Abstract
Platelet membrane glycoprotein IIIa (GPIIIa) is the most polymorphic integrin subunit in man, with at least seven recognized allelic isoforms present in the human gene pool. Whether these allelic variants of the GPIIb-IIIa complex differ in the ability to interact with the adhesive ligand fibrinogen (Fg) is still unknown. Since the Pena and Penb allelic forms of GPIIIa are distinguished by a single Arg143Gln amino acid substitution within the RGD binding domain of GPIIIa and anti-Pena human alloantibodies have been shown to bind GPIIb-IIIa on the platelet surface and inhibit ADP-induced platelet aggregation, we expressed both forms of this integrin in Chinese hamster ovary (CHO) cells and examined the relative adhesive properties. Both allelic forms of GPIIb-IIIa were expressed on the cell surface and were recognized by a well-characterized panel of murine and human monoclonal and polyclonal antibodies. Like Pena, the Penb form of GPIIb-IIIa could undergo conformational changes in response to RGD peptide binding, and could be induced by activating antibodies to bind Fg and the Fg mimetic antibody P1-55. The binding affinity for Fg of the Pena form of the GPIIb-IIIa complex was not significantly different from that of the Penb form, nor was its ability to signal to focal adhesion kinase, suggesting that Arg143Gln polymorphism has little or no effect on integrin function. Examination of the functional consequences of other integrin polymorphisms may be necessary to determine whether they constitute a risk factor for thrombosis or hemorrhage.
© 1998 by The American Society of Hematology.
THE INTEGRINS are a family of heterodimeric cell surface receptors that mediate cell-cell and cell-matrix interactions.1 A prototype integrin, platelet glycoprotein (GP)IIb-IIIa (αIIbβ3), is essential for normal hemostasis and plays a central role in platelet aggregation by binding to adhesive ligands such as fibrinogen (Fg) and von Willebrand factor. The GPIIb-IIIa complex is restricted to platelets and is the most abundant member of the integrin family found on the platelet surface, with 80,000 molecules per platelet.2 Mature GPIIIa is a single-chain polypeptide composed of 762 amino acids.3-5 GPIIIa contains 56 cysteine residues in highly conserved locations within the extracellular domain of the molecule, all of which are normally disulfide-linked to form 28 nonconsecutive disulfide bridges. Two large loops extending from amino acids Cys5-Cys435 and Cys405-Cys655 have been proposed by Calvete et al.6 Mature GPIIb has 1,008 amino acids containing nine disulfide bonds formed by 18 cysteines,7 and exists as a two-chain molecule consisting of a heavy and a light chain with a molecular mass of 125 and 22 kD, respectively.
In addition to their physiologic role, both GPIIb and GPIIIa are known to bear a number of clinically important alloantigenic determinants that can induce an alloimmune response in two immunopathologic syndromes, posttransfusion purpura (PTP) and neonatal alloimmune thrombocytopenic purpura (NATP).8 These antigenic determinants are mostly the result of single amino acid substitutions that result in subtle conformational changes in the platelet membrane GP that bears them.
Although the PlA1/PlA2 (Leu33Pro) polymorphism of GPIIIa is by far the one most often associated with development of PTP and NATP, the Pena and Penb antigenic epitopes, also found on GPIIIa,9 may be a more frequent cause of both alloimmune disorders in individuals of Asian descent. NATP, due to anti-Pen alloantibodies, is particularly severe. Moreover, human anti-Pena alloantibodies have been shown to bind to the surface of platelets and inhibit ADP-induced platelet aggregation. Taking these clues, we used RNA-polymerase chain reaction (PCR) to amplify the region of GPIIIa encoding the ligand binding domain of GPIIIa (amino acids 109 to 171) as identified by RGD cross-linking experiments,10 and found an Arg143Gln polymorphism that was responsible for formation of the Pena and Penballoantigenic epitopes.11 Interestingly, this amino acid substitution is only 24 residues away from an Asp119Tyr mutation that abolishes ligand binding and results in a variant form of Glanzmann thrombasthenia.12 Mutations of oxygenated residues within this binding domain of GPIIIa have also been shown to affect ligand binding function.13
Albeit still controversial, recent reports on the association of GPIIIa polymorphisms with the development of coronary disease suggest potential functional consequences of these platelet adhesive molecule polymorphisms.14 However, the relative paucity of information regarding the adhesive properties of these allelic forms of GPIIb-IIIa has left open the important issue of whether the GP polymorphisms are merely associated with or actually contribute to the increased risk of coronary disease in individuals that inherit them. Since the Pen polymorphism is located at GPIIIa amino acid residue 143 within the previously defined ligand binding site and anti-Pen antibodies bind to GPIIIa and block platelet aggregation, it is reasonable to hypothesize that changes in this region might have the potential to change the shape of the ligand binding pocket and thereby positively or negatively affect the affinity of the adhesive receptor for its ligand. Because individuals homozygous for the Penbform of GPIIIa are generally not available for study, we have produced Chinese hamster ovary (CHO) cell lines stably transfected with either the Pena (Arg143) or Penb(Gln143) forms of the GPIIb-IIIa complex, and examined the effects of this amino acid substitution on cell surface expression, signal transduction, and ligand binding properties.
MATERIALS AND METHODS
Monoclonal antibodies and peptides.
The monoclonal antibody Tab specific for GPIIb15 was kindly provided by Dr Rodger McEver (University of Oklahoma, Oklahoma City, OK). The GPIIb-IIIa complex-specific antibody AP2,16 which inhibits Fg binding to GPIIb-IIIa, and AP3,17 specific for GPIIIa, were produced at the Blood Research Institute (BRI) Hybrodoma Core Laboratory and purified by affinity chromatography. LIBS antibody D3,18 kindly provided by Dr Lisa Jennings (University of Memphis, Memphis, TN), has been mapped to a GPIIIa epitope at residue 422-692.19Pl-55, an activation-dependent antibody,20 was provided by Dr Beat Steiner (Hoffmann-La Roche Ltd, Basel, Switzerland). FITC-conjugated Pl-55 was labeled with FITC as follows: 1 mg Pl-55 in 0.1 mol/L sodium bicarbonate buffer, pH 9.0, was mixed with 2 mg FITC-Celite (Molecular Probes, Eugene, OR) in the dark at room temperature. After a 30-minute incubation, the reaction was stopped by adding 1 mol/L NH4Cl, and the mixture was dialyzed against phosphate-buffered saline (PBS). RGDW and RGEW peptides were synthesized and purified by high-performance liquid chromatography in the BRI Protein Core Laboratory. The mass of all peptides was verified by mass spectrometry before use.
Generation of stable cell lines expressing allelic forms of GPIIb-IIIa in CHO cells.
Allele-specific recombinant forms of GPIIIa cDNA were produced as described previously,11 and subcloned into theEcoRI cloning site of the mammalian expression vector EMC-3 containing a methotrexate resistance gene (generously provided by Dr Glenn Larsen, Genetics Institute, Cambridge, MA) or pcDNA3, which contains a neomycin resistance gene (Invitrogen, San Diego, CA). Wild-type full-length GPIIb cDNA was cloned into the EcoRI site of the same two vectors. Cotransfection of CHO cells with the GPIIb cDNA construct and either the Pena (Arg143) or Penb (Gln143) form of GPIIIa cDNA was performed using the calcium phosphate method.21 Forty-eight hours after transfection, cells were subjected to drug selection in media containing 500 to 600 μg/mL G418 (geneticin; GIBCO, Gaithersburg, MD) or α minimum essential media without ribonucleotides and deoxyribonucleotides, depending on the vector used, for 2 weeks. Resistant colonies were isolated with a cloning cylinder and assayed for expression of GPIIb-IIIa by flow cytometry using a variety of monoclonal antibodies against single subunits or the intact complex. To obtain a homogeneous population, cells were sorted in a FACStar (Becton Dickinson, San Jose, CA) using the complex-specific antibody AP2 and maintained in the same selection media.
Flow cytometric analysis.
Cultured cells were harvested by adding 3.5 mmol/L EDTA and 0.01% trypsin for 2 to 3 minutes. Trypsin was neutralized by adding serum-containing media, and the cells were washed twice in D-PBS (GIBCO). Cells (3 to 5 × 105) in PBS containing 0.5% bovine serum albumin (BSA) and 0.01% NaN3 were incubated with 20 μg/mL AP3, Tab, AP2, or an isotype control antibody for 1 hour at room temperature. The cells were then washed with PBS twice and incubated with a FITC-conjugated F(ab′)2 fragment of goat anti-mouse IgG for 30 minutes. The cells were washed, resuspended in PBS, and assayed with a FACS-scan (Becton Dickinson). Binding of the LIBS antibody D3 in the absence or presence of RGDW or RGEW peptides was assessed as already described. To determine the conformational state of the recombinant GPIIb-IIIa in CHO cells, binding of FITC-conjugated Pl-55 was measured in the absence or presence of the LIBS antibody D3. A non-LIBS antibody, AP3, was used as a negative control. Nonspecific binding of FITC-Pl-55 to the recombinant GPIIb-IIIa was defined by measuring the mean fluorescence intensity in the presence of RGDW peptide.
Immunoprecipitation analysis of cell lysates from transfected CHO cells.
Stably transfected or nontransfected CHO cells were washed twice with PBS. After washing, the cells were surface-labeled with 5 mmol/L NHS-LC-biotin in PBS for 30 minutes at room temperature and solubilized for 30 minutes on ice in lysis buffer containing 20 mmol/L Tris, 100 mmol/L NaCl, 1% Triton X-100, 2mmol/L PMSF, and 100 μg/mL leupeptin. The supernatant was obtained by centrifugation at 15,000g for 30 minutes at 4°C. Aliquots of labeled cell extracts were incubated overnight at 4°C with a variety of monoclonal antibodies, as well as human alloantibodies. Rabbit anti-mouse IgG was added to tubes containing mouse IgG, and the immune complexes were recovered with protein A Sepharose beads (Pharmacia, Uppsala, Sweden). The beads were washed five times with lysis buffer and resuspended in reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. The samples were separated on 7% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 3% BSA in Tris-buffered saline (TBS) for 1 hour at room temperature. After washing, the membrane was incubated for 1 hour with a 1:4,000 dilution of streptavidin conjugated to horseradish peroxidase (HRP), washed, and detected by ECL as described previously.22
Cell adhesion assays.
Ninety-six–well plates (Immunlon 2; Dynatech, Chantilly, VA) were coated with 0.1 mL PBS containing different concentrations of Fg, 10 μg/mL AP2, or 1% BSA at 4°C overnight and blocked with 1% BSA in PBS at room temperature for 1 hour before use. Transfected CHO cells were harvested as already described. Cells were washed, resuspended in media, and labeled with 2 μmol/L calcein-AM (Molecular Probes) at 37°C for 30 minutes. The cells were then washed and resuspended in serum-free media. One hundred microliters of cell suspension (2 × 105 cells) was added to each well. Cells were then allowed to adhere for 1 hour at 37°C. Following incubation, the plates were inverted and flicked to remove nonadherent cells. Excess liquid was blotted on paper towels. The wells were washed three times with media containing 1% BSA. Finally, 200 μL wash media was added to the wells, and the plates were read in a microplate fluorescence reader (CytoFluor II; PerSeptive Biosystem, Bedford, MA) at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. To assess the effects of antibody on cell adhesion, cells were pretreated with AP2 or an anti-PECAM-1 antibody at 20 μg/mL at room temperature for 30 minutes. The cells were then washed before plating onto coated wells.
Tyrosine phosphorylation of pp125FAK.
Tissue culture plates (100-mm) were coated overnight at 4°C with either Fg (100 μg/mL) or BSA (10 mg/mL). All plates were blocked with 1% BSA for 2 hours at room temperature. Transfected CHO cells in serum-free media were added to these plates and incubated at 37°C for 60 minutes. After washing, adherent cells were lysed in RIPA buffer containing 1% Triton X-100, 150 mmol/L NaCl, 10 mmol/L Tris, 1 mmol/L EGTA, 1 mmol/L Na3VO4, 0.5% Nonidet P40, 1% sodium deoxycholate, 2 mmol/L PMSF, and 10 μg/mL each of aprotinin and leupeptin. Lysates were clarified by centrifugation in a microfuge at 15,000g at 4°C for 30 minutes, and protein content was quantified using the BCA protein assay (Pierce Chemical Co, Rockford, IL). Equal amounts of protein from each lysate (500 μg) were immunoprecipitated with 4 μg polyclonal anti-pp125FAKantibody (Upstate Biotechnology) overnight at 4°C. The tyrosine phosphorylation state of pp125FAK was determined using HRP-conjugated PY20 as described previously.23
Fg labeling and binding to transfected CHO cells.
Fg was labeled with FITC as described for antibody labeling. After dialysis against PBS to remove free FITC, the concentration of labeled Fg was determined using a BCA reagent kit (Pierce). The clottability of labeled Fg was greater than 93% as determined by measuring the OD at 280 and 320 nm after Fg was incubated with human thrombin (1 U/mL) for 2 hours at 37°C. FITC-Fg (100 μg/mL) was incubated with transfected CHO cells in the presence of D3 for the times indicated. Then, aliquots of cells were analyzed in a FACScan.
Fg was also labeled with radioactive iodine using Iodo beads (Pierce) according to the manufacturer’s directions. Briefly, 5 mg Fg (peak I; kindly provided by Dr M. Mosesson, Mount Sinai Medical Center, Milwaukee, WI) was labeled with 0.3 mCi carrier-free sodium125I (Amersham Corp) using two Iodo beads per tube at room temperature for 10 minutes. Following iodination, labeled Fg was separated from free iodine by chromatography using Sephadex G-50 (PD10 column; Pharmacia Biotech, Uppsala, Sweden) equilibrated in Hanks’ buffered salt solution (HBSS). The specific activity of Fg used in the binding assays was approximately 50 to 80 dpm/ng. The clottability of labeled Fg was greater than 95% at 37°C in the presence of human thrombin (1 U/mL). The concentration of labeled Fg was determined spectrophotometrically at 280 nm assuming an extinction coefficient of 1.51 and a molecular mass of 340 kD. The labeled proteins were stored at −20°C until use. Transfected CHO cells were prepared as described earlier and suspended in HBSS buffer containing 2 mmol/L Ca2+, 1 mmol/L Mg2+, and 4 mg/mL BSA. Fg binding experiments were performed in triplicate in presiliconized 1.5-mL polypropylene tubes (Denville Scientific, Denville, NJ). The amount of antibody D3 used for activating GPIIb-IIIa was determined by preliminary studies, and the optimal concentration was 150 to 200 μg/mL. Cells (1 × 106) were preincubated with D3 (200 μg/mL) at room temperature for 30 minutes with occasional shaking. Increasing concentrations of 125I-labeled Fg were then added and incubated for 45 minutes in a total volume of 150 μL. After incubation, 40 μL of the reaction mixture was removed and layered onto 400 μL 20% sucrose in siliconized 1.5-mL polypropylene tubes, and cell-bound Fg was separated from free Fg by centrifugation at 15,000g for 3 minutes. After careful aspiration of the supernatant, the tip of each tube was cut off, and the bound radioactivity was determined using a gamma counter. For nonspecific binding, 3 mmol/L RGDW peptide and/or 10 mmol/L EDTA were added to the tubes with labeled fibrinogen Fg. In some cases, cells without D3 treatment were used to determine nonspecific binding. Specific binding was determined by subtracting nonspecific binding from total ligand binding. Specific binding isotherms were generated by plotting the specific binding of 125I-Fg to transfected CHO cells versus the 125I-Fg concentration using the GraphPad PRIZM program (Graph Pad Software Inc, San Diego, CA). Dissociation constants (kd) were determined from Scatchard plots using standard linear regression.
RESULTS
Expression of the Pena and Penb allelic forms of GPIIIa.
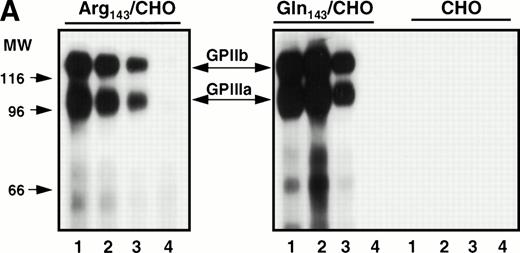
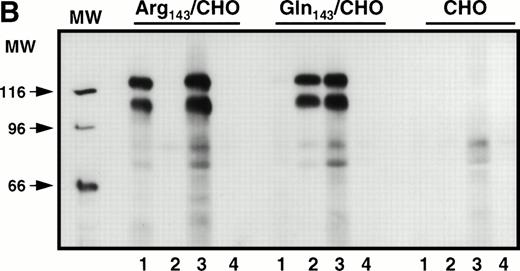
Stable cell lines expressing the Pena and Penballelic forms of GPIIb-IIIa were produced by cotransfection of GPIIb cDNA with either Pena (Arg143) or Penb (Gln143) GPIIIa cDNA into CHO cells. Surface-expressed GPIIb-IIIa was examined by immunoprecipitation and by immunofluorescence flow cytometric analysis using a variety of anti-GPIIb and anti-GPIIIa antibodies and human alloantibodies specific for the Pen alloantigenic determinants. Both allelic forms of GPIIb-IIIa were immunoprecipitated by monoclonal antibodies AP3 (specific for GPIIIa), Tab (specific for GPIIb), and AP2 (specific for the intact complex) but not by normal mouse IgG, as expected (Fig1A). Anti-PlA1 human alloantibodies reacted with GPIIIa independently of Pen phenotype (Fig1B). In contrast, human anti-Pena alloantisera reacted with the Arg143 form of GPIIIa but not with the Gln143 form, and human anti-Penb alloantibody bound to the Gln143 but not to the Arg143GPIIIa allele. Indirect immunofluorescence staining analysis using the complex-specific antibody AP2 showed that the GPIIb-IIIa complex was surface-expressed on both Pena and Penb stable cell lines (not shown). These data demonstrate that both the Pena and Penb forms of the GPIIb-IIIa complex are expressed normally on the cell surface and recognized by allele-specific anti-Pen alloantibodies.
Immunoprecipitation analysis of biotin-labeled surface-expressed proteins from transfected CHO cells expressing Pena (Arg143) or Penb(Gln143) alloantigens. Stably transfected CHO cells were surface-labeled with biotin. Cell extracts were immunoprecipitated with the indicated antibodies. (A) Surface-expressed GPIIb-IIIa was recognized by different monoclonal antibodies. MoAb AP3 (specific for GPIIIa, lane 1) and Tab (specific for GPIIb, lane 2) immunoprecipitated the respective subunit and its associated subunit. AP2 recognized the intact mature complex (lane 3). Normal mouse IgG was used as a negative control (lane 4). (B) Surface-expressed GPIIb-IIIa was recognized by allele-specific alloantibodies. Whereas PlA1 (lane 3) reacted with GPIIb-IIIa derived from both stable cell lines, anti-Pena alloantibodies (lane 1) recognized the Arg143 form of GPIIb-IIIa but not the Gln143form. Anti-Penb alloantibodies (lane 2) only reacted with the Gln143 form, as expected. Normal human IgG (lane 4) was used as a control.
Immunoprecipitation analysis of biotin-labeled surface-expressed proteins from transfected CHO cells expressing Pena (Arg143) or Penb(Gln143) alloantigens. Stably transfected CHO cells were surface-labeled with biotin. Cell extracts were immunoprecipitated with the indicated antibodies. (A) Surface-expressed GPIIb-IIIa was recognized by different monoclonal antibodies. MoAb AP3 (specific for GPIIIa, lane 1) and Tab (specific for GPIIb, lane 2) immunoprecipitated the respective subunit and its associated subunit. AP2 recognized the intact mature complex (lane 3). Normal mouse IgG was used as a negative control (lane 4). (B) Surface-expressed GPIIb-IIIa was recognized by allele-specific alloantibodies. Whereas PlA1 (lane 3) reacted with GPIIb-IIIa derived from both stable cell lines, anti-Pena alloantibodies (lane 1) recognized the Arg143 form of GPIIb-IIIa but not the Gln143form. Anti-Penb alloantibodies (lane 2) only reacted with the Gln143 form, as expected. Normal human IgG (lane 4) was used as a control.
Adhesive properties of the Pena and Penballelic forms of GPIIb-IIIa.
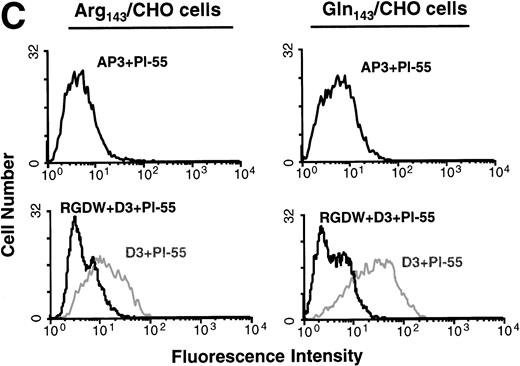
To examine whether the Pena and Penb allelic forms of GPIIb-IIIa could undergo conformational changes and bind to ligand in a similar manner, we measured the binding of the LIBS antibody D3 in the presence and absence of RGD peptides. D3 recognizes a conformation of the integrin complex induced by ligand binding.18 RGD, but not RGE, peptides induced exposure of the D3 epitope in both the Pena and Penballelic forms of GPIIIa (Fig 2). In contrast, binding of the non-LIBS antibody AP3 was not affected by either RGDW or RGEW peptides, as expected. We also measured the binding of the activation-dependent antibody Pl-55 to GPIIb-IIIa upon induction with LIBS antibody. Binding of the Pl-55 antibody to both Pena/CHO and Penb/CHO cells was increased by the LIBS antibody D3 but not by the non-LIBS antibody AP3 (Fig 2C). The binding of Pl-55 was specific, as it could be inhibited by RGD peptides. Thus, the Gln143 form of GPIIb-IIIa expressing the Penb antigenic determinant is capable of undergoing conformational changes and binding ligand.
Flow cytometric analysis of surface-expressed allelic forms of GPIIb-IIIa. (A) Binding of LIBS antibody D3 to Pena (Arg143)-transfected CHO cells. D3 binding was measured in the presence or absence of RGDW or RGEW peptides. Whereas RGDW peptides increased the binding of D3 to transfected CHO cells, RGEW did not. As a control, binding of AP3 to the cells was also measured. Neither RGDW nor RGEW affected the binding of AP3. (B) Binding of LIBS antibody D3 to Penb(Gln143)-transfected CHO cells. D3 binding to Penb-transfected CHO cells was increased in the presence of RGDW peptides. AP3 binding was not affected by RGDW peptides. The number above each histogram represents the mean fluorescence intensity. (C) Binding of activation-dependent antibody Pl-55. Transfected CHO cells were incubated with FITC-labeled Pl-55 in the presence of AP3 or D3 (200 μg/mL) for 30 minutes. The samples were diluted with PBS and analyzed by a FACScan. Nonspecific binding of the antibody was defined in the presence of 1 mmol/L RGD peptides. D3 increased the binding of Pl-55 to both Pena- and Penb-transfected CHO cells. Pl-55 binding was inhibited by 1 mmol/L RGDW peptides.
Flow cytometric analysis of surface-expressed allelic forms of GPIIb-IIIa. (A) Binding of LIBS antibody D3 to Pena (Arg143)-transfected CHO cells. D3 binding was measured in the presence or absence of RGDW or RGEW peptides. Whereas RGDW peptides increased the binding of D3 to transfected CHO cells, RGEW did not. As a control, binding of AP3 to the cells was also measured. Neither RGDW nor RGEW affected the binding of AP3. (B) Binding of LIBS antibody D3 to Penb(Gln143)-transfected CHO cells. D3 binding to Penb-transfected CHO cells was increased in the presence of RGDW peptides. AP3 binding was not affected by RGDW peptides. The number above each histogram represents the mean fluorescence intensity. (C) Binding of activation-dependent antibody Pl-55. Transfected CHO cells were incubated with FITC-labeled Pl-55 in the presence of AP3 or D3 (200 μg/mL) for 30 minutes. The samples were diluted with PBS and analyzed by a FACScan. Nonspecific binding of the antibody was defined in the presence of 1 mmol/L RGD peptides. D3 increased the binding of Pl-55 to both Pena- and Penb-transfected CHO cells. Pl-55 binding was inhibited by 1 mmol/L RGDW peptides.
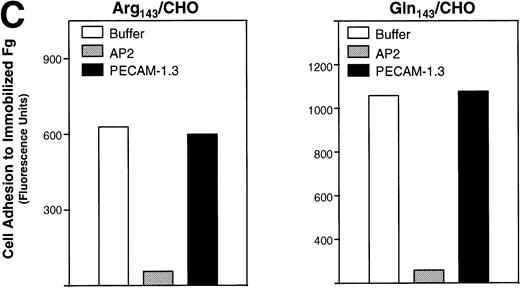
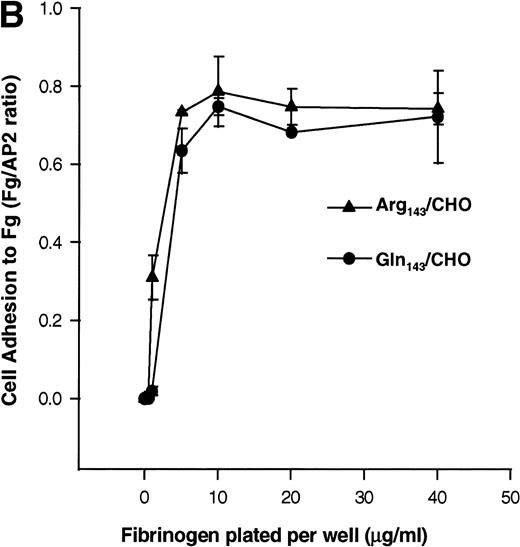
In addition, we examined the ability of the stable CHO cell transfectants to bind to soluble and immobilized Fg. Soluble Fg bound to both Pena and Penb CHO cells upon induction of the ligand binding site with the LIBS antibody D3 (Fig3A). Cell adhesion to immobilized Fg mediated by allele-specific forms of this integrin complex was examined using calcein AM–labeled cells. To directly compare the ability of these cells to adhere to substrates, cell adhesion to immobilized Fg was normalized to cell adhesion on AP2. Pena- and Penb-transfected CHO cells showed a similar pattern of adhesion to immobilized Fg following a 60-minute incubation (Fig 3B). Similar results were obtained when cells were allowed to adhere for only 10 or 30 minutes (not shown). Adhesion of these cells to immobilized Fg was mediated by GPIIb-IIIa, as the complex-specific antibody AP2 blocked attachment of cells to the matrix (Fig 3C).
Binding of transfected CHO cells to soluble and immobilized Fg. (A) Binding of transfected CHO cells to FITC-labeled Fg. Soluble Fg was labeled with FITC. Transfected CHO cells were incubated for 45 minutes at room temperature with FITC-labeled Fg at a concentration of 100 μg/mL in the presence or absence of D3 (200 μg/mL) followed by flow cytometry. The left panel shows the binding of FITC-Fg in the presence or absence of antibody D3. The right panel shows the surface expression of GPIIb-IIIa complexes as detected by AP2. (B) Cell adhesion to immobilized Fg ligand. Transfected CHO cells expressing different allelic forms of GPIIb-IIIa complex were labeled with calcein-AM at 37°C for 30 minutes. After washing, the cells were allowed to attach for 60 minutes to wells coated with various concentrations of Fg, BSA, or anti-GPIIb-IIIa complex antibody at 37°C. After adhesion, nonadherent cells were removed by washing, and adherent cells were measured in a fluorescence plate reader. Nonspecific cell adhesion on BSA-coated wells was subtracted. Cell adhesion was normalized to cell adhesion on AP2 (Fg/AP2 ratio). CHO cells expressing both allelic forms of GPIIb-IIIa bound to immobilized Fg in a dose-dependent manner. (C) Adhesion of transfected CHO cells to immobilized Fg is mediated by GPIIb-IIIa. Cells were pretreated with AP2 or anti-PECAM-1 antibodies (20 μg/mL) for 30 minutes at room temperature. After washing, cell adhesion to 2 μg/mL Fg was measured and plotted by fluorescence intensity. AP2 but not anti-PECAM-1 antibody blocked cell adhesion mediated by both allelic forms of GPIIb-IIIa to immobilized Fg.
Binding of transfected CHO cells to soluble and immobilized Fg. (A) Binding of transfected CHO cells to FITC-labeled Fg. Soluble Fg was labeled with FITC. Transfected CHO cells were incubated for 45 minutes at room temperature with FITC-labeled Fg at a concentration of 100 μg/mL in the presence or absence of D3 (200 μg/mL) followed by flow cytometry. The left panel shows the binding of FITC-Fg in the presence or absence of antibody D3. The right panel shows the surface expression of GPIIb-IIIa complexes as detected by AP2. (B) Cell adhesion to immobilized Fg ligand. Transfected CHO cells expressing different allelic forms of GPIIb-IIIa complex were labeled with calcein-AM at 37°C for 30 minutes. After washing, the cells were allowed to attach for 60 minutes to wells coated with various concentrations of Fg, BSA, or anti-GPIIb-IIIa complex antibody at 37°C. After adhesion, nonadherent cells were removed by washing, and adherent cells were measured in a fluorescence plate reader. Nonspecific cell adhesion on BSA-coated wells was subtracted. Cell adhesion was normalized to cell adhesion on AP2 (Fg/AP2 ratio). CHO cells expressing both allelic forms of GPIIb-IIIa bound to immobilized Fg in a dose-dependent manner. (C) Adhesion of transfected CHO cells to immobilized Fg is mediated by GPIIb-IIIa. Cells were pretreated with AP2 or anti-PECAM-1 antibodies (20 μg/mL) for 30 minutes at room temperature. After washing, cell adhesion to 2 μg/mL Fg was measured and plotted by fluorescence intensity. AP2 but not anti-PECAM-1 antibody blocked cell adhesion mediated by both allelic forms of GPIIb-IIIa to immobilized Fg.
Determination of affinity constants of the Pena and Penb allelic forms of the GPIIb-IIIa complex for soluble Fg.
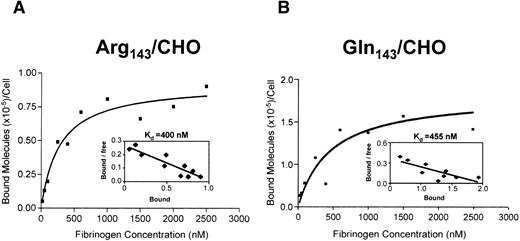
To quantitatively examine the affinity of these two forms of GPIIb-IIIa for soluble ligand, we performed binding isotherm analysis using125I-labeled Fg in the presence of the activating LIBS antibody D3. Recombinant GPIIb-IIIa expressed on CHO cells or COS-7 is in the resting state, as these cells cannot bind to soluble Fg, but can be induced into an activated conformation using LIBS antibodies.24 LIBS antibody–activated GPIIb-IIIa complex binds to Fg in a manner similar to physiologically activated GPIIb-IIIa.25 To directly measure the affinity of the receptor for Fg, we used the LIBS antibody D3 to activate and induce Fg binding to the GPIIb-IIIa complex. Specific and saturable binding of Fg to GPIIb-IIIa–transfected CHO cells was reached at a concentration of 2,000 nmol/L (Fig 4). Scatchard plot analyses of Fg binding to both Pena and Penbforms of GPIIb-IIIa showed a kd of 400 nmol/L for Pena/CHO cells and 455 nmol/L for Penb/CHO cells, respectively (Fig4, inset). Data from five experiments are summarized in Fig 4C. The Pena and Penb forms of the GPIIb-IIIa complex have a similar affinity for Fg (P = .78).
Saturation binding isotherm and Scatchard plot analysis of 125I-Fg binding to D3-activated transfected CHO cells. Transfected CHO cells expressing (A) Pena or (B) Penb forms of GPIIb-IIIa were treated with D3 at a concentration of 200 μg/mL and then incubated with increasing concentrations of 125I-Fg at room temperature for 45 minutes. Data are plotted as molecules of 125I-Fg specifically bound versus fibrinogen concentration. Scatchard plot analyses of the specific binding data are shown in the insets. Data are replotted according to the Scatchard method using the GraphPad linear regression program. kd Values for both Pena and Penb forms of GPIIb-IIIa are 400 and 455 nmol/L, respectively. (C) Summary of kd values from five different experiments. kd Values derived from the Scatchard plots are summarized. Both forms of GPIIb-IIIa bound to Fg with similar affinity, 428 nmol/L for Pena- and 457 nmol/L for Penb-transfected CHO cells, respectively (P = .78). Note that there is no significant difference in affinity for Fg between the Arg143 (Pena) and Gln143(Penb) forms of the GPIIb-IIIa complex.
Saturation binding isotherm and Scatchard plot analysis of 125I-Fg binding to D3-activated transfected CHO cells. Transfected CHO cells expressing (A) Pena or (B) Penb forms of GPIIb-IIIa were treated with D3 at a concentration of 200 μg/mL and then incubated with increasing concentrations of 125I-Fg at room temperature for 45 minutes. Data are plotted as molecules of 125I-Fg specifically bound versus fibrinogen concentration. Scatchard plot analyses of the specific binding data are shown in the insets. Data are replotted according to the Scatchard method using the GraphPad linear regression program. kd Values for both Pena and Penb forms of GPIIb-IIIa are 400 and 455 nmol/L, respectively. (C) Summary of kd values from five different experiments. kd Values derived from the Scatchard plots are summarized. Both forms of GPIIb-IIIa bound to Fg with similar affinity, 428 nmol/L for Pena- and 457 nmol/L for Penb-transfected CHO cells, respectively (P = .78). Note that there is no significant difference in affinity for Fg between the Arg143 (Pena) and Gln143(Penb) forms of the GPIIb-IIIa complex.
Signaling properties of the Pena and Penballelic forms of GPIIb-IIIa.
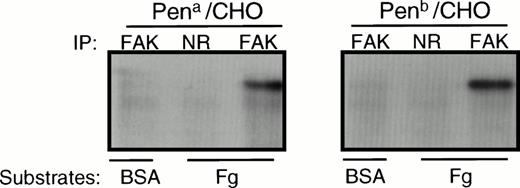
Previous studies have shown that adhesion to immobilized ligands of CHO cells expressing GPIIb-IIIa induces tyrosine phosphorylation of pp125FAK.26 To examine whether the Pen polymorphism affects the signaling properties of the integrin, we measured tyrosine phosphorylation of pp125FAK after cell adhesion to immobilized Fg. pp125FAK became tyrosine-phosphorylated in both adherent Pena- and Penb-transfected CHO cells, but not in nonadherent cells over a BSA-coated surface (Fig 5). Similar results were observed when GPIIb-IIIa–transfected CHO cells adhered to an immobilized monoclonal antibody to GPIIb-IIIa (not shown). Thus, not only are the adhesive properties of the Pena and Penb allelic forms of GPIIIa similar, but outside-in signaling appears to be unaffected by the polymorphism as well.
Tyrosine phosphorylation of pp125FAKfollowing cell adhesion to immobilized Fg. Adherent CHO cells bound to Fg-coated wells or nonadherent cells on BSA-coated wells were lysed in RIPA buffer and immunoprecipitated using an anti-FAK antibody (FAK) or normal rabbit IgG (NR). Immunoprecipitates were separated on SDS-PAGE, transferred to a PVDF membrane, and probed with HRP-conjugated PY20 to assess the tyrosine phosphorylation state of FAK. Note that adhesion of Pena and Penb transfectants to immobilized Fg induces similar levels of tyrosine phosphorylation of FAK. The gel is representative of 3 experiments.
Tyrosine phosphorylation of pp125FAKfollowing cell adhesion to immobilized Fg. Adherent CHO cells bound to Fg-coated wells or nonadherent cells on BSA-coated wells were lysed in RIPA buffer and immunoprecipitated using an anti-FAK antibody (FAK) or normal rabbit IgG (NR). Immunoprecipitates were separated on SDS-PAGE, transferred to a PVDF membrane, and probed with HRP-conjugated PY20 to assess the tyrosine phosphorylation state of FAK. Note that adhesion of Pena and Penb transfectants to immobilized Fg induces similar levels of tyrosine phosphorylation of FAK. The gel is representative of 3 experiments.
DISCUSSION
The GPIIb-IIIa complex is the most abundant protein present on the platelet surface. As a receptor for Fg and von Willebrand factor, the GPIIb-IIIa complex plays an important role in platelet aggregation and adhesion during the process of normal hemostasis and the pathogenesis of thrombotic diseases. Certain amino acid changes in the receptor resulting in a loss of either the surface expression or function of the integrin complex have been implicated in an inherited bleeding disorder, Glanzmann’s thrombasthenia (GT). In contrast, other amino acid changes create polymorphisms in the receptor, which can be recognized as alloantigens or autoantigens in an immunopathologic response. To date, there are currently seven known molecular variants of GPIIIa in the human gene pool, including Leu33(PlA1), Pro33(PlA2),27Pro33Arg40,28 Gln143(Penb),11,29 Ala407(Mo),30 Gln489 (Ca),22 and Cys636 (Sra).31 Unlike the dysfunctional GPIIb-IIIa found in GT platelets, these variant forms of GPIIb-IIIa seem to function normally, as the individuals bearing these alleles show no known difference in normal hemostasis. However, the fact that certain antibodies directed against these polymorphisms, for example, anti-Pena antibodies, are potent inhibitors of platelet aggregation implies the importance of these regions containing the polymorphisms in receptor function. Indeed, an Arg143→ Gln143 dimorphism within the RGD binding domain of GPIIIa has been shown to be responsible for the formation of the Pena and Penb alloantigenic epitopes.11
Weiss et al14 reported that individuals bearing the Pro33 variant form of GPIIIa (PlA2) might be at increased risk to develop coronary thrombosis, especially individuals under the age of 60. This finding poses an interesting question about the role of receptor polymorphism in integrin function. However, a recent study in a large cohort of apparently healthy men has indicated that inheritance of the GPIIIa PlA2 allele may not be associated with an increase in the subsequent risk of myocardial infarction, stroke, or venous thrombosis.32 Although the association between the PlA2 allele and coronary heart disease is still controversial, the functional consequence of the PlA2 allelic form of GPIIIa in contributing to the formation of a more adherent Fg receptor remains to be elucidated. Expression of PlA1 and PlA2 antigens is controlled by a single point mutation at GPIIIa nucleotide 196, resulting in a Leu33Pro dimorphism at the amino-terminal region of GPIIIa.27 However, the actual structural and functional differences between the GPs bearing these two antigens have not been identified.
The purpose of the present investigation was to identify potential functional differences between the Pena and Penb forms of GPIIb-IIIa in a controlled mammalian expression system. Previously, we demonstrated that residue 143 of GPIIIa is both necessary and sufficient to control expression of the Pena and Penb epitopes in a single GPIIIa subunit expression system.11 To determine whether these epitopes could be expressed in a GPIIb-IIIa complex on the surface of CHO cells, we established two stable cell lines expressing these two allelic forms of GPIIb-IIIa by cotransfection of GPIIb with either the Pena or Penb form of GPIIIa cDNA. Immunoprecipitation and flow cytometric analysis demonstrated that Pena and Penb forms of GPIIb-IIIa were expressed normally on the CHO cell surface and recognized by alloantibodies specific for each allelic form of GPIIIa (Fig 1).
The Pen polymorphism is located proximal to one of the previously defined ligand binding domains of the GPIIb-IIIa complex (residues 109 to 171 of GPIIIa), where both an Asp119Tyr substitution and a flanking Ser162Leu amino acid change have been shown to abolish ligand binding or affect complex maturation in two GT patients.12 33Whether the Gln143 form of GPIIIa (Penb) has an advantage or disadvantage over the Arg143(Pena) form with respect to affinity for Fg has not been established. In this study, we examined the function of the Penb form of GPIIIa in ligand binding and post–ligand binding events of GPIIb-IIIa. Using a LIBS antibody as a reporter for conformational changes in the receptor upon ligand occupancy, we found that the Penb form of GPIIb-IIIa was capable of assuming an activated conformational state upon ligand binding. We also demonstrated that the Penb form of GPIIb-IIIa could be induced into an activated state capable of binding soluble ligand in an RGD-inhibitable manner. Furthermore, the Penb form of GPIIb-IIIa could mediate downstream signaling events such as cell spreading and tyrosine phosphorylation of pp125FAK. Together, these data suggest that the Penb form of GPIIb-IIIa is functionally normal compared with the Penaform.
To examine more precisely the adhesive properties of these two stable cell lines for soluble Fg, we performed ligand binding isotherm analysis using radioactively labeled Fg. We found that the binding affinity for Fg of the Pena form of the GPIIb-IIIa complex was not significantly different from that of the Penb form, suggesting that the Arg143Gln polymorphism has little or no effect on integrin function. This observation is consistent with our previous finding that platelets derived from a Penb/b homozygous individual aggregate normally in response to ADP.11
We would have liked to study the adhesive properties of these two integrin variants using human platelets. Unfortunately, Penb/b homozygous individuals are rare in the Western hemisphere and difficult to obtain in Asia. Therefore, we expressed the Pena (Arg143) or Penb(Gln143) forms of the GPIIb-IIIa complex on CHO cells. This approach has been widely used for studying integrin function, including ligand binding and signal transduction. Effects of many naturally occurring mutations of GPIIb or GPIIIa on integrin function have been recapitulated in CHO cell lines expressing mutant forms of integrin constructs,23,34,35 despite the fact that a few differences exist between GPIIb-IIIa expressed on CHO cells versus naturally presenting cells such as platelets. For example, CHO cell GPIIb-IIIa is unable to become activated from within the cell and bind to soluble ligand.24 However, activation of GPIIb-IIIa on CHO cells can be achieved from outside the cell using activating LIBS antibody, and the GPIIb-IIIa complex activated in this fashion has been shown to bind Fg in a manner similar to physiologically activated GPIIb-IIIa.25 By using LIBS antibodies as activators, we examined the adhesive properties of the Pena and Penb allelic forms of GPIIIa with respect to the ability to bind to soluble or surface-bound ligands (Figs 3 and 4). Despite the location of this GPIIIa polymorphism within the ligand binding site, no significant difference in ligand binding was observed between these two allelic forms of GPIIb-IIIa.
In summary, we have produced and functionally characterized stable cell lines expressing the Pena and Penb allelic forms of GPIIb-IIIa in CHO cells. These stable cell lines should provide useful diagnostic reagents for serologic determination of platelet alloantigenic epitopes. By direct determination of the ligand binding affinity of different allelic forms of GPIIb-IIIa for Fg, we have found that the Arg143Gln polymorphism located proximal to the important ligand binding site of GPIIIa has little or no effect on integrin function. The use of this approach should permit other investigators to directly measure the ligand binding affinity of the other allelic forms of GPIIb-IIIa, and address the important mechanistic issue of whether molecular polymorphisms affect integrin function and subsequently contribute to the development of coronary artery disease.
ACKNOWLEDGMENT
We thank Dr Lisa Jennings and Dr Beat Steiner for the generous gift of monoclonal antibodies D3 and P1-55, and Dr Mike Mosesson for the generous supply of peak I Fg.
Supported in part by National Institutes of Health Grant No. HL-44612 (P.J.N.) and American Heart Association (Wisconsin Affiliate) Predoctoral Fellowship Award No. 95F-Pre-16 (R.W.).
Presented in abstract form at the 38th Annual Meeting of the American Society of Hematology, Orlando, FL, December 6-10, 1996.
Address reprint requests to Ronggang Wang, MD, PhD, Blood Research Institute, The Blood Center of Southeastern Wisconsin, 638 N 18th St, PO Box 2178, Milwaukee, WI 53201-2178; e-mail:rwang@smtpgate.bcsew.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal