNA binding sequence has been defined, regulated genes have not been characterized.
The present studies point to other proteins that interact with CHOP. The Far Western assay (Fig 9) suggested that CHOP’s binding partners change and become more numerous during differentiation. Other systems have shown complex and often cell-specific interactions between different C/EBP family members. There is potential for homodimerization and heterodimerization of some of the C/EBP isoforms and the possibility of regulatory interactions in trans (eg, the promoter of the c/ebp β gene has a C/EBPα binding site). In addition, interactions between C/EBP and non-C/EBP proteins have been documented.40-42 In this regard, additional data obtained by us using the yeast two hybrid system confirms that erythroid cells (and, presumably, other cells) express non-C/EBP proteins that interact specifically with CHOP in vitro and in vivo53 54(manuscripts in preparation). Further studies in this area will help to elucidate the role of CHOP in erythropoiesis and may identify novel regulatory pathways.
Because of the association of chop upregulation and ER stress, Zinszner et al55 performed targeted disruption ofchop in murine ES cells and produced animals with a chop −/− phenotype.55 Somewhat surprisingly, these mice were described as phenotypically normal and exhibited normal fertility. Unfortunately, no data regarding the hematopoietic system were reported. However, these animals, as well as embryonic fibroblasts derived from them, exhibited a defect in the development of apoptosis after treatment with agents that cause ER stress. This observation that a lack of CHOP prevents apoptosis would seem to contradict the demonstration that Epo, which increases chop expression, prevents apoptosis of erythroid cells. This apparent paradox mirrors the dissimilar effects of chop upregulation in adipogenic differentiation and in erythropoiesis and further supports the hypothesis that CHOP’s role in cell growth and differentiation is dependent on the context of cell lineage and stage as well as the other binding partners available to it.
ACKNOWLEDGMENT
The authors thank Alan Friedman for the generous gift of thec/ebp α, β, and δ cDNAs and Cindy Miller for her tutorial on methyl cellulose culture. We also acknowledge the expert editorial work of Rosemary Panza.
Supported by National Institutes of Health (NIH) NRSA F32 DK08986 (M.C.), NRSA F32 DK09201 (K.C.), NRSA F32 HL08563 (J.C.K.), and NIH R01 DK38841 and by US Navy Grant No. N00014-93-1-0776 (A.J.S.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Arthur J. Sytkowski, MD, Laboratory for Cell and Molecular Biology, Division of Hematology and Oncology, Department of Medicine, Beth Israel Deaconess Medical Center, 1 Deaconess Rd, 21-27 Burlington Bldg, Room 548, Harvard Medical School, Boston, MA 02215.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal