Abstract
To examine whether transfer of γ globin genes into mouse erythroleukemia cells can be used for the analysis of regulatory elements of γ globin gene promoter, Aγ gene constructs carrying promoter truncations that have been previously analyzed in transgenic mice were used for production of stably transfected mouse erythroleukemia (MEL) cell clones and pools. We found that constructs, which contain a microlocus control region (μLCR) that efficiently protects globin gene expression from the effects of the position of integration in transgenic mice, display position-dependent globin gene expression in MEL cell clones. Aγ globin gene expression among MEL cell clones carrying the μLCR(−201)Aγ and μLCR(−382)Aγ gene constructs ranged 15.5-fold and 17.6-fold, respectively, and there was no correlation between theAγ mRNA levels and the copies of the transgene (r= .28, P = .18). There was significant variation in per copy Aγ globin gene expression among MEL cell pools composed of 10 clones, but not among pools composed of 50 clones, indicating that position effects are averaged in pools composed by large numbers of clones. The overall pattern of Aγ globin gene expression in MEL cell pools resembled that observed in transgenic mice indicating that MEL cell transfections can be used in the study ofcis elements controlling γ globin gene expression. MEL cell transfections, however, are not appropriate for investigation of cis elements, which either sensitize or protect the globin transgenes from position effects.
© 1998 by The American Society of Hematology.
ANALYSIS OF THE cis control of globin gene expression has greatly benefited from studies of transgenic mice.1 A major advance in this field has been the discovery of the locus control region (LCR), the major regulatory element of the β locus, which resides 6 to 20 kb upstream of the ε globin gene.2 The LCR activates the β locus chromatin domain, it serves as a chromatin “insulator” by protecting globin transgenes from the effects of integration in inactive chromatin, and it is a powerful enhancer of globin gene expression.1,2 The discovery of the LCR triggered the extensive use of transgenic mice for the analysis of globin gene expression. Studies in transgenic mice have provided information on the function of the individual DNase I hypersensitive sites of the LCR,3-14 have shown that competition between genes for interaction with the LCR plays a role in the control of globin gene switching,15,16 have led to the discovery of cis elements involved in ε17,18 and γ19,20 gene silencing, and have provided insights on the function of downstream regulatory elements21-23 and on the interaction between transcriptional factors and globin gene regulatory motifs.24
Another experimental system for analysis of the cis control of globin gene expression uses transfections of human genes into established erythroleukemia cell lines. Mouse erythroleukemia (MEL) cells, although arrested at a differentiation stage before globin gene transcription, they are capable of chemically induced erythroid maturation during which high levels of human globin gene expression are observed. Thus, they have been used for studies of induction of globin gene expression by various compounds and in the analysis of regulatory elements of the β globin locus.25-30 Typically, in these studies, the cells are transfected either transiently or stably with recombinant human globin genes and are induced to terminal maturation by exposure to various chemical agents like dimethyl sulfoxide (DMSO) or hexamethylane-bis-acetamide (HMBA). Measurements of globin gene expression before and after MEL cell induction provide information on the regulatory role of the sequences contained in the transferred gene.
MEL cells are an adult erythroleukemia cell line characterized by synthesis of adult βmajor and βminor globin chains; there is no transcription of murine embryonic (βh1 and εy) globin genes. There is only adult human globin production when the chromosomal human globin genes from adult erythroid cells or from lymphoblasts or fibroblasts are transferred into the MEL cells by cell fusion.31,32Although MEL cells are derived from the adult hematopoietic lineage, their transcriptional environment is permissive of human γ globin gene expression. Thus, when the chromosome 11 of human fetal erythroblasts is transferred into MEL cells, the human γ globin genes continue to be expressed.32 These MELxhuman fetal erythroid cell hybrids initially express predominantly or exclusively human γ globin and over time in culture they switch to predominantly or exclusively β globin expression.32 γ Gene expression also continues when human chromosomes containing either the −117Aγ hereditary persistence of fetal hemoglobin (HPFH) gene or a GγAγ deletion HPFH gene are transferred into MEL cells by cell fusion.33
We were interested to assess whether MEL cells can substitute the transgenic mice for the analysis of the cis elements, which regulate human γ globin gene expression. To obtain this information, we analyzed γ gene expression in MEL cells stably transfected with various Aγ globin gene promoter truncation constructs, which have been previously characterized in transgenic mice.19 Our results suggest that MEL cells can be used for the analysis of cis elements whose mutations produce severe deficiency in Aγ globin mRNA. This cell line is less useful for the study of cis elements that have moderate effects on γ gene expression or for studies of the effects of position of integration on the expression of transferred γ globin genes.
MATERIALS AND METHODS
Recombinant constructs.
Four constructs, which contain a 2.5-kb μLCR cassette linked to various Aγ gene promoter truncations, were used.19 These Aγ gene recombinants have the same 3′ end at the HindIII position 530 bp downstream to the polyadenylation site of the human Aγ gene, while they differ regarding their 5′ ends. The 5′ end of the μLCR(−141)Aγ construct is in the Nco I site at position −141. The γ promoter of the μLCR(−201)Aγ construct extends to the ApaI site at position −201, while in the μLCR(−382)Aγ and μLCR(−730)Aγ construct, the promoter extends to the StuI and SspI sites at positions −382 and −730, respectively.
MEL cell culture and DNA transfection.
MEL 585 cells were maintained in RPMI 1640 medium with 10% fetal calf serum. Cells, 10 × 106, were cotransfected with 1 μg TKneo and a 3-fold to 10-fold molar excess of linearizedAγ gene fragments by electroporation at 0.6 kV, 1 μF using a Bio-Rad Gene Pulser set (Bio-Rad Laboratories, Hercules, CA). After electroporation, individual cell populations were allowed to grow without selection for 48 hours, then selection was applied using medium supplemented with 700 μg/mL G418. After about 7 to 10 days in culture, populations of rapidly growing cells are observed. Individual clones for each of the transfected constructs were produced from a pool using limiting dilution assay. It is known that prolonged culture of a pool, even a large one, may result in the predominance of one or two clones. For this reason, the clones were generated shortly after the production of the cell pools to ensure the presence of different cell clones. This has also been confirmed by the Southern analysis, which showed the presence of various copy numbers of the recombinant in the clones studied. Cells were induced with 3 mmol/L HMBA/10 μmol/L hemin for 3 days before harvesting for RNA analysis.
In another set of experiments after electroporation, the cells were plated in 48-well plates at approximately 105 cells per well in selection medium containing 700 μg/mL G418. Cell concentrations were such as to yield ≤1 positive clone per well based on estimated transfection efficiency. After approximately 14 days, individual G418-resistant clones were picked and pooled for the generation of cell populations with defined number of clones (10 or 50). The cells were subsequently expanded, induced to differentiate as described above, for 3 days, and harvested for RNA analysis.
Copy number determination.
Genomic DNA was isolated from the pooled cells by standard procedures. DNA samples were digested overnight with EcoRI restriction enzyme. About 2 to 10 μg of the digested DNA (as determined by fluorometry) was resolved by electrophoresis in 1× TAE buffer over 1% agarose and transferred onto nylon filter. The filter was subsequently hybridized with a 0.8-kbAγ globin probe and signals were quantitated on a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). Copy numbers were determined by comparing the signals from a given MEL cell sample with those of human genomic (ie, two-copy) DNA (Promega, Madison, WI).
RNA analysis and quantitation.
Total cellular RNA was isolated by the acid-phenol method described previously.12 Human Aγ and murine α globin mRNAs were quantitated by RNase protection assay. Antisense RNA probes were synthesized from linearized DNA templates using T7 polymerase. Probe pT7Aγ detects a 170-bp protected fragment derived from exon 2 of the Aγ mRNA. Probe pT7Mα, directed against murine α globin mRNA, was derived from pSP6Mα through the replacement of the SP6 promoter with the T7 promoter. Probe pTRIRNA 18s (Ambion, Austin, TX) was used to identify an 80-bp protected fragment.
The levels of human Aγ and murine α globin mRNA were quantitated by Phosphorimager analysis. To maximize the accuracy of RNase protection assays, at least two independent assays were performed for measurement of globin mRNA levels in each pool or clone of MEL cells. Aγ mRNA levels were corrected for RNA loading using the levels of 18S RNA as a standard.
RESULTS
Globin transgene expression in MEL cell clones is strongly influenced by the position of integration.
Constructs which contain globin gene sequences that participate in the interaction between globin genes and the LCR display copy number-dependent, position of integration-independent expression of globin genes in transgenic mice. Cis elements, which are located in the Aγ globin gene promoter and either confer position-independent Aγ globin gene expression [μLCR(−201) or μLCR(−382) Aγ construct] or position-dependent expression [μLCR(−730) or μLCR(−1350) Aγ construct], have been previously identified by studies in transgenic mice.19 To examine whether MEL cells can be used for the study of elements involved in LCR/globin gene interaction, we measured Aγ globin mRNA levels among individual MEL cell clones containing the μLCR(−201)Aγ, μLCR(−382)Aγ, and μLCR(−730)Aγ constructs. Each MEL cell clone was induced to terminal maturation with HMBA and hemin andAγ mRNA levels in induced cells were measured by quantitative RNase protection assay. Aγ mRNA levels were expressed as percentage of the murine α globin mRNA after correction for the number of copies of the μLCRAγ recombinant contained in each clone.
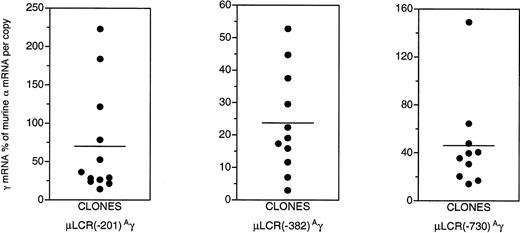
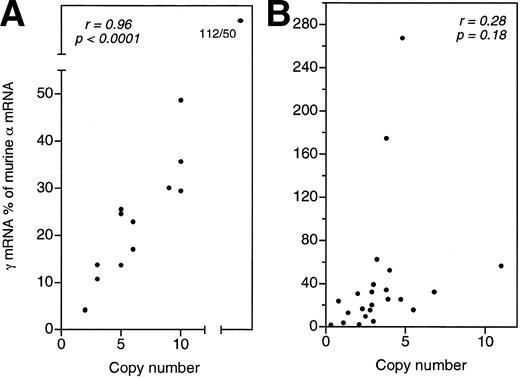
As shown in Fig 1, a striking degree of variation in Aγ gene expression is characteristic of the clones carrying these constructs. Large variation in Aγ gene expression is not expected for the μLCR(−201)Aγ and the μLCR(−382)Aγ constructs, which in transgenic mice are characterized by copy number-dependent, position-independent expression. Aγ mRNA levels among the μLCR(−201)Aγ and μLCR(−382)Aγ MEL cell clones ranged 15.5-fold (from 14.4% to 223.1% of murine α mRNA per copy) and 17.6-fold (from 3.0% to 52.8% per copy), respectively. While in transgenic mice, there is excellent correlation (r = .95, P ≤ .0001) between the Aγ mRNA levels and the number of copies of the integrated μLCR(−201)Aγ and μLCR(−382)Aγ constructs (Fig 2A), there is no correlation (r = .28, P = .18) between theAγ mRNA levels and the number of copies of the integrated μLCR(−201)Aγ and μLCR(−382)Aγ constructs in MEL cell clones (Fig2B). These results indicate that globin gene constructs, which are not influenced by the position of integration in transgenic mice, become sensitive to position effects when they are transferred into MEL cells.
Aγ gene expression in clones of MEL cells carrying the μLCR(−201)Aγ, μLCR(−382)Aγ, and μLCR(−730)Aγ constructs. Notice the striking degree of variation in Aγ gene expression among clones carrying the same construct. Horizontal lines represent mean Aγ mRNA values.
Aγ gene expression in clones of MEL cells carrying the μLCR(−201)Aγ, μLCR(−382)Aγ, and μLCR(−730)Aγ constructs. Notice the striking degree of variation in Aγ gene expression among clones carrying the same construct. Horizontal lines represent mean Aγ mRNA values.
Correlation between total Aγ mRNA levels and transgene copy numbers in transgenic mouse lines (A) or MEL cell clones (B) carrying the μLCR(−201)Aγ and μLCR(−382)Aγ constructs. The excellent correlation observed in transgenic mice (r = .96, P < .0001) does not exist in MEL cell clones (r = .28, P = .18).
Correlation between total Aγ mRNA levels and transgene copy numbers in transgenic mouse lines (A) or MEL cell clones (B) carrying the μLCR(−201)Aγ and μLCR(−382)Aγ constructs. The excellent correlation observed in transgenic mice (r = .96, P < .0001) does not exist in MEL cell clones (r = .28, P = .18).
Comparison of Aγ gene expression in MEL cell pools and transgenic mice.
Four recombinants carrying Aγ gene promoter truncations [μLCR(−141)Aγ, μLCR(−201)Aγ, μLCR (−382)Aγ, and μLCR(−730)Aγ] were transferred to MEL cells and pools of stably transfected cells were generated and induced to terminal maturation with HMBA and hemin. Levels of Aγ mRNA in uninduced and induced cells were expressed as percentage of the murine α mRNA after correction for the average number of copies of the μLCRAγ recombinant contained in each pool and for the number of copies of the endogenous murine α globin genes. Results are summarized in Table 1.
γ Globin Gene Expression in MEL Cell Pools Transfected With Four Aγ Promoter Truncation Mutants
| Construct . | Pool No. . | Copy No. . | % γ/α mRNA per Copy . |
|---|---|---|---|
| μLCR(−141)Aγ | A | 15.1 | 2.8 |
| B | 20.8 | 2.4 | |
| C | 9.2 | 4.8 | |
| D | 7.8 | 2.3 | |
| E | 8.2 | 2.5 | |
| F | 9.9 | 1.6 | |
| Mean ± SD | 2.7 ± 1.1 | ||
| Range | 3x | ||
| μLCR(−201)Aγ | A | 1.8 | 11.7 |
| B | 2.7 | 22.1 | |
| C | 2.6 | 73.0 | |
| D | 7.4 | 19.0 | |
| E | 6.2 | 29.6 | |
| F | 5.4 | 23.7 | |
| Mean ± SD | 29.9 ± 21.9 | ||
| Range | 6.2x | ||
| μLCR(−382)Aγ | A | 1.6 | 30.0 |
| B | 1 | 26.9 | |
| C | 2 | 29.6 | |
| D | 4.9 | 42.3 | |
| E | 7.8 | 29.6 | |
| F | 6.4 | 18.0 | |
| Mean ± SD | 29.4 ± 7.8 | ||
| Range | 2.4x | ||
| μLCR(−730)Aγ | A | 0.7 | 9.7 |
| B | 1.5 | 25.8 | |
| C | 2.5 | 96.4 | |
| D | 8.4 | 13.8 | |
| E | 1.4 | 18.6 | |
| F | 6.7 | 17.6 | |
| Mean ± SD | 30.3 ± 32.8 | ||
| Range | 9.9x |
| Construct . | Pool No. . | Copy No. . | % γ/α mRNA per Copy . |
|---|---|---|---|
| μLCR(−141)Aγ | A | 15.1 | 2.8 |
| B | 20.8 | 2.4 | |
| C | 9.2 | 4.8 | |
| D | 7.8 | 2.3 | |
| E | 8.2 | 2.5 | |
| F | 9.9 | 1.6 | |
| Mean ± SD | 2.7 ± 1.1 | ||
| Range | 3x | ||
| μLCR(−201)Aγ | A | 1.8 | 11.7 |
| B | 2.7 | 22.1 | |
| C | 2.6 | 73.0 | |
| D | 7.4 | 19.0 | |
| E | 6.2 | 29.6 | |
| F | 5.4 | 23.7 | |
| Mean ± SD | 29.9 ± 21.9 | ||
| Range | 6.2x | ||
| μLCR(−382)Aγ | A | 1.6 | 30.0 |
| B | 1 | 26.9 | |
| C | 2 | 29.6 | |
| D | 4.9 | 42.3 | |
| E | 7.8 | 29.6 | |
| F | 6.4 | 18.0 | |
| Mean ± SD | 29.4 ± 7.8 | ||
| Range | 2.4x | ||
| μLCR(−730)Aγ | A | 0.7 | 9.7 |
| B | 1.5 | 25.8 | |
| C | 2.5 | 96.4 | |
| D | 8.4 | 13.8 | |
| E | 1.4 | 18.6 | |
| F | 6.7 | 17.6 | |
| Mean ± SD | 30.3 ± 32.8 | ||
| Range | 9.9x |
The μLCR(−141)Aγ pools display the lowest level of Aγ transgene expression (range from 1.6% to 4.8% of murine α mRNA per copy; mean value, 2.7%). In the μLCR(−201)Aγ pools, the levels ofAγ mRNA were about one order of magnitude higher compared with the (−141)Aγ pools (mean value, 29.9%; range from 11.7% to 73% of murine α). The μLCR(−201)Aγ, μLCR(−382)Aγ, and μLCR(−730)Aγ pools had similar meanAγ mRNA levels (29.9%, 29.4%, 30.3% of murine α mRNA per copy, respectively). Aγ mRNA levels varied by 6.2-fold among the μLCR(−201)Aγ pools, 2.4-fold among the μLCR(−382)Aγ pools, and 9.9-fold among the μLCR(−730)Aγ pools. The variation inAγ mRNA levels among pools was significantly lower than among clones carrying the same contructs.
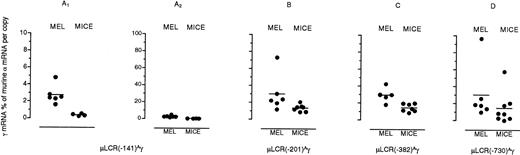
In Fig 3, we compare Aγ mRNA levels in the MEL cell pools and transgenic mice. Each data point in the transgenic mice columns corresponds to the mean value ofAγ mRNA in several adult animals of each transgenic line. The overall pattern of globin gene expression between MEL cell pools resembles that in transgenic mice. In both MEL cells and transgenic mice, disruption of the Aγ gene CACCC box, as in the μLCR(−141)Aγ construct, severely affectsAγ gene expression. Addition of 5′ sequences, as in the μLCR(−201)Aγ or the μLCR(−382)Aγ constructs, restoresAγ gene expression to about 30% of murine α. Several differences in globin gene expression between transgenic mice and MEL cell pools are also noted. Thus, the μLCR(−141)Aγ recombinant, which in the adult transgenic mice shows barely measurableAγ mRNA levels, displays considerable expression in MEL cells. The mean Aγ mRNA levels in MEL cell pools carrying the μLCR(−201)Aγ, μLCR(−382)Aγ, and μLCR(−730)Aγ constructs are almost twofold higher than those in adult mice carrying the same transgenes. WhileAγ mRNA expression in the μLCR(−201)Aγ transgenic mice is position-independent, as shown by the small degree of variation of theAγ mRNA levels among transgenic lines, Aγ mRNA levels in the μLCR(−201)Aγ MEL cell pools display about sixfold variation, indicating that Aγ gene expression is position-dependent (Fig 3B).
Aγ gene expression for fourAγ promoter truncation constructs in MEL cell pools and transgenic mice. Levels of Aγ mRNA are expressed as percentage of murine mRNA per copy of transgene and copy of murine gene. Horizontal lines represent mean Aγ mRNA values. Notice that in panels A1 and A2, mRNA levels for the μLCR(−141)Aγ construct are plotted in a different scale to demonstrate the observed difference in expression between cells and mice.
Aγ gene expression for fourAγ promoter truncation constructs in MEL cell pools and transgenic mice. Levels of Aγ mRNA are expressed as percentage of murine mRNA per copy of transgene and copy of murine gene. Horizontal lines represent mean Aγ mRNA values. Notice that in panels A1 and A2, mRNA levels for the μLCR(−141)Aγ construct are plotted in a different scale to demonstrate the observed difference in expression between cells and mice.
The variation in Aγ/α mRNA levels among MEL cell pools could be caused by the presence of small numbers of clones comprising each pool. Pools containing few clones are expected to be randomly affected by the large variation of Aγ globin gene expression among clones. When the pools are comprised of a large number of clones, any position effects on Aγ globin gene expression in the clones should be “averaged,” resulting in smaller variation in Aγ/α gene expression among pools. To examine this possibility, MEL cell pools carrying the μLCR(−382)Aγ construct consisting of either 10 or 50 single clones each were generated and globin mRNA levels were measured in induced cells. As shown in Table 2, pools of 10 clones displayed a 14.1-fold variation in the level of Aγ mRNA (range from 1.5% to 21.1% of murine α mRNA per copy). Aγ mRNA levels among pools composed of 50 clones varied by only 2.2-fold (from 15.4% to 33.6% of murine α). These data show that a significant number of individual clones (at least 50) must be present in each MEL cell pool to adjust for the effects of position of integration on the expression of the transgene.
γ Globin Gene Expression in MEL Cell Pools Containing 10 or 50 Individual Clones Carrying the μLCR(−382)Aγ Construct
| No. of Clones per Pool . | Pool No. . | Copy No. . | % γ/α mRNA per Copy . |
|---|---|---|---|
| 10 clones | A | 20.6 | 1.5 |
| B | 3.5 | 4.0 | |
| C | 1.3 | 5.5 | |
| D | 2.3 | 9.9 | |
| E | 19.9 | 20.2 | |
| F | 9.0 | 21.1 | |
| Mean ± SD | 10.4 ± 8.4 | ||
| Range | 14.1x | ||
| 50 clones | A | 12.0 | 15.4 |
| B | 13.8 | 22.4 | |
| C | 5.3 | 27.7 | |
| D | 7.0 | 28.7 | |
| E | 6.2 | 30.9 | |
| F | 11.5 | 33.5 | |
| G | 6.9 | 33.6 | |
| Mean ± SD | 27.5 ± 6.6 | ||
| Range | 2.2x |
| No. of Clones per Pool . | Pool No. . | Copy No. . | % γ/α mRNA per Copy . |
|---|---|---|---|
| 10 clones | A | 20.6 | 1.5 |
| B | 3.5 | 4.0 | |
| C | 1.3 | 5.5 | |
| D | 2.3 | 9.9 | |
| E | 19.9 | 20.2 | |
| F | 9.0 | 21.1 | |
| Mean ± SD | 10.4 ± 8.4 | ||
| Range | 14.1x | ||
| 50 clones | A | 12.0 | 15.4 |
| B | 13.8 | 22.4 | |
| C | 5.3 | 27.7 | |
| D | 7.0 | 28.7 | |
| E | 6.2 | 30.9 | |
| F | 11.5 | 33.5 | |
| G | 6.9 | 33.6 | |
| Mean ± SD | 27.5 ± 6.6 | ||
| Range | 2.2x |
Coordinate induction of exogenous and endogenous globin genes.
For studies of globin genes transferred into MEL cells, the cells are cultured in the presence of HMBA and hemin for 72 hours during which terminal maturation occurs and hemoglobin is produced. The process of MEL cell induction is empirical and experiments may vary in the degree of MEL cell induction, as judged by the degree of hemoglobinization. When the levels of transgene mRNA are expressed as percent of the endogenous murine α gene, variation in MEL cell inducibility between experiments could contribute to variation in expression if the human transgene and the endogenous gene are not induced in a coordinate fashion. Discordant human Aγ and murine α globin gene induction will result in random variation of human/mouse mRNA ratios among MEL cells carrying the same construct.
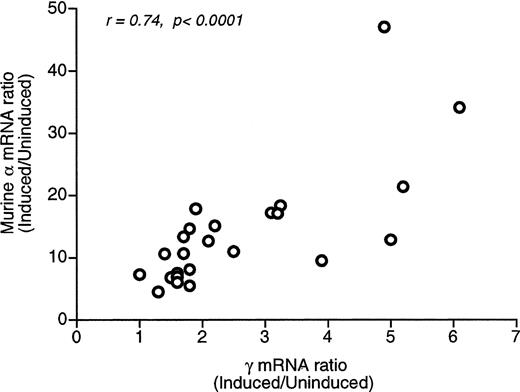
To examine whether induction of the human Aγ and murine α genes is coordinate, we calculated the ratio of Aγ and α mRNAs in induced (I) and uninduced (U) MEL cells. There was considerable variation among MEL cell pools in I/U Aγ mRNA ratios (up to 4.9-fold) and an even larger variation in I/U α mRNA ratios (10.4-fold). However, as shown in Fig 4, there is a statistically significant correlation between the I/U Aγ mRNA and the I/U α mRNA ratios (r = 0.74, P < .0001), suggesting that the exogenous Aγ and the endogenous α globin genes are induced in a coordinate fashion. Therefore, random differences in the degree of Aγ and α globin gene induction do not significantly contribute to the observed variation ofAγ/α mRNA levels.
Regression analysis of the induced/uninducedAγ and mRNA ratios. A statistically significant correlation is observed (r = .74, P < .0001), indicating that the exogenous and endogenous genes are induced coordinately.
Regression analysis of the induced/uninducedAγ and mRNA ratios. A statistically significant correlation is observed (r = .74, P < .0001), indicating that the exogenous and endogenous genes are induced coordinately.
DISCUSSION
Transgenic mice have been extensively used for elucidation of thecis elements that control globin gene expression,3-23,34-37 but studies with this model system are expensive and time-consuming. Using transgenic mice, we have previously obtained evidence that the Aγ promoter region between nucleotides −382 and −730 relative to the cap site harbors a position-dependent γ gene silencer.19 To further delineate this element and demarcate the sequences that contain the putative γ gene silencer, the function of a large number ofAγ gene promoter mutations needs to be determined. To test whether stable transfections of MEL cells could be used for that purpose, we transfected MEL cells with Aγ globin gene constructs whose function has been well defined in transgenic mice and asked whether the results obtained in transgenic mice can be reproduced in MEL cell clones and/or pools. We found that the MEL cell system is not useful for studies of elements which protect globin gene expression from position effects. Typically, such studies require clonal analysis and measurement of gene expression per copy of the transgene. Copy number-dependent expression indicates that gene expression is not influenced by the position of integration of the transgene, while copy number-independent expression indicates that the transgene is sensitive to position effects. As we have shown in the present study, constructs that in transgenic mice display position-independent, copy number-dependent expression, become sensitive to position effects in MEL cells. Therefore, it is unlikely that MEL cell transfectants can provide useful information on globin gene sequences, which participate in LCR-globin gene interactions. Another implication of our observations concerns the evaluation of globin gene retroviral vectors. Such retroviral vectors developed for the purpose of gene therapy for hemoglobinopathies, usually contain sequences of the human β or γ globin genes linked to various LCR cassettes.38-43 Studies in transduced MEL cell clones are performed to test whether the LCR cassette contained in the retroviral vector enhances globin gene expression and protects it from position effects. Our results suggest that variation in globin gene expression among transduced MEL cell clones does not necessarily signify that a retroviral vector is sensitive to position effects.
The great majority of the clones used in this study contained multiple copies of integrants. It has been suggested that the presence of multiple transgene copies may adversely affect the level of transgene expression.26 Although no correlation between transgene copy number and expression per copy was observed in this study, it has been recently reported44 that single copy transgenes demonstrate consistent and reproducible expression in stably transfected MEL cells. It is possible that the presence of multiple integrants accounts for the observed variation in Aγ globin gene expression among MEL cell clones.
The comparison of globin gene expression between MEL cell pools and transgenic mice shows that MEL cell pools may be useful in identifyingcis elements whose mutations severely affect γ globin gene expression. Thus, the deleterious effect of CACC box disruption [in the μLCR(−141)Aγ pools] on Aγ gene expression and the restoration of expression upon addition of upstream sequences [in the μLCR(−201)Aγ pools], which were demonstrated in transgenic mice, were also observed in MEL cell pools. However, studies in MEL cells pools cannot identify elements that moderately affect globin gene expression. This is apparent from the findings in transgenic mice and MEL cell pools carrying the μLCR(−730)Aγ construct. In transgenic mice, this construct displayed 500-fold variation among lines and significant decrease in expression in the adult mice, showing the presence of a position-dependent γ globin gene silencer. Such effects were not observed in the MEL cell pools transfected with this construct.
Supported by Grants No. HL46557, HL53750, and DK45365 from the National Institutes of Health, an International Fogarty Fellowship Award to G.V., and a Cooley’s Anemia Foundation Award to E.S.
Address reprint requests to G. Stamatoyannopoulos, MD, Medical Genetics, Box 357720, University of Washington, Seattle, WA 98195; e-mail: gstam@u.washington.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal