Patients who survive hematopoietic cell transplantation (HCT) have multiple risk factors for chronic liver disease, including hepatitis virus infection, iron overload, and chronic graft-versus-host disease (GVHD). We studied 3,721 patients who had survived 1 or more years after HCT at a single center and identified patients with histologic or clinical evidence of cirrhosis. Risk factors for the development of cirrhosis were evaluated and compared with a group of matched control subjects. Cirrhosis was identified in 31 of 3,721 patients surviving 1 or more years after HCT, 23 of 1,850 patients surviving 5 or more years, and in 19 of 860 patients surviving 10 or more years. Cumulative incidence after 10 years was estimated to be 0.6% and after 20 years was 3.8%. The median time from HCT to the diagnosis of cirrhosis was 10.1 years (range, 1.2 to 24.9 years). Twenty-three patients presented with complications of portal hypertension, and 1 presented with hepatocellular carcinoma. Thirteen patients have died from complications of liver disease, and 2 died of other causes. Three patients have undergone orthotopic liver transplantation. Hepatitis C virus infection was present in 25 of 31 (81%) of patients with cirrhosis and in 14 of 31 (45%) of controls (P = .01). Cirrhosis was attibutable to hepatitis C infection in 15 of 16 patients presenting more than 10 years after HCT. There was no difference in the prevalence of acute or chronic GVHD, duration of posttransplant immunosuppression, or posttransplant marrow iron stores between cases and controls. Cirrhosis is an important late complication of hematopoietic cell transplantation and in most cases is due to chronic hepatitis C. Long-term survivors should be evaluated for the presence of abnormal liver function and hepatitis virus infection.
LIVER INJURY IS COMMON early after hematopoietic cell transplantation (HCT), and etiologies include hepatic venocclusive disease (VOD), graft-versus-host disease (GVHD), viral and fungal infections, tumor invasion, and cholestatic disorders.1 The natural history of most of these hepatic disorders is either progression to death or reversal. Among long-term survivors, the prevalence of chronic liver disease, and particularly cirrhosis and its complications, is largely unknown.
Long-term survivors of HCT may have predisposition to chronic liver disease. Hepatitis C infection among patients transplanted before the introduction of blood product screening is estimated at 5% to 70% of surviving patients, depending on the endemic seroprevalence.2-5 In Seattle, hepatitis C infection was present posttransplant in 113 of 355 (32%) patients who underwent HCT in 1987-1988.6 Natural history studies in nontransplant patients contracting posttransfusion hepatitis C infection indicate that at least 20% of patients surviving 20 years will develop cirrhosis.7,8 It would be anticipated therefore that marrow transplant survivors would also have a high likelihood of developing cirrhosis. Furthermore, transplant-related complications, high-dose immunosuppression, and transfusional iron overload might further adversely affect the natural history of hepatitis C in these patients. There is precedent for the delayed appearance of liver damage among patients with VOD caused by toxin ingestion and among patients treated with multiple courses of chemotherapy.9-11 Liver dysfunction may also be a manifestation of chronic GVHD12; however, it is not apparent that this process leads to the development of cirrhosis.
The aim of this study of long-term survivors of HCT is to determine the prevalence and the clinical features of cirrhosis and to define the risk factors for its development.
MATERIALS AND METHODS
Techniques of Hematopoietic Transplantation
The techniques of HCT have evolved over the years represented in this study (1969 through 1995). Patients underwent cytoablative therapy, hematopoietic cell infusion, GVHD prophylaxis, and prophylaxis against viral and fungal infections in Seattle, according to research protocols at the time, which have been previously described in detail.13-19
Evaluation of Long-Term Survivors
The Long-Term Follow-Up (LTFU) staff of the Fred Hutchinson Cancer Research Center (FHCRC) conduct active monitoring of all hematopoietic cell recipients by a process of periodic on-site examinations and annual questionnaires sent to both patients and referring physicians.20 Information is gathered regarding all hospitalizations, invasive procedures, new diagnoses, medications, and results of blood testing. By this approach, updated data are available from the past 2 years on greater than 90% of long-term survivors.20 This information is coded by a modification of the Systemized Nomenclature of Medicine (SNOMED) and entered onto a computerized database. As of March 30, 1998, follow-up is available for 3,721 patients who survived more than 1 year posttransplant, for 1,850 who survived more than 5 years, and for 860 who survived more than 10 years after HCT.
Patient Selection
Cases with cirrhosis.
Transplant survivors with either histologic evidence of cirrhosis (defined as diffuse fibrosis with architecturally abnormal regenerative nodules21) or clinical evidence of severe chronic liver disease (portal hypertension and/or liver failure) were identified from the center’s LTFU database. Patients were excluded from analysis if they died of any cause within 1 year of HCT or if they died of nonhepatic complications of multisystem chronic GVHD. Thirty-one patients fulfilled criteria for inclusion in this study. For each patient, demographic information, details of transplant-related factors, and follow-up clinical and laboratory data were obtained from review of hospital records, yearly follow-up questionnaires, and correspondence with primary physicians under protocols approved by the FHCRC Institutional Review Board.
Matched control subjects.
For each case, 1 control subject was selected from among all surviving patients. Control subjects were matched according to the year of transplantation, type of HCT (allogeneic, syngeneic, or autologous), underlying disease, and age at transplantation. From the list of potential controls, the surviving individual who matched most closely with the patient with cirrhosis was selected.
Risk Factors Analyzed in Cases and Controls
VOD of the liver.
The diagnosis of VOD was made according to previously established criteria.22 Criteria included occurrence of two of the following events within 20 days of transplantation: hyperbilirubinemia (total serum bilirubin >2 mg/dL), hepatomegaly or right upper quadrant pain of liver origin, and sudden weight gain (>2% of baseline body weight) because of fluid accumulation. As an index of the severity of VOD, the peak total serum bilirubin before day 20 was used.22
Acute and chronic GVHD.
The diagnosis of acute GVHD required the appearance of a distinctive syndrome of cutaneous, gastrointestinal, and/or hepatic dysfunction proven by biopsy in at least one site.23,24 Grading of acute GVHD was by a published method.23 A diagnosis of hepatic chronic GVHD was made when allograft recipients beyond day 100 posttransplant developed cholestatic liver disease (elevated bilirubin and alkaline phosphatase) associated with clinical and histological evidence of chronic GVHD in other organ systems.12 When liver biopsy specimens were available, a histologic diagnosis of chronic GVHD was based on characteristic bile duct abnormalities, cholestasis, and portal inflammation.25
Immunosuppressive therapy after day 100.
Regimens to prevent acute GVHD most commonly included intermittent methotrexate (administered to day 102) or a combination of methotrexate (to day 11) and cyclosporine (to day 180 posttransplant). Patients received chronic immunosuppressive treatment of chronic GVHD according to research protocols that were active at the time.26-30This therapy included corticosteroids, methotrexate, azathioprine, cyclosporine, or anti-thymocyte globulin, used alone or in combination. As an index of the burden of immunosuppressive drugs from day 100 posttransplant to the diagnosis of cirrhosis (or to June 1997 in controls), we noted the number of months during which any immunosuppressive drugs were administered and whether patients received immunosuppressive agents for longer than 1 year.
Hepatitis viruses.
All patients were analyzed for the presence or absence of hepatitis B virus (HBV) and hepatitis C virus (HCV). Stored sera from approximately day 60 posttransplant was available from 27 of 31 (87%) cases and from 29 of 31 (94%) controls. Recent serum specimens were also available from 27 cases and 24 controls, obtained at a median of 5 years (range, 1 to 20 years) and 2 years (range, 1 to 13 years) posttransplant, respectively. Overall, 29 cases and 30 controls had at least one posttransplant serum sample available. Polymerase chain reaction (PCR) for the detection of HCV RNA and HBV DNA was performed as described previously.31 32 In 2 cirrhosis patients and 1 control patient in whom stored sera were not available for analysis, second generation hepatitis C antibody testing reported in the medical record was taken as evidence of the presence or absence of infection. Results of HBV DNA and/or hepatitis B surface antigen (HBsAg) testing performed by outside laboratories were recorded in the medical records of all 3 patients and controls who did not have stored serum available. Patients were designated as hepatitis C positive on the basis of any positive PCR (or serologic) result and hepatitis B positive on the basis of HBsAg and/or HBV DNA tests positive on the most recently available sample.
Tissue iron stores.
Bone marrow aspirate samples were routinely obtained at day 80 posttransplant and marrow iron stores were assessed by a computerized morphometric method. We have previously demonstrated a significant relationship between marrow iron stores determined by this method and hepatic iron stores in HCT patients.33 In the current study, we used this methodology to provide an estimate of the degree of tissue iron overload that patients had around the time of discharge from Seattle. In brief, a single 5-μm section was cut from a paraffin-embedded marrow particle preparation. If no satisfactory marrow specimen was available from day 80, another specimen from as close in time to day 80 as possible was selected. Marrow specimens suitable for morphometric analysis were available in 25 cases and 29 controls. The histologic slides were then stained with Perl’s Prussian-blue (potassium ferrocyanide) with nuclear fast red as a counterstain. Ten separate fields from each slide were photographed using a digital camera mounted on a microscope. The total area of iron particles was determined and expressed relative to the total area of hematopoietic cell nuclei.
Statistical Analysis
The frequency of putative risk factors for cirrhosis was compared in patients with cirrhosis and in the cohort of matched controls. The Wilcoxon signed rank test was used for continuous variables, and the paired sign (McNemar’s) test was used for analysis of nominal variables using the software program, StatView 4.0 (Abacus Concepts, Berkeley, CA).
RESULTS
Prevalence of Cirrhosis in Long-Term Survivors
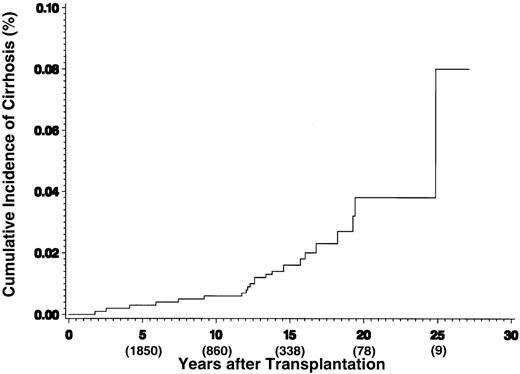
Clinical or histologic evidence of cirrhosis was identified in 31 of 3,721 patients surviving 1 or more years after HCT, 23 of 1,850 patients surviving 5 or more years, and in 19 of 860 of patients surviving 10 or more years. The cumulative incidence of diagnosis of cirrhosis after HCT is shown in Fig 1 and is estimated to be 0.6% at 10 years and 3.8% at 20 years.
Cumulative incidence of cirrhosis after HCT. The numbers in parentheses reflect the number of patients at risk at each time point.
Cumulative incidence of cirrhosis after HCT. The numbers in parentheses reflect the number of patients at risk at each time point.
Clinical Features of 31 Patients With Cirrhosis
The characteristics of the patients are shown in Table 1. The majority of patients (65%) had a pretransplant diagnosis of leukemia and 23% had severe aplastic anemia. Twenty-seven (87%) patients received marrow from an allogeneic donor. A variety of pretransplant cytoreductive regimens were used; however, the majority of patients received cyclophosphamide-based regimens. Most allogeneic recipients received methotrexate with or without cyclosporine for prevention of acute GVHD.
Patient Characteristics
| . | Cirrhosis Cases (n = 31) . | Controls (n = 31) . |
|---|---|---|
| Age at transplant* (yrs) | ||
| Median | 30 | 28 |
| Range | 2.5-56 | 2.5-54 |
| Sex (M:F) | 14:17 | 12:19 |
| Years since first marrow transplant* (yrs) | ||
| Median | 15 | 16 |
| Range | 5-25 | 5-27 |
| Primary disease* | ||
| Hematologic malignancy | 24 | 24 |
| Chronic myelogenous leukemia | 9 | 9 |
| Acute myelogenous leukemia | 8 | 8 |
| Acute lymphocytic leukemia | 3 | 2 |
| Myelodysplastic syndrome | 1 | 1 |
| Lymphoma | 3 | 4 |
| Aplastic anemia | 7 | 7 |
| Donor type* | ||
| Allogeneic | 27 | 28 |
| HLA-matched related | 22 | 26 |
| HLA-mismatched related | 3 | 1 |
| HLA-matched unrelated | 2 | 1 |
| Syngeneic | 2 | 1 |
| Autologous | 2 | 2 |
| Conditioning regimen | ||
| TBI containing | 17 | 20 |
| Cyclophosphamide + TBI ± other drugs | 16 | 19 |
| Other TBI regimen | 1 | 1 |
| Non-TBI containing | 14 | 11 |
| Cyclophosphamide alone | 5 | 7 |
| Busulfan + cyclophosphamide | 5 | 3 |
| Anti-thymocyte globulin | 2 | 0 |
| None | 1† | 0 |
| Other | 1 | 1 |
| GVHD prophylaxis | ||
| Methotrexate only | 8 | 14 |
| Methotrexate + cyclosporine | 8 | 9 |
| Cyclosporine ± prednisone | 6 | 3 |
| Other regimens | 2 | 2 |
| None | 7‡ | 3 |
| . | Cirrhosis Cases (n = 31) . | Controls (n = 31) . |
|---|---|---|
| Age at transplant* (yrs) | ||
| Median | 30 | 28 |
| Range | 2.5-56 | 2.5-54 |
| Sex (M:F) | 14:17 | 12:19 |
| Years since first marrow transplant* (yrs) | ||
| Median | 15 | 16 |
| Range | 5-25 | 5-27 |
| Primary disease* | ||
| Hematologic malignancy | 24 | 24 |
| Chronic myelogenous leukemia | 9 | 9 |
| Acute myelogenous leukemia | 8 | 8 |
| Acute lymphocytic leukemia | 3 | 2 |
| Myelodysplastic syndrome | 1 | 1 |
| Lymphoma | 3 | 4 |
| Aplastic anemia | 7 | 7 |
| Donor type* | ||
| Allogeneic | 27 | 28 |
| HLA-matched related | 22 | 26 |
| HLA-mismatched related | 3 | 1 |
| HLA-matched unrelated | 2 | 1 |
| Syngeneic | 2 | 1 |
| Autologous | 2 | 2 |
| Conditioning regimen | ||
| TBI containing | 17 | 20 |
| Cyclophosphamide + TBI ± other drugs | 16 | 19 |
| Other TBI regimen | 1 | 1 |
| Non-TBI containing | 14 | 11 |
| Cyclophosphamide alone | 5 | 7 |
| Busulfan + cyclophosphamide | 5 | 3 |
| Anti-thymocyte globulin | 2 | 0 |
| None | 1† | 0 |
| Other | 1 | 1 |
| GVHD prophylaxis | ||
| Methotrexate only | 8 | 14 |
| Methotrexate + cyclosporine | 8 | 9 |
| Cyclosporine ± prednisone | 6 | 3 |
| Other regimens | 2 | 2 |
| None | 7‡ | 3 |
Except where indicated, all figures refer to the number of patients.
Abbreviation: TBI, total body irradiation.
Characteristic matched in selection of control subjects.
Patient with aplastic anemia who received a syngeneic graft.
Including 3 allograft recipients.
Fifteen (48%) patients developing cirrhosis have died at a median of 5 years (range, 2 to 19 years) after HCT. Three individuals have undergone orthotopic liver transplantation for decompensated cirrhosis, 2 of whom are currently surviving. The median follow-up of currently surviving cirrhosis patients is 15.8 years (range, 8 to 25 years).
Table 2 shows the clinical and histologic features of liver disease in individuals with cirrhosis. The median time from HCT to the diagnosis of cirrhosis was 10.1 years (range, 1.2 to 24.9 years). Twenty-three patients presented with complications of portal hypertension (ascites [n = 11], variceal hemorrhage [n = 4], encephalopathy [n = 6], or hypersplenism [n = 7]). One patient presented with a large hepatic lesion that proved to be hepatocellular carcinoma complicating unsuspected cirrhosis. In 8 patients without clinical evidence of cirrhosis, the initial diagnosis was made on liver biopsy. The median time since transplantation in these 8 patients was 13 years (range, 6 to 25 years). Of 16 surviving patients with cirrhosis, 12 (75%) have persistent elevation of serum aminotransferases to greater than twice the upper limit of normal.
Clinical and Histologic Features of 31 Patients With Cirrhosis
| UPN . | Age at BMT (yrs) . | Gender . | Years Posttransplant to Diagnosis of Cirrhosis . | Clinical Features of Liver Disease at Presentation . | Hepatitis Viruses . | Liver Histology . | Years Posttransplant to Death,* Follow-Up, or OLT† . | Cause of Death . |
|---|---|---|---|---|---|---|---|---|
| 209 | 16 | F | 24.9 | Firm, fibrotic liver at laparotomy‡ | None | Early cirrhosis/steatosis | 25.2 | |
| 756 | 37 | M | 19.4 | Jaundice, ascites, metastatic adenocarcinoma stomach | HCV + HBV | Cirrhosis | 19.4* | Adenocarcinoma stomach |
| 299 | 19 | M | 19.3 | Variceal hemorrhage | HCV | Cirrhosis/CH | 24.0 | |
| 765 | 29 | F | 18.2 | Variceal hemorrhage | HCV | CH | 19.6 | |
| 387 | 30 | F | 16.8 | Asymptomatic, abnormal LFTs | HCV | Cirrhosis/CH | 22.4 | |
| 911 | 28 | M | 16.0 | Asymptomatic, abnormal LFTs | HCV | Early cirrhosis/CH | 18.6 | |
| 171 | 21 | M | 15.7 | Ascites, SBP, encephalopathy, thrombocytopenia | HCV + HBV | Cirrhosis/CH | 17.0* | Liver failure |
| 1028 | 26 | M | 14.6 | Ascites, jaundice | HCV | Cirrhosis/CH | 16.0† | OLT complications |
| 859 | 32 | M | 13.8 | Asymptomatic, abnormal LFTs | HCV | Cirrhosis | 19.0 | |
| 264 | 28 | M | 13.4 | Mass in liver | HCV | Cirrhosis/HCC | 15.5* | Hepatocellular carcinoma |
| 838 | 30 | F | 12.6 | Liver failure | HCV | Cirrhosis/CH | 16.3† | |
| 1782 | 22 | F | 12.3 | Asymptomatic, abnormal LFTs | HCV | Cirrhosis/siderosis | 14.8 | |
| 1391 | 27 | F | 12.2 | Splenomegaly | HCV | Cirrhosis/CH | 16.2 | |
| 1055 | 2.5 | F | 12.0 | Ascites, encephalopathy | HCV | Cirrhosis | 12.1* | Liver failure |
| 1989 | 24 | M | 11.7 | Asymptomatic, abnormal LFTs | HCV + HBV | Early cirrhosis/CH | 14.2 | |
| 2393 | 33 | M | 10.1 | Asymptomatic, abnormal LFTs | HCV | Cirrhosis/siderosis | 13.2 | |
| 1595 | 46 | M | 9.2 | Hypersplenism | HCV + HBV | Cirrhosis | 15.4 | |
| 3859 | 46 | M | 7.5 | Ascites, esophageal varices, hypersplenism | HCV | Cirrhosis/CH | 9.6† | |
| 1244 | 56 | F | 7.4 | Ascites, SBP, encephalopathy, hypersplenism | None | No biopsy | 16.9* | Liver failure |
| 4669 | 13 | F | 5.9 | Asymptomatic, abnormal LFTs | HCV | Early cirrhosis | 8.5 | |
| 5084 | 34 | F | 5.4 | Jaundice, splenomegaly | None | cGHVD/marked siderosis | 7.9 | |
| 3331 | 32 | F | 4.6 | Ascites, SBP, encephalopathy, coagulopathy, renal failure | HCV | Cirrhosis/CH | 4.9* | Liver failure |
| 3254 | 41 | F | 4.1 | Ascites, jaundice, encephalopathy | HCV + HBV | Cirrhosis/CH | 4.4* | Liver failure |
| 2831 | 37 | F | 3.7 | Jaundice, encephalopathy | HCV | Cirrhosis/cGVHD | 3.7* | Liver failure |
| 3576 | 23 | F | 3.0 | Hypersplenism | HCV | Cirrhosis | 10.5 | |
| 69772-153 | 26 | F | 2.5 | Jaundice | None | Cirrhosis/cGVHD | 2.6* | Liver failure |
| 4978 | 53 | F | 2.0 | Ascites, varices | HCV | Cirrhosis/CH | 5.3* | Ovarian carcinoma |
| 1971 | 34 | M | 1.8 | Variceal hemorrhage, multiorgan failure | HCV | Early cirrhosis/cGVHD | 2.0* | Liver failure |
| 36622-155 | 23 | M | 1.8 | Ascites, SBP, renal failure | HCV | Cirrhosis | 2.0* | Liver failure |
| 6616 | 40 | M | 1.8 | Variceal hemorrhage, ascites, renal failure | None | cGVHD, dense portal tract fibrosis | 2.9* | Liver failure |
| 72292-154 | 42 | F | 1.2 | Jaundice, ascites | None | Severe ductopenia, diffuse fibrosis | 2.9* | Liver failure |
| UPN . | Age at BMT (yrs) . | Gender . | Years Posttransplant to Diagnosis of Cirrhosis . | Clinical Features of Liver Disease at Presentation . | Hepatitis Viruses . | Liver Histology . | Years Posttransplant to Death,* Follow-Up, or OLT† . | Cause of Death . |
|---|---|---|---|---|---|---|---|---|
| 209 | 16 | F | 24.9 | Firm, fibrotic liver at laparotomy‡ | None | Early cirrhosis/steatosis | 25.2 | |
| 756 | 37 | M | 19.4 | Jaundice, ascites, metastatic adenocarcinoma stomach | HCV + HBV | Cirrhosis | 19.4* | Adenocarcinoma stomach |
| 299 | 19 | M | 19.3 | Variceal hemorrhage | HCV | Cirrhosis/CH | 24.0 | |
| 765 | 29 | F | 18.2 | Variceal hemorrhage | HCV | CH | 19.6 | |
| 387 | 30 | F | 16.8 | Asymptomatic, abnormal LFTs | HCV | Cirrhosis/CH | 22.4 | |
| 911 | 28 | M | 16.0 | Asymptomatic, abnormal LFTs | HCV | Early cirrhosis/CH | 18.6 | |
| 171 | 21 | M | 15.7 | Ascites, SBP, encephalopathy, thrombocytopenia | HCV + HBV | Cirrhosis/CH | 17.0* | Liver failure |
| 1028 | 26 | M | 14.6 | Ascites, jaundice | HCV | Cirrhosis/CH | 16.0† | OLT complications |
| 859 | 32 | M | 13.8 | Asymptomatic, abnormal LFTs | HCV | Cirrhosis | 19.0 | |
| 264 | 28 | M | 13.4 | Mass in liver | HCV | Cirrhosis/HCC | 15.5* | Hepatocellular carcinoma |
| 838 | 30 | F | 12.6 | Liver failure | HCV | Cirrhosis/CH | 16.3† | |
| 1782 | 22 | F | 12.3 | Asymptomatic, abnormal LFTs | HCV | Cirrhosis/siderosis | 14.8 | |
| 1391 | 27 | F | 12.2 | Splenomegaly | HCV | Cirrhosis/CH | 16.2 | |
| 1055 | 2.5 | F | 12.0 | Ascites, encephalopathy | HCV | Cirrhosis | 12.1* | Liver failure |
| 1989 | 24 | M | 11.7 | Asymptomatic, abnormal LFTs | HCV + HBV | Early cirrhosis/CH | 14.2 | |
| 2393 | 33 | M | 10.1 | Asymptomatic, abnormal LFTs | HCV | Cirrhosis/siderosis | 13.2 | |
| 1595 | 46 | M | 9.2 | Hypersplenism | HCV + HBV | Cirrhosis | 15.4 | |
| 3859 | 46 | M | 7.5 | Ascites, esophageal varices, hypersplenism | HCV | Cirrhosis/CH | 9.6† | |
| 1244 | 56 | F | 7.4 | Ascites, SBP, encephalopathy, hypersplenism | None | No biopsy | 16.9* | Liver failure |
| 4669 | 13 | F | 5.9 | Asymptomatic, abnormal LFTs | HCV | Early cirrhosis | 8.5 | |
| 5084 | 34 | F | 5.4 | Jaundice, splenomegaly | None | cGHVD/marked siderosis | 7.9 | |
| 3331 | 32 | F | 4.6 | Ascites, SBP, encephalopathy, coagulopathy, renal failure | HCV | Cirrhosis/CH | 4.9* | Liver failure |
| 3254 | 41 | F | 4.1 | Ascites, jaundice, encephalopathy | HCV + HBV | Cirrhosis/CH | 4.4* | Liver failure |
| 2831 | 37 | F | 3.7 | Jaundice, encephalopathy | HCV | Cirrhosis/cGVHD | 3.7* | Liver failure |
| 3576 | 23 | F | 3.0 | Hypersplenism | HCV | Cirrhosis | 10.5 | |
| 69772-153 | 26 | F | 2.5 | Jaundice | None | Cirrhosis/cGVHD | 2.6* | Liver failure |
| 4978 | 53 | F | 2.0 | Ascites, varices | HCV | Cirrhosis/CH | 5.3* | Ovarian carcinoma |
| 1971 | 34 | M | 1.8 | Variceal hemorrhage, multiorgan failure | HCV | Early cirrhosis/cGVHD | 2.0* | Liver failure |
| 36622-155 | 23 | M | 1.8 | Ascites, SBP, renal failure | HCV | Cirrhosis | 2.0* | Liver failure |
| 6616 | 40 | M | 1.8 | Variceal hemorrhage, ascites, renal failure | None | cGVHD, dense portal tract fibrosis | 2.9* | Liver failure |
| 72292-154 | 42 | F | 1.2 | Jaundice, ascites | None | Severe ductopenia, diffuse fibrosis | 2.9* | Liver failure |
Abbreviations: BMT, bone marrow transplantation; cGVHD, chronic graft-versus-host disease; CH, chronic hepatitis; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LFTs, liver function tests; OLT, orthotopic liver transplantation; SBP, spontaneous bacterial peritonitis; UPN, unique patient number.
Patients who died.
Patients who had OLT.
Patient with stage IV breast adenocarcinoma. Several hepatic lesions on imaging presumed due to metastases. Laparotomy performed for attempted resection; however, cirrhosis demonstrated on frozen section biopsy and procedure abandoned. No evidence of hepatic decompensation or portal hypertension.
Patient developed severe autoimmune hepatitis 16 months posttransplant after treatment with interferon-α for cytogenetic relapse of chronic myelogenous leukemia. Hepatitis resolved with corticosteroids. At 29 months posttransplant, another episode of cytogenetic relapse was treated with donor lymphocyte infusion that was complicated by severe liver GVHD leading to progressive liver failure and death.
Patient had history of heavy alcohol consumption for many years before marrow transplantation.
Patient developed progressive jaundice and ascites 4 weeks after treatment with amoxicillin/clavulanic acid (Augmentin). There was no evidence of GVHD in other organs and there was poor response to therapy with cyclosporine and prednisone.
All but 2 of the deaths in patients with cirrhosis were due to complications of liver disease. Eleven patients died of liver failure (progressive jaundice, coagulopathy, ascites, encephalopathy, hepatorenal syndrome), 1 died within 1 week of liver transplantation, and 1 died of hepatocellular carcinoma. Two patients who died of recurrent malignancy had evidence of portal hypertension before death.
Liver biopsy was performed during long-term follow-up in 30 of the 31 patients. In 26 patients, a definitive histologic diagnosis of cirrhosis or early cirrhosis was made. In 5 patients, the diagnosis of cirrhosis was based on nonhistologic criteria. One patient did not have a liver biopsy performed; however, she had clear clinical evidence of cirrhosis with peripheral stigmata of chronic liver disease, ascites complicated by secondary bacterial peritonitis, splenomegaly, thrombocytopenia, and hepatic encephalopathy. Three patients met clinical criteria of hepatic failure or portal hypertension, 2 of whom had dense fibrosis associated with severe bile duct abnormalities characteristic of GVHD but without definitive regenerative nodules. The third patient had not had a biopsy in the past 6 years, but had obviously progressed clinically. Another patient presented with bleeding esophageal varices. In this patient, extensive work-up excluded extrahepatic causes of portal hypertension; however, a single, small liver biopsy showed only chronic hepatitis without diagnostic features of cirrhosis.
Clinical Features of 31 Control Subjects
Characteristics of the control subjects and details of their preparative regimens and GVHD prophylaxis are also shown in Table 1. Cases and their controls were closely matched for age at transplantation, year of transplant, primary disease, type of transplant, conditioning therapy, and GVHD prophylaxis.
Case-Control Analysis of Risk Factors (Table3)
VOD of the liver had developed in 11 (35%) cases and 4 (13%) controls (P = .09). Mean peak serum bilirubin levels before day 20 were not significantly different. No cirrhosis patient or control subject had developed severe VOD. There was no difference in the prevalence of either acute or chronic GVHD involving the liver or in the duration of immunosuppression administered for the treatment of GVHD between cases and control subjects. Marrow iron content at day 80 posttransplant did not differ in cases and controls.
Univariable Analysis of Risk Factors for Chronic Liver Disease in Patients With Cirrhosis and Their Matched Controls
| . | Cases (n = 31) . | Controls (n = 31) . | P Value . |
|---|---|---|---|
| VOD | |||
| No. of patients (%) | 11 (35%) | 4 (13%) | .09 |
| Peak serum bilirubin (mg/dL) before day +20 (mean ± SEM) | 4.6 ± 1.3 | 2.3 ± 0.3 | NS |
| GVHD | |||
| Acute GVHD grade II-IV (%) | 9 (29%) | 12 (39%) | NS |
| Acute liver GVHD (%) | 7 (23%) | 7 (23%) | NS |
| Chronic liver GVHD (%) | 16 (52%) | 15 (48%) | NS |
| Immunosuppression | |||
| Median duration after day +100, months | 11.0 | 4.4 | NS |
| Patients treated for more than 1 yr | 15 (48%) | 13 (42%) | NS |
| Marrow iron stores at day 80 post-BMT3-150 (mean % ± SEM) | 45.4 ± 5.5 | 42.9 ± 5.3 | NS |
| HCV infection | |||
| HCV positive (%) | 25 (81%) | 14 (45%) | .01 |
| HCV genotype 1 (% of HCV positives) | 18 (86%) | 11 (79%) | NS |
| HCV genotype 2/3 (% of HCV positives) | 3 (14%) | 3 (21%) | NS |
| HBV infection | |||
| HBV positive (%) | 5 (16%)3-151 | 1 (3%)3-151 | NS |
| Other identifiable causes of liver disease | 23-152 | 0 |
| . | Cases (n = 31) . | Controls (n = 31) . | P Value . |
|---|---|---|---|
| VOD | |||
| No. of patients (%) | 11 (35%) | 4 (13%) | .09 |
| Peak serum bilirubin (mg/dL) before day +20 (mean ± SEM) | 4.6 ± 1.3 | 2.3 ± 0.3 | NS |
| GVHD | |||
| Acute GVHD grade II-IV (%) | 9 (29%) | 12 (39%) | NS |
| Acute liver GVHD (%) | 7 (23%) | 7 (23%) | NS |
| Chronic liver GVHD (%) | 16 (52%) | 15 (48%) | NS |
| Immunosuppression | |||
| Median duration after day +100, months | 11.0 | 4.4 | NS |
| Patients treated for more than 1 yr | 15 (48%) | 13 (42%) | NS |
| Marrow iron stores at day 80 post-BMT3-150 (mean % ± SEM) | 45.4 ± 5.5 | 42.9 ± 5.3 | NS |
| HCV infection | |||
| HCV positive (%) | 25 (81%) | 14 (45%) | .01 |
| HCV genotype 1 (% of HCV positives) | 18 (86%) | 11 (79%) | NS |
| HCV genotype 2/3 (% of HCV positives) | 3 (14%) | 3 (21%) | NS |
| HBV infection | |||
| HBV positive (%) | 5 (16%)3-151 | 1 (3%)3-151 | NS |
| Other identifiable causes of liver disease | 23-152 | 0 |
Except where indicated, figures refer to the number of patients.
Abbreviation: NS, not significant.
By morphometric method; expressed as total area of iron particles as a percentage of total area of hematopoietic cell nuclei.
All were also HCV positive.
Autoimmune hepatitis (UPN 6977) and amoxicillin-clavulanic acid-induced liver disease (UPN 7229) were each thought to contribute to severity of liver disease in 2 patients with chronic GVHD of the liver.
The most significant factor related to the development of cirrhosis was chronic viral hepatitis, particularly HCV infection. In 25 of 31 cases (81%), cirrhosis could be attributed to viral hepatitis, either as HCV infection alone (20 patients) or in the presence of HBV (5 patients). In patients presenting with liver disease more than 10 years after transplantation, HCV infection was present in 15 of 16 (94%). Hepatitis C infection was also evident in 7 of 10 patients presenting with cirrhosis within 5 years of transplantation; however, other contributing causes of liver disease were more likely to be present in these cases. In these 7 patients, chronic liver GVHD (n = 3), alcoholic liver disease (n = 1), and hepatitis B infection (n = 1) may have contributed to the rapid progression of liver disease. In only 2 of the patients whose cirrhosis was diagnosed before 5 years posttransplant was HCV thought to be the only cause for cirrhosis; both had abnormal liver function tests before transplant and 1 had bridging fibrosis on a pretransplant liver biopsy, suggesting that their liver disease predated transplantation. In 3 HCV-negative patients, liver failure was due to GVHD alone (n = 1), GVHD plus drug (amoxicillin-clavulanic acid)-induced liver disease (n = 1), or GVHD plus autoimmune hepatitis (n = 1).
HCV infection was present in 14 of 31 (45%) control subjects, a significantly lower rate of infection than in cirrhosis patients (P = .01). Only 2 control subjects had undergone liver biopsy; 1 showed mild chronic hepatitis and the other marked portal fibrosis without diagnostic features of cirrhosis. Three control subjects had received interferon therapy for chronic hepatitis C. An elevated serum AST or ALT (>2× upper limit of normal) was present at last follow-up in 4 control subjects, all of whom had HCV infection. The distribution of HCV genotypes did not differ between cases and controls, suggesting that a specific viral genotype may not be important in determining progression to more severe liver disease.
DISCUSSION
This study shows that cirrhosis of the liver is an important late complication of hematopoietic cell transplantation, with a cumulative incidence reaching 3.8% by 20 years after HCT. This figure very likely represents an underestimate of the true incidence of cirrhosis, particularly compensated cirrhosis, because the majority of long-term survivors have not been subjected to liver biopsy, even in the presence of persistently abnormal liver function tests. In the patients found to have cirrhosis, the time from transplantation to diagnosis was variable, ranging from 1 to 25 years, with approximately half of the 31 cases being diagnosed more than 10 years posttransplant. Complications of cirrhosis, including liver failure, portal hypertension, and hepatocellular carcinoma, were observed in 23 patients, and 13 of the 31 individuals have died of hepatic causes.
The major risk factor for the development of cirrhosis was chronic hepatitis C infection. Short-term studies of the natural history of hepatitis C infection after marrow transplantation have demonstrated that patients commonly have fluctuating levels of serum aminotransferases3; however, progression to cirrhosis during this time period has not been previously demonstrated. This is not surprising, because natural history studies in other patient groups, such as those with posttransfusion hepatitis, have shown that the progression of liver disease is quite delayed, with cirrhosis and hepatic decompensation commonly not developing until the second decade or later.7,34,35 We estimate that approximately 30% to 35% of our patients transplanted before 1991 became infected with hepatitis C.6 Therefore, of 860 patients surviving 10 or more years after transplantation in Seattle, 250 to 300 are likely to be chronically infected with hepatitis C and are at risk over the coming years for the development of cirrhosis and its complications.
There are few studies that examine the late course of viral hepatitis in patients who have survived more than 10 years after hematopoietic transplantation. Nontransplant childhood cancer survivors with chronic hepatitis C have not developed liver failure or hepatocellular carcinoma despite more than 14 years of follow-up,36,37 yet asymptomatic cirrhosis was observed, particularly in patients with hepatitis C and hepatitis B coinfection.36 In contrast to children treated with standard chemotherapy, many of the patients reported here presented with decompensated liver disease after a relatively short duration of follow-up (median time to diagnosis, 10.1 years posttransplant), suggesting that transplant-specific factors may be important in accelerating the course of infection. For example, patients receive myeloablative therapy before transplant, resulting in virtual absence of cellular and humoral immunity. In this setting, titers of circulating viruses can increase to very high levels.38 Also unique to the allogeneic transplant setting is the routine use of immunosuppressive drugs to prevent and treat acute and chronic GVHD. Viral titers can be elevated during immunosuppressive therapies, and an immune rebound acute hepatitis C has been described when these drugs are discontinued (usually around day 80 to 150).31 39-41 Full recovery of the immunological repertoire may not occur until 3 to 6 months posttransplant, and in patients with chronic GVHD, deficits in cellular immunity may persist for years. However, in the current analysis, we could not discern a difference between cases and controls with regard to prior GVHD or duration of immunosuppressive therapy administered after transplant.
Nonvirological factors may have contributed to the accelerated rate of progression to cirrhosis in this cohort. VOD of the liver was more commonly associated with cirrhosis cases than controls (35% v13%, P = .09). Within a few weeks of onset of VOD, liver histology commonly shows marked pericentral and sinusoidal fibrosis,42 paralleled by the appearance of circulating markers of fibrogenesis and reduction of markers of fibrolysis.43-46 There are no long term follow-up liver biopsy data available to determine whether the fibrosis resolves or persists after resolution of the clinical syndrome. In an analogous condition, hepatic VOD caused by the ingestion of pyrrolizidine alkaloids in India is associated with micronodular cirrhosis and hepatic failure in some long-term survivors.9 None of our cirrhosis patients or control subjects had a history of severe VOD, but it is possible that even the amount of liver damage associated with mild or moderate VOD may contribute to late hepatic fibrosis through activation of stellate cells and deposition of extracellular matrix.47,48 Activation of stellate cells may contribute to the progression of other liver diseases, particularly chronic viral hepatitis. Alternatively, nodular regenerative hyperplasia has been described as a sequela of pretransplant cytoreductive therapy, and this disease may also present with signs of portal hypertension.10 11
Iron overload is another well-recognized cause of hepatic fibrosis, cirrhosis, and hepatocellular carcinoma.49,50 The role of iron overload in the pathogenesis of liver disease is well established in patients with genetic hemochromatosis51 and those with thalassemia who have secondary iron overload from large numbers of red blood cell transfusions and hyperabsorption of dietary iron.52 Marrow transplant recipients who often have a long transfusional support history for hematologic malignancy or aplastic anemia commonly develop tissue iron overload, with marked hemosiderosis on liver histopathology and elevations of hepatic iron concentration on biochemical determination.33 In many cases, iron overload is disproportionate to the burden of transfusions, suggesting prolonged ineffective erthropoiesis and increased absorption of iron. The role of hepatic iron overload in the production of late liver damage and fibrosis in the marrow transplant setting has not been established, but experience in patients with chronic hepatitis C suggests that iron overload is an important cofactor in progressive hepatic fibrosis and that phlebotomy may lead to improvement.
We sought to determine if the peritransplant iron burden influenced the subsequent development of cirrhosis. Because very few patients early after transplant had liver biopsy material available for hepatic iron quantitation and because the serum ferritin is unreliable immediately posttransplant (due to frequent infection, GVHD, and inflammation), we used determination of marrow iron content using a computerized morphometric method. We had found a correlation between marrow iron content and the hepatic iron index in patients dying early after transplant.33 In the current study, excessive marrow iron at day 80 was a common finding in both the cases with cirrhosis and their matched controls, and no differences in the quantity of marrow iron were seen between the two groups. However, liver iron overload could contribute to the relatively accelerated rate of progression to cirrhosis in patients with chronic viral hepatitis.
We observed that marrow recipients developed cirrhosis at a median of 10 years after transplantation, due in most cases to hepatitis C acquired before or at the time of transplantation. Considering that the majority of patients were young adults or children at the time of transplantation, the rate of progression of disease is more rapid in our series than would be expected in immunocompetent individuals of the same age.53 However, our patients clearly demonstrated a slower rate of decompensation than other groups of severely immunosuppresssed patients with chronic hepatitis C infection.54-58 The rate of progression to cirrhosis after marrow transplantation appears comparable to that seen in hemophiliacs or human immunodeficiency virus (HIV)-infected individuals with fluctuating levels of immune suppression, where progression to cirrhosis and liver failure occurs over 20 years.59 60 This suggests that the period of immunosuppression related to the transplant procedure and its complications does alter the course of chronic hepatitis C, but that subsequent restoration of normal immune function prevents rapid progression to cirrhosis and liver failure.
Although viral hepatitis appeared to be the predominant cause of liver disease among patients presenting more than 10 years after HCT, other causes of liver disease were frequent among patients presenting earlier than 10 years. At the time of presentation with cirrhosis, chronic GVHD of the liver was a contributing cause of liver disease in 6 patients; however, in only 2 patients (UPN 5084 and UPN 6616) was it the only apparent cause of liver disease. Although there are case reports in the literature of hepatic chronic GVHD progressing to cirrhosis,61 62 these reports predate the ability to diagnose HCV infection. Reported cases had bile duct changes and a clinical history consistent with chronic GVHD; however, it is possible that viral hepatitis contributed to the pathogenesis of fibrosis. If hepatic chronic GVHD alone can progress to cirrhosis and hepatic decompensation, it would appear to be an extremely rare complication. Our data support the need to search for other causes of liver disease in long-term survivors with chronic GVHD who develop cirrhosis.
With current testing of blood products, the risk of acquiring hepatitis C infection before transplant is extremely small,63 but there remains a number of patients in the community who were transplanted before the introduction of routine hepatitis screening. Most of these patients have been cured of their underlying hematologic disease and have gone on to normal and active lives; however, there exists the possibility of unsuspected liver disease with the potential of progression to overt liver failure and hepatocellular carcinoma. Chronic viral hepatitis may contribute to fatigue, a symptom frequently noted in quality of life surveys of long-term survivors.64,65 Accordingly, we recommend that long-term transplant survivors should be evaluated for the presence of liver disease and for the presence of hepatitis B and C infection. Particularly in patients with abnormal liver enzymes, hepatitis C testing should include HCV RNA determination, because patients acquiring the disease around the time of transplant may remain anti-HCV–negative despite viremia.3
This present study demonstrates that cirrhosis is an important, albeit uncommon, late complication of hematopoietic cell transplantation. As with secondary neoplasia, careful life-long monitoring is recommended to detect rare events late after transplantation.66 This study should alert clinicians to the potential consequences of chronic viral hepatitis after blood and marrow transplantation. Early detection and appropriate treatment may slow or eliminate progression to hepatic failure and death.
Supported by National Institutes of Health Grants No. CA18029, CA15704, CA18221, and HL36444. S.I.S. was supported by an Astra-Merck Research Fellowship.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal